All published articles of this journal are available on ScienceDirect.
Studying the Response of Anethum graveolens L. to the Stress of Salinity and Foliar Spray with Alhagi Maurorum Aqueous Extract
Abstract
Background
Weed infestation and salinity are both biotic and abiotic stresses that are highly present in the Basrah governorate in Iraq and widely influence crop growth
Methods
The present work was conducted to evaluate the effect of salinity at (0, 6, 12 dS/m) and aqueous extract of Alhagi maurorum at (0, 3 g/L) on growth and biochemical parameters as well as phytocomponents of Dill plant Anethum graveolens L.
Results
Results revealed that growth parameters and photopigment content increased with salinity at 12 dS/m and reduced with Alhagi maurorum aqueous extract. Besides that, carbohydrate content was reduced with both stresses, and proline content increased with Alhagi maurorum aqueous extract. GC-MS chromatogram of Anethum graveolens methanolic extract displayed high alteration in phytocomponents quantity post salinity and camelthorn extract treatments.
Conclusion
Current work inferred that the Dill plant has high tolerance properties to salinity contrary to weed infestation stress. Stresses combination declined growth parameters and reduced metabolite content of Dill plants.
1. INTRODUCTION
Soil salinity is a major global issue in the world due to its massive adverse impact on agricultural productivity and sustainability. In Iraq, irrigation with salt water is considered a major source of salt increase in the soil [1]. The rising of water salinity, especially in the southern of Iraq and specifically in Basrah is due to industrial and urban waste discharge in the water bodies, low rainfall, and high evapotranspiration [2]. In addition, the elevation of the salt tide in the Shatt Al-Arab River (the main source of water in Basrah) owing to the mismanagement of water resources and the aquatic policy of neighboring countries exacerbated the problem extent [3]. The last evaluation study for Shatt Al-Arab water reported that it is not suitable for drinking and irrigation [4]. Plants can be divided into two categories that are related to salinity tolerance: glycophytes and halophytes. Glycophytes are extremely sensitive to soil salinity and could be included in a majority of plant life and all major crops; halophytes are salt tolerant and often grow in salty environments. The effectiveness of salt stress can be identified in two main ways: osmotic stress and ionic toxicity. The stress and toxicity could affect all major plant processes, such as photosynthesis, cellular metabolism, and plant nutrition [5].
Alhagi maurorum, or Camelthorn, is a perennial plant that belongs to the Fabaceae family and is native to North Africa, the Middle East and southeast Europe [6]. A. maurorum is a wild plant that grows in different types of soil [7], and it can be seen approximately in all areas in Basrah. The constituents of A. maurorum and their biological activity are widely investigated in various studies. Plant extract exhibited numerous bioactive metabolites, including flavonoids, coumarins, glycosides, fatty acids, unsaturated sterols, sterols, steroids, vitamins, resins, alkaloids, tannins, carbohydrates, and triterpenes that permit the plant pharmacological and therapeutic properties [8-10]. However, the highest phenolic compounds were observed in leaves and flower extract [50 and 32 mg/g) respectively [11]. The presence of phenolic compounds in a high content is the main reason for the allelopathic act of A. maurorum against plant growth [12].
Apiaceae or Umbelliferae family involves approximately 450 genera and 3700 species worldwide [13]. Apiaceae family plants are commonly consumed as fresh vegetables or in culinary or for therapy purposes due to the high content of nutritional, aromatic and active components [14]. Anethum graveolens or Shipth, (the common Arabic name of Dill) is consumed widely in Iraq. Green parts are used to prepare the known traditional dishes, while seeds are used as a spice or an ingredient in the therapeutic mixture. Dill is rich with phytocompounds that identified 17 components, including volatile compounds, such as Dillapiole, Carvone, Limonene, Anethole, and Eugenol, as well as Flavonoids, Coumarins, α-Phellandrene, and Phenols [15]. The biological potency of the extract and essential oil of Dill has been reported in numerous studies. Aqueous extract of seeds inhibited significantly broad-spectrum of bacteria, such as S. aureus, E. coli, P. aeruginosa, S. typhimurium, Shigella flexneri and Salmonella typhi, that cause nosocomial infections [16]. Similarity, essential oil exhibited a high inhibition activity against gram-positive and gram-negative bacteria and the inhibitory effect increased with the manner of essential oil concentration [17]. The main therapeutic use of A. graveolens seeds is relieving digestive problems, as well as relieving intestinal convulsion and griping, helping to settle colic, ameliorate appetite, reduce gas and aid digestion [18, 19]. Seeds are also used to stimulate milk flow in lactating mothers and are often added to cattle fodder for this reason [20].
The aim of a current study is to investigate the impact of two types of stresses that are prevailing in Basrah: biotic stress A. maurorum infestation and abiotic stress salinity on growth, biochemical traits and phytocomponents content of A. graveolens as well as determination for which Dill plants are more tolerant.
2. MATERIALS AND METHODS
2.1. A. Maurorum Extraction and Salt Solutions Preparation
Camelthorn plants were uprooted before the flowering stage from areas of the University of Basrah location, washed with tap water, dried at room temperature, and ground to a fine powder. About 3 g powder was macerated with warm water over night, filtrated, and extract used for foliar application on Dill plants later (R1). Stock salt water was collected from ponds of rainfall water drainage in the University of Basrah location; 6 and 12 dS/m concentrations were obtained from stock and utilized for plant irrigation (S1 and S2) respectively.
2.2. A. Graveolens Cultivation and Treatment
Dill seeds were obtained from the local market and sown in trays with peatmoss substrate. Post seedlings reached 5 cm, and they were transplanted in pots 35 cm in diameter with a 3:1 ratio of soil to peatmoss substrate. For one weak post-plant transplantation, irrigation with salt water was applied on plants at 400 ml for each pot once a week. Later, the number of irrigation times increased according to the climate and plant requirements. Foliar spray with A. maurorum extract was applied 3 times on plants during growth season.
2.3. Growth Criteria
Vegetative and floral growth criteria were recorded for Dill plants, including plant height, branches number, leaves number, inflorescences number, flower number, fruit set time, plant fresh weight, and biomass of seeds inflorescence.
2.4. Biochemical Criteria
Dill plants were ground to tiny particles and utilized for proline and carbohydrate content assessment as well as GC-MS analysis.
2.4.1. Photosynthetic Pigment
Chlorophyll A, Chlorophyll B, total Chlorophyll, and Carotenoids were determined in leaves 100 as described earlier by [21]. About 0.5 g of plant leaf was incubated with 10 ml of 80% acetone overnight at 4 0C. Next day, filtrate was suspended and made up to a final volume of 30 ml. The absorbance measured at 663, 645, and 452 nm against blank and pigments concentration was calculated according to [22] using the following equations:
Chlorophyll a (mg/100g FW)
=(10.3 x A663-0.92 x A645)/1000 x 30/0.5 x100
Chlorophyll b (mg/100g FW)
=(19.7 x A645-3.87 x A663)/1000 x 30/0.5 x100
Total chlorophyll (mg/100g FW)
Chl a + Chl b
Carotenoids (mg/100g FW)
=(4.2 x A452)-((0.026 x Chl a)+(0.426 x Chl b)) /1000 x 30/ 0.5 x 100
2.4.2. Proline Content
Free proline content was estimated as described earlier [23]. About 0.25 g of dried sample was homogenized in 10 ml of 3% aqueous sulfosalicylic acid and filtrated; 2 ml of filtrate was reacted with 2 ml of cold acid-ninhydrin (1.25 g of ninhydrin in 30 ml glacial acetic acid and 20 ml of 6 M phosphoric acid) and 2 ml of glacial acetic acid in a test tube at 100°C water. Post 1 hour, the reaction terminated in the ice bath, and then 4 ml of toluene was added to the tubes, mixed well, and waited for phase separation. The separated aqueous phase was warmed to room temperature, and the absorbance was read at 520 nm using toluene as a blank. The proline concentration was estimated according to the standard curve and calculated on a dry weight basis as per the following formula:
µmoles proline/g=[(µg proline/ml x ml toluene) / 115.5 µg/mole]/[(g sample)/ 5]
2.4.3. Carbohydrates Content
The content of carbohydrates was estimated to, as described earlier [24], 0.5 of the dried sample was homogenized with 70 ml of distilled water and heated in a water bath at 70 0C for 1 hour. Then, it was cooled to room temperature and filtrated; 5 ml of filtrate was placed in a separate tube and diluted with 25 ml of distilled water. 1 mL of solution was mixed with 1 mL of 5% phenol in a test tube. Subsequently, 5 mL of concentrated sulfuric acid was added rapidly to the mixture, vortexed and placed in the water bath for 20 min for color development. The absorption was recorded by spectrometry at 490 nm, and carbohydrate concentration was determined from the sugar standard curve and calculated in dry weight following the equation:
Carbohydrates content mg/g=(mg glucose/ml x final sample volume ml) / sample weight g
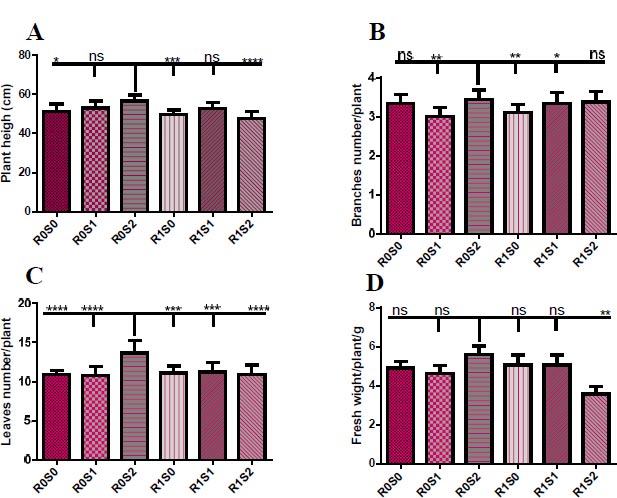
(A) Representative the effect of treatment with Camelthorn extract and salinity on the height of Dill plants.. R0S0 control or untreated plants. R0S1plants that irrigated with salinity at 6 dS/m. R0S2 plants that irrigated with salinity at 12 dS/m. R1S0 plants that sprayed with 3 g/L of A. maurorum extract. R1S1 plants that sprayed with 3 g/L of A. maurorum extract and irrigated with salinity at 6 dS/m. R1S2 plants that sprayed with 3 g/L of A. maurorum extract and irrigated with salinity at 12 dS/m. (B) Representative of the effect of treatment with Camelthorn extract and salinity on branches number of Dill plants. (C) Representative of the effect of treatment with Camelthorn extract and salinity on leaves number of Dill plants. (D) Representative of the effect of treatment with Camelthorn extract and salinity on fresh weight of Dill plants. A multiple ANOVA was performed using ordinary one-way ANOVA multiple comparisons to compare the averages of treatments. Significance was designated as follows: *p < 0.05, **P < 0.01***P < 0.001, ****p < 0.0001.
2.5. Experiment Design and Statistical Analysis
The experiment was designed as a randomized complete block design (R.C.B.D.) in three blocks with three replicates. The results were analyzed by Graph Prism program version 6.01, and the averages of treatment were compared at the probability level 0.05 by one-way analysis of variance ANOVA.
3. RESULTS
Fig. (1) illustrates the vegetative growth parameters of treated plants with camelthorn extract and salinity concentrations. Plants that were irrigated with 12 dS/m of salt water R0S2 recorded the highest height of plants compared to other plants. While plants that were treated with 3 g/L of camelthorn extract and irrigated with 12 dS/m of salinity R1S2 recorded the lowest plant height (Fig. 1A). The number of branches also increased in irrigated plants with 12 dS/m salt water R0S2 compared to other plants, particularly to 6 dS/m-irrigated plants R0S1 and 3 g/L-treated plants (Fig. 1B). For the leaves number parameter, irrigation plants with 12 dS/m R0S2 caused an increase in the leaves number of Dill plants compared to other plants (Fig. 1C). Similarly, 12 dS/m-irrigated plants showed an increase in fresh weight compared to other plants, especially plants that were treated with camelthorn extract and irrigated with 12 dS/m of salinity R1S2 (Fig. 1D)
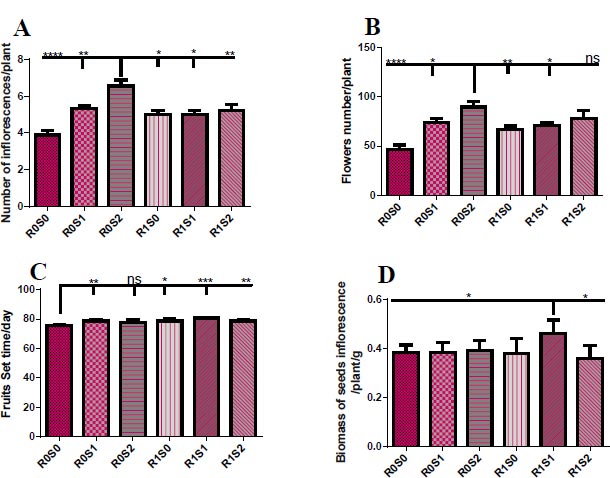
(A) Representative the effect of treatment with Camelthorn extract and salinity on inflorescences number of Dill plants. R0S0 control or untreated plants. R0S1 plants that irrigated with salinity at 6 dS/m. R0S2 plants that irrigated with salinity at 12 dS/m. R1S0 plants that sprayed with 3 g/L of A. maurorum extract. R1S1 plants that sprayed with 3 g/L of A. maurorum extract and irrigated with salinity at 6 dS/m. R1S2 plants that sprayed with 3 g/L of A. maurorum extract and irrigated with salinity at 12 dS/m. (B) Representative of the effect of treatment with Camelthorn extract and salinity on flower number of Dill plants. (C.) Representative of the effect of treatment with Camelthorn extract and salinity on fruit set time of Dill plants. (D) Representative of the effect of treatment with Camelthorn extract and salinity on seeds inflorescences biomass of Dill plants. A multiple ANOVA was performed using ordinary one-way ANOVA multiple comparisons to compare the averages of treatments. Significance was designated as follows: *p < 0.05, **P < 0.01, ***P < 0.001, ****p < 0.0001.
Fig. (2) elucidates the floral growth parameters of treated plants with camelthorn extract and salinity concentration. 12 dS/m-irrigated plants exhibited an increase in the number of inflorescences and flowers per plant compared to other plants (Fig. 2A, B). For the set time of Dill fruits, control plants R0S0 showed early set fruit time compared to treated plants (Fig. 2C). While treatment with 3 g/L of camelthorn extract and 6 dS/m salinity R1S1 caused an increase in the weight of seeds inflorescence compared to other plants (Fig. 2D).
Fig. (3) illustrates the effect of foliar spray with camelthorn extract and irrigation with salt water on biochemical features. Plants that were irrigated with 12 dS/m of salinity R0S2 showed a rise in Chlorophyll A, Chlorophyll B, total Chlorophyll, and Carotenoids compared to other plants (Fig. 3A, B, C, D). A significant increase in proline content is obtained in plants treated with 3 g/L of camelthorn extract R1S0 compared to other plants (Fig. 3E). On another hand, carbohydrates content raised in control plants R0S0 compared to other treated plants (Fig. 3F). GC-MS result of methanolic extract of A. graveolens displayed numerous of phytoconstituents that increased or decreased due to the treatments (Table 1). Monoterpenes p-Cymene and D-Limonene increased in R0S1, R0S2 and R1S2-treated plants compared to R0S0 control plants (Fig. 4A, B, C, F). However, these compounds vanished, and other monoterpenes compounds alpha-Phellandrene and Cyclohexene,1-methyl-4-(1-methylethenyl)- detected with A. maurorum extract treatment in R1S0 and R1S1-treated plants (Fig. 4D, E).

(A) Representative the effect of treatment with Camelthorn extract and salinity on leaf content of chlorophyll A. R0S0 control or untreated plants. R0S1 plants that irrigated with salinity at 6 dS/m. R0S2 plants that irrigated with salinity at 12 dS/m. R1S0 plants that sprayed with 3 g/L of A. maurorum extract. R1S1 plants that sprayed with 3 g/L of A. maurorum extract and irrigated with salinity at 6 dS/m. R1S2 plants that sprayed with 3 g/L of A. maurorum extract and irrigated with salinity at 12 dS/m. (B) Representative of the effect of treatment with Camelthorn extract and salinity on leaf content of chlorophyll B. (C) Representative the effect of treatment with Camelthorn extract and salinity on leaf content of total chlorophyll. (D). Representative of the effect of treatment with Camelthorn extract and salinity on leaf content of Carotenoids. (E) Representative of the effect of treatment with Camelthorn extract and salinity on proline content. (F) Representative of the effect of treatment with Camelthorn extract and salinity on carbohydrate content. A multiple ANOVA was performed using ordinary one-way ANOVA multiple comparisons to compare the averages of treatments. Significance was designated as follows: *p < 0.05, **P < 0.01, ***P < 0.001, ****p < 0.0001.
Table 1.
| Peak Area % | ||||||||
|---|---|---|---|---|---|---|---|---|
| NO. | R.T. | Compound Name | R0S0 | R0S1 | R0S2 | R1S0 | R1S1 | R1S2 |
| 1 | 5.93 | p-Cymene alpha-Phellandrene |
0.999 - |
1.220 - |
1.853 - |
- 2.692 |
- 1.365 |
1.162 - |
| 2 | 5.968 | D-Limonene Cyclohexene,1-methyl-4-(1-methylethenyl)- |
1.305 - |
2.236 - |
5.181 - |
- 14.824 |
- 8.822 |
4.4646 |
| 3 | 7.621 | Dill ether | 1.261 | 1.285 | 0.870 | 1.536 | 0.478 | 1.038 |
| 4 | 7.95 | 5-Hydroxymethylfurfural | 4.033 | 2.146 | - | - | - | - |
| 5 | 8.085 | (-)-Carvone | 8.545 | 8.545 | 8.794 | 10.320 | 16.417 | 4.235 |
| 6 | 9.596 | 4-Heptadecanone | 4.360 | - | - | - | - | - |
| 7 | 10.008 | Phenol, 4-ethenyl-2,6-dimethoxy- | 0.791 | 0.874 | 1.954 | 2.672 | 0.989 | 0.959 |
| 8 | 10.33 | Dillapiole | 18.327 | 18.695 | 24.342 | 33.807 | 27.059 | 13.902 |
| 9 | 11.699 | n-Hexadecanoic acid | 4.944 | 6.538 | 7.042 | 7.500 | 4.516 | 9.388 |
| 10 | 12.432 | cis-13-Octadecenoic acid 9-Octadecenoic acid |
7.27 | 5.4577 - |
4.237 - |
- 5.028 |
- 4.927 |
- 3.008 |
| 11 | 18.021 | Nonacosan-10-one | 4.623 | 7.251 | 6.009 | 5.071 | 6.872 | 5.0342 |
| 12 | 19.443 | Stigmasterol | 3.776 | 3.645 | 3.144 | 2.236 | 2.694 | 2.483 |
| 13 | 19.899 | gamma.-Sitosterol | 2.992 | 3.498 | 3.498 | 2.908 | 3.366 | 3.104 |
Also, a monoterpene Carvone increased with all treatments except R1S2-treated plants. On the other hand, aldehyde compound 5-Hydroxymethylfurfural and saturated hydrocarbon 4-Heptadecanone declined or vanished with all salinity and A. maurorum extract treatments. Dillapiole is an essential oil and the most present compound in Dill extract, except for R1S2-treated plants. Dillapiole highly increased with treatments special in plants sprayed with camelthorn extract R1S0. Moreover, monounsaturated fatty acid cis-13-Octadecenoic increased in R0S1 and R0S2-treated plants compared to R0S0 control plants (Fig. 4A, B, C) and replaced in profile data of R1S0, R1S1, and R1S2-treated plants with another unsaturated fatty acid 9-Octadecenoic acid (Fig. 4D, E, F). The sterol compounds Stigmasterol and gamma.-sitosterol increased and declined with treatments, respectively.

(A) Representative GC-MS profile of methanolic extract of R0S0 control or untreated plants. (B) Representative GC-MS profile of methanolic extract of R0S1 plants that irrigated with salinity at 6 dS/m. (C) Representative GC-MS profile of methanolic extract of R0S2 plants that irrigated with salinity at 12 dS/m. (D) Representative GC-MS profile of methanolic extract of R1S0 plants sprayed with 3 g/L of A. maurorum extract. (E) Representative GC-MS profile of methanolic extract of R1S1 plants sprayed with 3 g/L of A. maurorum extract and irrigated with salinity at 6 dS/m. (F) Representative GC-MS profile of methanolic extract of R1S2 plants sprayed with 3 g/L of A. maurorum extract and irrigated with salinity at 12 dS/m.
4. DISCUSSION
The vegetative growth of A. graveolens is used widely in culinary due to its desirable flavor. From the results, there is a remarkable increase in vegetative growth with irrigation at 12 dS/m of salt water. Obtained results highly suggest that A. graveolens is not only tolerant to salinity, but it could be reached to optimal growth level under salinity conditions (12 dS/m or up). The obtained result agree with the findings of Kasem and Yousif for A. graveolens and Tetragonia tetragonioides respectively [25, 26]. On the other hand, individual treatment with A. maurorum extract or with salinities special at 12 dS/m inhibited vegetative growth of Dill plants significantly. The inhibition effect of Camelthorn extract could be attributed to the massive presence of phenolic compounds, such as Hydroquinone and Sinapyl alcohol, that represent 33% and 25% of the compound profile of A. maurorum aqueous extract, respectively [27]. Phenolic compounds have the ability to passively affect polar auxin transport, which plays a remarkable role in plant hormones' coordination and plant life cycle processes [28].
A. graveolens has umbrella-shaped inflorescences that hold clusters of small yellow-green flowers with distinctive aroma. In the current study, the increase in the number of inflorescences and total flower number per plant with 12 dS/m salinity irrigation R0S2 was obtained. The increase may be attributed to increasing of plant branch numbers with saltwater treatment or due to leaf numbers increasing with this treatment, which consequently raised photosynthesis efficiency and increased phytohormones export from leaves to apical meristems, which positively influenced floral induction [29]. For fruit set time, control plants R0S0 exhibited early set fruit time compared to treated plants, which may be due to converting the utilization of accumulated carbohydrates by untreated plants to early fruit set instead of the growth [30]. While plants were treated with both stress agents, 3 g/L and 6 dS/m-treated plants were the most delayed for fruit set time. S1R1 plants also recorded a significant increase in the biomass of seed inflorescence per plant. This increase may be attributed to a slight lack in the seeds shedding because of the late time of fruit setting or to the role of flavonoids in A. maurorum extract, which slightly improved the biomass of seed inflorescence.
Photosynthetic pigments in plants are considered a sensitive indicator for salinity tolerance. Chlorophyll A, B, total Chlorophyll and Carotenoids underwent a gradual salt-dependent increase from 6 to 12 dS/m. Under the salinity condition, an increase in photopigmented content is observed in some tolerant genotypes of rice and halophyte plants [31, 32]. This increase was one of the plant’s strategies for salinity tolerance as well as the ratio of Chlorophyll A content to Chlorophyll B content and the increase of Carotenoids under salinity status suggests a rearrangement mechanism of photosystem composition in order to avoid photoinhibition risks [32]. Increasing photopigment content drove consequently the growth promoting in salinity-treated plants. Treated plants with 3 g/L of A. maurorum extract and 12 dS/m salinity exhibited a high reduction in pigments due to the severe impact of stress combination on plant metabolism. Proline accumulation in Dill plants increased significantly in treated plants with 3 g/L of A. maurorum extract. Proline is a recognized stress marker that provides protection and induces tolerance against biotic and abiotic stresses via acting as an excellent osmolyte, antioxidative defense molecule, metal chelator and signaling molecule [33]. The interesting part of our result is that irrigation with salinity reduced proline accumulation in a pattern. The reduction may be due to converting the utilization of nitrogen to growth enhancement in these plants instead of proline synthesizing as a stress protection agent [34]. Carbohydrate content was reduced under salinity and A. maurorum extract treatment compared to control plants, which could be attributed to the utilization of these plants' carbohydrates to recover stress effect by synthesis of osmotic adjustment metabolites, specific proteins, and certain free radical scavenging enzymes [35].
From the GC-MS result of A. graveolens methanolic extract, we obtained interesting alterations in the peak area percentage of phytocomponents due to the treatments. For instance, volatile compounds, such as p-Cymene, D-Limonene, alpha-Phellandrene Cyclohexene,1-methyl-4-(1-methyl ethenyl)- and Carvone highly altered according to salinity and Camelthorn treatment due to the stress effect [36]. Treatment with Camelthorn extract caused an increase in several active compounds, including alpha-Phellandrene, Cyclohexene,1-methyl-4-(1-methylethenyl)- Dill ether and Dillapiole, which is similar to the reported finding of Khalil and his team [27]. Treatment with salinity and Camelthorn extract reduced approximately the majority of phytocomponents in Dill plants due to the diminishing growth of plants caused by both stress types.
CONCLUSION
Our work concluded that A. graveolens was not only tolerant to salinity but also reached optimal growth under salinity conditions. On the other hand, Dill plants were sensitive to weed infestation and were affected passively by A. maurorum aqueous extract treatment. Moreover, the combination of stress types reduced the growth and metabolite content of Dill plants massively.
AUTHORS’ CONTRIBUTION
The methodology was given by W.H.N., F. A. H., F. M. J., and A. J. H; formal analysis was done by W.H.N.; writing was done by W. H. N. funding was looked over by W. H. N., and F. A. H. All authors read and agreed to the published version of the manuscript.
LIST OF ABBREVIATIONS
| DS/m | = Decisiemens per metre |
| G/L | = Gram per Liter |
| µmoles | = Micromoles |
| Mg | = Mele gram |
| µg | = Microgram |
| Ml | = Mele |
| GC-MS | = Gas Chromatography-Mass Spectrometry |
| ANOVA | = Analysis of Variance |
| R.C.B.D. | = Randomized Complete Block Design |
| A | = Absorbance |
| Chl a | = Chlorophyll a |
| Chl b | = Chlorophyll b |
| FW | = Fresh Weight |
| Fig | = Figure |
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the [PRIDE] at [https://www.ebi.ac.uk/pride/ markdownpage/globus], reference number [1-20231216- 141515-3135956].
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Najla Jaber and Dr. Rashad Imran, the faculty in the Soil and Water Resources Department/ University of Basrah, for the provided facilities.


