All published articles of this journal are available on ScienceDirect.
In vitro Methodology to Assess Quantitative Resistance in Plant-fungus Pathosystems
Abstract
Background:
Quantitative resistance (QR) to fungal pathogens remains a primary focus of all major host breeding programs. Field screenings for resistant plants, although accurate and effective, demand significant time and a sizable workforce to accomplish. Moreover, weather conditions in the field may not always be favorable for uniform disease spread, which eventually may lead to the failure of the overall experiment. The development of a more efficient and reliable bioassay to screen for resistance to fungal pathogens would be advantageous for any breeding program working on disease resistance; however, only if it correlates with field screening trials.
Objective:
The objective of this review is to combine novel findings related to rapid screening methods to evaluate QR, which are needed to accelerate the progress in developing fungal disease resistance in cultivars. Insights into the in vitro quantitative components of the host-pathogen interaction, factors affecting in vitro evaluation in young plant materials, as well as molecular pathways for the association between the in vitro and adult plant responses to fungal infection are also reviewed.
Conclusion:
An in vitro method was found to be efficient and successful in terms of inoculum volume, plant samples, and working space. The main advantage of this method is its predictive ability for adult plant disease. In addition, it provides reproducible results and is found to be a simple and reliable method. The in vitro assay allows rapid and early determination of resistance/susceptibility to fungal pathogens, which can be incorporated into a breeding program for identifying resistant plants.
1. INTRODUCTION
When infection is possible, interactions between fungal pathogens and plants can be characterized by several quantitative traits related to disease development [1]. Overall, the values arising from these quantitative traits result from the host effect (quantitative resistance [QR]), the pathogen effect (quantitative pathogenicity, often called aggressiveness in the plant pathology literature), and their interaction. QR in agricultural crops against fungal pathogens is characterized by a continuous variation ranging from very low to moderate levels of resistance [2]. QR is characterized by a reduced rate of epidemic development in the host population by altering spore infection efficiency, time from infection to sporulation, lesion development rate, and the abundance of spores produced, and therefore the severity of disease (DS) [3]. These quantitative disease resistance components follow directly from the differential-difference equation proposed by Van der Plank [4] as a model for epidemic development. QR is generally thought to be more durable than qualitative disease resistance and is therefore being used to an increasing extent in crop protection [5]. Qualitative resistance, determined by the presence of R genes, only reduces the initial inoculum by removing avirulent spores [4]. QR has, until recently, been used less in breeding programs than qualitative resistance, mainly because the latter has been easier to incorporate into desirable agronomical genotypes [6].
Disease resistance is a sustainable solution to plant disease control due to environmental and economic factors [2, 5]. Among several methods used to assess the level of resistance among plants or genotypes, field screening through natural or artificial infection is preferred as it matches the situation that a producer confronts while growing crops [4]. However, field screening has limitations since it depends on proper environmental conditions, such as it can often be carried out only once a year, is time-consuming and expensive [7]. In addition, field screening demands substantial time, area and workforce to maintain the number of plants required for an experiment [8]. Moreover, uncontrollable environmental conditions, such as humidity, temperature, and the simultaneous presence of other pathogens in the field, may not always be favorable for disease propagation, which may lead to the failure of the overall experiment [9]. The whole plant inoculation method in the greenhouse has some major logistical disadvantages in terms of resources and destructive phenotyping [10]. Only a limited number of cultivars can be evaluated because of space limitations, and the risk of cross-contamination also reduces the number of assays that can be performed at a given time. More often, such limitations restrict quick progress in breeding plants for disease resistance [11-13].
The development of a more efficient, simple and reliable bioassay for resistance screening of facultative and obligate parasites without the limitations posed by field and greenhouse trials would be advantageous for any breeding program working on disease resistance [14]. An in vitro assay, being a non-destructive (for the whole plant) disease evaluation method, can facilitate mass and rapid germplasm screening [15-18], one of the key elements in plant-breeding programs [19]. It would require much less space and inoculum and allows a specified plant to be evaluated with multiple pathogens [20]. In several plant-fungal pathosystems, in vitro assays appear more precise and reproducible than field screenings; thus, the results are not prone to fluctuations in environmental conditions [21-23]. It allows the screening of a single plant against multiple pathogens at a time. Moreover, this method can be utilized while screening a segregating population where each plant has unique genetics; therefore, seeds from those plants can be saved for further breeding purposes. In vitro assays have been used to differentiate the level of resistance in several plants, ranging from herbaceous crops to large tree species, with a high success rate [24-26]. In vitro assays are reliable and efficient alternatives to field and greenhouse screening methods and may be used for mass screening of host plants to facilitate fungal resistance breeding [27-29]. Furthermore, cultivars identified as resistant based on these rapid screening methods must be evaluated under field conditions as the final confirmatory test [30]. The detached leaf disease phenotyping technique has been extensively used and reported as a rapid and alternative tool for screening several plant species for resistance against numerous biotrophic, hemibiotrophic and necrotrophic fungal pathogens [7-10, 30-33]. The objective of this review is to combine novel findings related to quick screening methodologies to assess QR, which are requested to speed up the progress in developing resistant cultivars to fungal infection. Insights into the in vitro quantitative components of the host-pathogen interaction, factors affecting in vitro evaluation in young plant materials, as well as molecular pathways for the association between the in vitro and adult plant responses to fungal infection are also reviewed.
2. INSIGHTS INTO THE IN VITRO METHODOLOGIES USED IN THE HOST-FUNGAL PATHOGEN PATHOSYSTEMS
Resistance against fungal pathogens remains a primary focus of all major host breeding programs [2]. The development of genetically resistant cultivars is a cost-effective and desirable option to reduce yield losses caused by fungal pathogens [5]. Although QR to fungal diseases has been identified, it is considerably more challenging to assess than qualitative resistance [3, 5]. In the process of resistance breeding, screening for fungal resistance needs to be performed with accuracy, which is conducted either in a greenhouse or in a field by applying the whole plant assay technique [4]. However, both involve tedious procedures and have challenges [34-41]. Experimental efficacy for the fungal-plant host pathosystem could be improved considerably by reducing inoculum production time, increasing the reliability of inoculum production, and increasing the number of cultivars that can be evaluated in each experiment, especially when large numbers of fungal isolates are to be screened for comparisons of pathogenicity [42-49].
The advanced in vitro assay allows better control over humidity, temperature and a large number of replicates with less inoculum and may therefore be an option to eliminate the limitations [50-57]. In vitro techniques have the advantage of being more efficient in terms of the time of execution and the number of revealed components of QR, such as the disease intensity, latent period, and incubation period [7-10]. In addition, the inoculation of various living plant parts, such as seeds, fruits/heads, stems, leaves, and roots, has been used successfully in many plant-fungal systems. The required host material for each in vitro assay is small compared to the whole plant disease evaluation method (Fig. 1).
The advantageous features of this method reside in its predictive ability of adult plant disease; the results from the in vitro assay are considerably correlated and comparable with the results of screening under greenhouse and field conditions in many fungal-plant intersections [7-10]. Using ranking studies, the relative resistance levels of host cultivars under in vitro experiments appear comparable to resistance levels in the field or greenhouse, indicating that in vitro method predicts resistance occurring at the earliest and latest host development stages during disease infection [7-10]. The in vitro assay can prevent unnecessary interactions between pathogens during host evaluation for resistance to different pathogens or different isolates/strains of the same fungus [34]. With this method of screening, a host plant can be screened repeatedly because inoculations can be done at any growth stage without affecting the growth and seed production of the rest of the plant. This method also saves time in breeding for resistance and works well on newly expanding leaves because they appear to have less contamination [34]. The in vitro method can be used during the simultaneous evaluation of progenies for multiple resistance as a single plant can be evaluated several times at any growth stage without affecting the growth of the plant, thus, facilitating a selection program at a reduced cost [36].
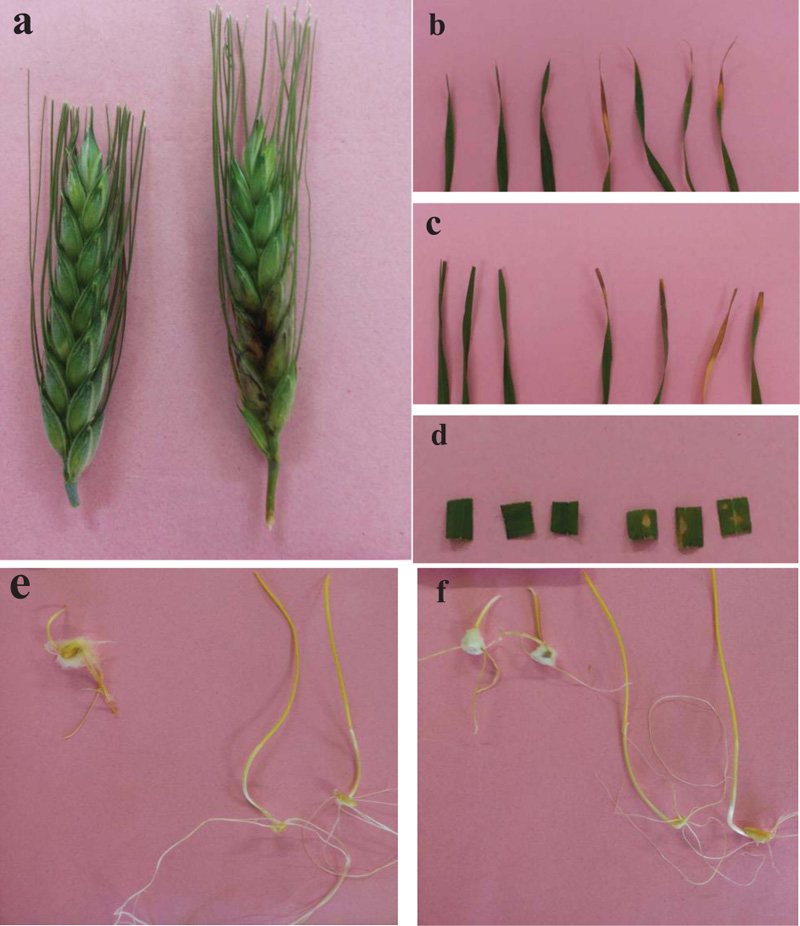
3. MEASURING IN VITRO QUANTITATIVE COMPONENTS OF THE HOST-PATHOGEN INTERACTION
QR can be selected in breeding programs either based on overall symptom expression or by prior identification of resistance mechanisms or correlated variables, followed by selection for these mechanisms. In the latter, the first step towards efficient selection for QR is the determination of in vitro resistance components. No single measure is suitable, and each plant–fungus interaction is likely to be different [58]. The three in vitro components include reduced infection frequency or density, reduced lesion size or concentration, and reduced propagule production per unit of host tissue over a period [59]. In most plant–fungus interactions involving necrotrophs, hemibiotrophs and biotrophs, the major epidemiological components of QR are infection efficiency (IE), latent period (LP), sporulation rate (SR), and lesion length (LL) [60]. Some in vitro components are more important than others in characterizing QR, and it is possible for some host–fungus interactions to recognize one component of QR, or a set of subcomponents, that adequately represent the QR in the field. For example, in Puccinia hordei/barley leaf rust and Phytophthora infestans/potato late blight, LP and LL were the most suitable in vitro components to assess field QR, respectively [61, 62]. The identification of single components may be especially useful in material that has been selected for QR over a long period but less in recently accessed material [63]. In populations that have not been strongly selected for QR, no or negative correlation of components is expected.
3.1. Infection Efficiency
IE is defined as the probability that a spore deposited on a receptive host surface produces a lesion in the absence of competitive interactions. It is usually measured as a percentage of successful infections resulting from a controlled number of deposited spores [64]. For practical reasons, IE may be indirectly measured by the observed numbers of chlorotic flecks or lesions per unit of leaf area [65]. This trait is difficult to precisely estimate because it depends on the number of spores deposited as well as on microclimatic conditions, which are difficult to control [66].
3.2. Latent Period
LP is the time interval between infection and the onset of sporulation from that infection. It determines the duration of epidemic cycles and thus largely controls the rate of epidemic development. The definition of LP is clear when applied to a single lesion. In most experimental studies, however, artificial inoculations result in many infections per leaf, and the variation in observed LP among infection sites on a leaf may be considerable [67]. To cope with this difficulty, several criteria are used to estimate LP, such as the time from inoculation to first sporulation [68] or the time needed for half of the final number of lesions to sporulate [69] or to show apparent sporulation structures [70]. The most precise method for estimating the time needed for half of the final number of lesions to sporulate was proposed by Shaner [67] and is based on an adjustment of the dynamics of lesion emergence to a sigmoid curve. Since LP is highly dependent on temperature, it is recommended to express the time in degree-days to allow comparisons between different experiments [71]. It has been observed that using different methods to measure LP (i.e., the time needed for half of the final number of lesions to sporulate vs. the time to first sporulation) could lead to differences in its estimation as observed for Puccinia triticina/wheat [65] and Phytophthora infestans/potato [69]. Such differences might, of course, result from uncontrolled environmental effects. A more interesting alternative is that variability in LP among fungal isolates could reveal heterogeneity in both the time at which the first sporulation occurs and the dynamics of lesion maturation [72].
3.3. Sporulation Rate
SR is the number of spores produced per lesion and per unit of time [73, 74]. In practice, spores are either weighed [75] or counted [76]. Sporulation is sometimes expressed in spore production per unit area of diseased leaf [75] or relative to lesion size [76]. Spore production per lesion is highly density-dependent [73]. It can be useful to consider the spore production per unit area of sporulating tissue [76], which is considerably less density-dependent [77]. The number of spores produced by a diseased plant will determine the quantity of inoculum that can infect neighboring plants but also indicates the interaction between the host plant and the parasite since the quantity of spores produced depends on the aggressiveness of the parasite and the level of host QR [4]. It also reflects the capacity of the fungus to invade host tissues during the incubation period.
The infectious period is the time from the beginning to the end of sporulation. This component is difficult to precisely estimate since sporulation often shows an early peak, followed by an asymptotic decrease [77]; however, more irregular patterns may be obtained [78]. For cereal rusts, sporulation can last for more than 40 days under controlled conditions on adult plants [77].
Since many pathogen species have two or more forms of propagule related to sexual or asexual reproduction [79], infection efficiency, sporulation rate, latent period, and infectious period may, in principle, be measured for each type of spore [80]. Nevertheless, a given parameter may not have the same meaning when measured on sexual and asexual spores. For example, the latent period associated with sexual spores is very different from that of asexual spores because it depends on the fortuitous encounter and merger of two sexually compatible lesions. Therefore, while there seems to be room for adaptive adjustment of asexual latency, sexual latency is expected to be highly dependent on environmental stochasticity [1]. Moreover, organs resulting from sexual reproduction often ensure inter-season survival, as in Blumeria graminis or Leptosphaeria maculans, and the latent period, as defined above, has no meaning in such cases [1].
3.4. Lesion Length
LL is another quantitative trait that is measured as a QR component [81]. It is generally defined as the surface area that produces spores. For some pathogens, such as Puccinia triticina/leaf rust, lesion size remains limited, but it can dramatically increase in some species, such as Puccinia striiformis var. striiformis/stripe rust or Phytophthora infestans/late blight, for which lesion growth is semisystemic [82]. In this case, LL accounts for a large part of the quantitative development of epidemics and lesion growth rate is a key factor in pathogen competition for available host tissue. LL is not easy to precisely determine for pathogens, such as Mycosphaerella graminicola/Septoria blotch, that induce necrosis on the host leaf [83]. Moreover, such pathogens often indirectly cause apical necrosis on the leaves that can be confused with a diseased area.
3.5. Dwarfing
Dwarfing is a symptom characteristic of plants systemically infected by fungal pathogens and is explained by a decrease in the concentration of growth hormone in infected tissue [4]. This decrease in size can be observed at a very early stage.
3.6. Disease Severity
QR is sometimes estimated through disease severity, measured as the percentage of the infected plant organ (root, leaf or fruit/spike) covered by pathogen lesions [84]. DS is a composite variable resulting from the integrated effect of infection efficiency and lesion size, but also, when assessed at the crop scale, sporulation and dispersal.
In any case, it is essential to measure quantitative traits that can be clearly related to disease dynamics and pathogen evolution. For instance, a few in vitro traits have been identified that may be useful for the characterization of quantitative resistance by making comparisons with two cultivars that are susceptible to the pathogen in the field. The collective effects of each or some of the in vitro traits are particularly important.
4. EXAMPLES OF IN VITRO STUDIES ON DIFFERENT CROPS AND FUNGI
Under in vitro tests, commonly used parameters for resistance assessment are lesion growth rate, i.e., the rate of necrosis extension, infection efficiency, i.e., the percentage of successful infections, lesion size, latent period, and spore density [7-57]. These traits are well adapted to the description of the epidemic phase of polycyclic pathogens that belong to the fungi and oomycete groups [1]. Moreover, the in vitro traits mentioned above can easily be applied to pathogens with specific biological features [1]. In vitro assays have been used as a tool for investigating QR in the whole plant for a wide range of important fungal diseases, including rust, powdery mildew, downy mildew, Fusarium head blight, etc. (Table 1). Table 1 presents in vitro applications used to assess quantitative resistance in several fungal-plant pathosystems.
4.1. A Necrotrophic Fungus
A detached leaf assay was used to determine the reaction of five barley genotypes to spot blotch caused by Cochliobolus sativus [25]. The estimation of the infected leaf area enabled discrimination among barley genotypes differing in their susceptibility to the pathogen. Significant correlations were found (P=0.001) between in vitro values in both seedling (r=0.89) and adult (r=0.95) plants. The established in vitro assay enabled a fast assessment of the susceptibility of barley to spot blotch and should be useful for many types of studies on this disease [25].
4.2. A Hemibiotrophic Fungus
The detached leaf assay was successful in the identification of an important component of the resistance of Fusarium species, causing head blight (FHB) in the European wheat germplasm. This in vitro method may be a useful mechanism to discriminate amongst different types of resistance in a breeding program [8]. Michodochium majus is used in the detached leaf assays to detect leaf symptoms for observing QR components (incubation period, latent period and lesion length) [12]. Lesion length is one of the components of QR measured as an indicator of fungal pathogenicity and aggressiveness. QR components detected in the M. majus detached leaf assay have been correlated to FHB resistance in wheat inoculated with F. culmorum and F. graminearum [8, 12]. FHB resistance in a seed germination assay was highly correlated in F. graminearum, F. avenaceaum, F. culmorum, M. majus and Microdochium nivale, indicating common resistance between M. Majus and other Fusarium spp. in the in vitro assay [12]. Browne and Cooke [13] reported that QR components (incubation periods, longer latent periods and shorter lesion lengths) in the detached leaf assay and higher germination rates in the seed germination assay were related to greater FHB resistance (Type II). However, the exotic wheat germplasms, which provide highly effective resistances to FHB resistance, do not appear to be detected in the detached leaf or seed germination assays [8, 12].
| Host Plant |
Fungal Pathogen/ Disease |
Targeted Young Plant Materials |
Analyzed in vitro Components | Reliability of in vitro Test to Predict Resistance at Adult Plant Stage | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wheat | Several Fusarium and Microdochium species/ Fusarium head blight |
Detached leaves Seedlings Detached heads |
Latent period (LP), incubation period (IP) and lesion length (LL) Area under disease progress Curve (AUDPC), coleoptile length reduction and LL Disease incidence and disease severity (DS) |
Yes Yes Yes |
[8, 12, 13] [11, 14-16] [17] |
||||||
| Septoria tritici/blotch | Detached leaves | The percentage leaf area covered by lesions bearing pycnidia |
Yes | [18] | |||||||
| Septoria nodorum/blotch | Detached seedling leaves |
DS | Yes | [19] | |||||||
| Puccinia triticina/leaf rust | Detached leaves | Disease symptoms and the rate of pustule development Incubation and latent period |
Yes Yes |
[20] [21] |
|||||||
| Barley | Several Fusarium and Microdochium species/Fusarium head blight |
Detached leaves Detached leaves Seedlings Seedlings Detached heads |
LP and IP LP, LL, AUDPC and percentage of infected seedlings Root weight, coleoptile length, coleoptile weight and seed germination reductions Disease incidence and severity |
Yes Yes Yes Yes |
[13] [22] [23] [10] |
||||||
|
Cochliobolus sativus/common root rot |
Seedlings | Percentage infected area of the sub-crown underdone |
Yes | [24] | |||||||
| Cochliobolus sativus/spot blotch | Detached leaves | Infected leaf area | Yes | [25] | |||||||
|
Puccinia striiformis f.sp. hordei/ stripe rust |
Detached leaves | DS | Not analyzed | [26] | |||||||
| Oat |
Microdochium nivale/Fusarium head blight |
Detached leaves | LP | Yes | [13] | ||||||
| Maize | Puccinia sorghi/rust | Detached leaves | DS | Not analyzed | [27] | ||||||
|
Bipolaris maydis/southern corn leaf blight |
Detached leaves | Percentage leaf area | Yes | [28] | |||||||
| Rice | Magnaporthe grisea/blast | Detached leaves | DS | Not analyzed | [29] | ||||||
| Banana |
Mycosphaerella fijiensis/black leaf streak |
Detached leaves | IP and leaf areas infected | Yes | [30] | ||||||
| St. Augustine grass |
Magnaporthe oryzae/grey leaf spot | Detached stolon and detached leaves |
LL and DS | Yes | [31] | ||||||
| Faba beans | Uromyces viciae-fabae/rust | Detached leaves | Percentage leaf area | Yes | [32] | ||||||
| Ascochyta fabae/ascochyta blight | Detached leaves and stem segments with attached leaves |
Number and size of lesions and the number of lesions bearing pycnidia |
Yes | [33] | |||||||
|
Colletotrichum lindemuthianum/ anthracnose |
Detached leaves | DS | Not analyzed | [34] | |||||||
| Botrytis fabae/chocolate spot | Detached leaves | DS | Not analyzed | [35] | |||||||
| Pea | Erysiphe pisi/powdery mildew | Leaflet Detached leaves |
Infection efficiency and spore production Percentage of leaf area affected with disease |
Yes Yes |
[6, 36] | ||||||
| Chickpea | Ascochyta rabiei/ascochyta blight | Detached leaves | DS | Yes | [37] | ||||||
| Alfalfa | Sclerotinia trifoliorum/clover rot | Excised leaf tissues |
Rate and extent of necrosis | Yes | [38] | ||||||
| Soybean | Phakopsora pachyrhizi/rust | Detached leaves | Lesion size and number of spores |
Yes. | [9] | ||||||
| Rose |
Diplocarpon rosae/black spot, Podosphaera pannosa/ powdery mildew and Peronospora Sparsa/downy mildew |
Detached leaves | DS | Yes | [39] | ||||||
| Sweet cherry |
Podosphaera clandestine/ powdery mildew |
Detached leaves | DS | Yes | [40] | ||||||
| Grapevine | Erysiphe necator/powdery mildew | Detached leaves | Percentage of inoculating spots leading to a colony and the mean diameter of colonies |
Not analyzed | [41] | ||||||
| Plasmopara viticola/downy mildew | Detached leaves | DS | Yes | [10] | |||||||
| Tomato | Phytophthora infestans/late blight | Detached leaves | LL | Yes | [41] | ||||||
| Alternaria solani/early blight | Detached leaflets | Lesion radius, rate of lesion expansion, and final DS |
Yes | [43] | |||||||
| Tobacco |
Phytophthora parasitica var. nicotianae/black shank |
Detached leaves | Number of lesions/leaf and percentage of leaf area infected |
Yes | [44] | ||||||
| Potato | Phytophthora infestans/late blight | detached leaves and intact plants |
DS | Yes | [45] | ||||||
| Cucumber |
Sphaerotheca fuliginea/ powdery mildew |
Leaf blades | DS | Not analyzed | [46] | ||||||
|
Didymella bryoniae/gummy stem blight |
Detached leaves | DS | No | [7] | |||||||
| Sunflower |
Plasmopara halstedii/downy mildew |
Seedlings | Percentage infection, LP, sporulation density and reduction of hypocotyls length |
Yes | [47] | ||||||
| Spinach | Peronospora effuse/downy mildew | Detached leaves and cotyledons |
DS | Yes | [48] | ||||||
| Quinoa |
Peronospora farinose/downy mildew |
Detached leaves | AUDPC, IP and LP | Yes | [49] | ||||||
| Lucerne |
Phytophthora medicaginis/Phytophthora root rot |
Detached leaves and intact cotyledons |
DS | Cotyledon test predicted resistance at the adult plant stage |
[50] | ||||||
| White lupin |
Diaporthe toxica/phomopsis leaf blight |
Detached leaves | DS | Yes | [51] | ||||||
| Cocoa |
Phytophthora palmivora and Phytophthora megakarya/ black pod |
Detached leaves | DS | Yes | [52] | ||||||
| Fenugreek |
Cercospora traversiana/ Cercospora leaf spot |
Detached leaves | The number of lesions and LL |
Yes | [53] | ||||||
| Oak |
Phytophthora ramorum/sudden oak death |
Detached leaves | LL | Yes | [54] | ||||||
| Spindle tree |
Colletotrichum gloeosporioides/ leaf anthracnose |
Detached leaves | Chlorotic spots | Not analyzed | [55] | ||||||
| Taro | Phytophthora colocasiae/leaf blight | Detached leaves | LL | Yes | [56] | ||||||
| Durian | Phytophthora palmivora/root rot | Detached leaves | DS | Yes | [57] |
4.3. A Biotrophic Fungus
In vitro components of quantitative resistance in sunflower (Helianthus annuus) to Plasmopara halstedii [47], i.e., percentage infection, latent period, sporulation density, and reduction of hypocotyl length, were compared between two sunflower cultivars and showed different levels of quantitative resistance in the field. The moderately susceptible inbred line showed a higher percentage of infection, a higher sporulation density, a shorter latent period and less reduced hypocotyl length than the moderately resistant inbred line. The percentage infection of FU was 1.4% less than BT, the latent period of BT was 12.4% less than FU, the sporulation density of FU was 22.3% less than BT, and the reduced hypocotyl length of BT was 15.3% less than FU. It seems that the criteria, such as latent period, sporulation density and reduction of hypocotyl length, may be used to measure quantitative resistance in sunflowers to P. halstedii [47].
The above-mentioned studies have generally concluded that in vitro traits can be applied for measuring QR in several plant species infected with biotrophic, hemibiotrophic, and necrotrophic fungal pathogens.
5. FACTORS AFFECTING IN VITRO EVALUATION IN YOUNG PLANT MATERIALS
Several factors varying in importance affect in vitro evaluation in young plant materials, which include growth stage, senescence of plant parts, amending growth medium with plant hormones, incubation conditions, and the origin of genetic host and fungal materials.
5.1. Growth Stage
The level of expression of QR is known to vary with plant development [3, 4]. For example, QR is greater in late expanding leaves against ascochyta blight in chickpeas and faba beans [33, 37], powdery mildew and crown rust in oat [85, 86], powdery mildew in sweet cherries and rusts in grapes [40, 41], rust and Fusarium head blight wheat [87, 88], powdery mildew in leaf blight in taro [56] and southern corn leaf blight in maize [28]. The effect of leaf position was considerably more pronounced in the powdery mildew-grapevine interactions; the magnitude of differences between susceptible and resistant cultivars increased with leaves at positions 3-5. Data suggested that leaves at position 3 must be inoculated to clearly distinguish resistant from susceptible cultivars [41]. Senescing grapevine leaves became more resistant to grapevine powdery mildew, and this effect was evident in both the success of infection and the diameter of the colony [41]. Conversely, older cucumber leaves were more susceptible to gummy stem blight than younger leaves in the field, greenhouse, and detached-leaf tests [7]. Arraiano et al. [18], using a detached leaf assay in wheat, found that secondary leaves were more susceptible to Septoria tritici than first-expanding leaves. Important factors for downy mildew/Plasmopara viticola resistance screens include leaf age; the fourth fully expanded leaf corresponds well with vineyard ratings [89].
5.2. Senescence of Plant Parts
A critical aspect of the in vitro assay is the prevention of senescence of leaf pieces before the duration required to express symptom stages and disease severity levels necessary to differentiate among cultivar responses. The leaf senescence may have had an inhibiting effect on sporulation for a period before sporulation occurred profusely over leaf stomata [41]. The youngest tissues can be infected, but the development of the fungus is generally arrested during the aging of tissues before sporulation occurs [41]. Senescence of leaf tissue before symptom development altered the accuracy with which QR resistance was determined, which has implications for using detached leaf assays to determine resistance to other diseases [88]. Using whole plants, which prevents the senescence associated with detached leaves, Singh and Huerta-Espino [87] found that cultivar differences were most clearly observed in later-forming leaves, which showed greater resistance. In a detached leaf assay, Arraiano et al. [18] inoculated seedling leaves with an S. tritici, which showed a relatively long period between inoculation and symptom development prior to detaching the leaves and mounting them on water agar.
5.3. Amending Growth Medium With Plant Hormones
Amending agar medium with plant hormones aided the retention of green leaf color in detached leaves [9, 12, 26, 90]. Benzimidazole and cytokinin have been used in the incubation medium to prevent chlorophyll degradation in detached leaves. Detached wheat and barley leaves were kept green for 14 to 34 days on benzimidazole agar [26], detached wheat leaves were kept green for at least 14 days using kinetin [12], and detached oat leaves were kept green for up to 10 days using kinetin and 6-benzylaminopurine treatments [86], indicating that maintaining green detached leaves for much longer periods is essential to propagate fungi. Soybean leaf pieces in a medium containing kinetin at 10 mg/liter had 5% chlorosis 18 days after plating compared to leaf pieces in media amended with all other plant hormones, which had higher levels of chlorosis [9]. Longer periods of healthy leaf tissue are important for the short-term use of rust isolates [90].
5.4. Incubation Conditions
The incubation conditions of detached potato leaves in closed trays rather than detachment itself appeared to affect the late blight resistance expression, concluding that the lower expression of resistance in the detached leaf tests was due to differences in environmental conditions [45]. The constant, highly favorable environment a pathogen finds in the closed trays apparently enhances infection by the Phytophthora infestans zoospores. Incubating detached leaves in closed trays appears to decrease resistance expression. Therefore, a suitable experimental condition must be chosen depending on the aim of the experiment. When the expression of resistance is to be examined on detached leaves, the reduced level of resistance should be measured against the low infection frequency inherent to intact plants. Inoculation of intact plants is preferred, but in most cases, the inoculation of detached leaves incubated in covered trays appears to be an adequate alternative [45]. Relative humidity during incubation in closed containers was 85% and may have proved lethal to the more fragile Phytophthora colocasiae zoospores (taro leaf blight) without an agar drop to protect them [56].
5.5. Origin Of Genetic Host And Fungal Materials
The origin of genetic host and fungal materials plays a critical role in evaluating in vitro resistance reactions. Brown and Cooke [12] did not find a relationship between the detached leaf assay and whole plant FHB resistance in International Maize and Wheat Improvement Center (CIMMYT) genotypes containing more diverse sources of resistant germplasms in their pedigree. The detached leaf assay did not appear to detect the whole plant resistance of Sumai 3, a Chinese wheat cultivar, because it displayed shorter incubation and latent periods and longer lesion lengths relative to European cultivars. Moreover, the detached leaf technique did not detect the greater FHB resistance of Frontana wheat cultivar because this genotype had a comparable incubation period, latent period, and lesion length to the most resistant Irish and UK cultivars possessed only moderate FHB resistance [12]. Although considerable differences in mean Plasmopara viticola/downy mildew severity separated Vitis vinifera, Vitis hybrid, V. riparia, and V. labrusca during in vitro inoculations (from susceptible to resistant), notable intra-species variation was identified for all well-represented species [89]. The effect of the pathogen source on resistance ratings could reflect genetic variation in P. viticola; the lack of disease severity was much more likely due to resistance against the single isolates screened [89].
Certain precautions are required while conducting detached-leaf assays. Leaves should be free from mites because they forage on rust spores, which in turn, reduce the efficiency of infection. Surface sterilization of leaves with 0.1% NaOCl or 70% ethanol should also be avoided because it causes patches of necrosis that interfere with pathogen development [9].
6. MOLECULAR PATHWAYS FOR THE CORRELATION BETWEEN THE IN VITRO AND WHOLE PLANT REACTIONS TO FUNGAL INFECTION
Molecular pathways for the relationship between the in vitro and whole plant responses to fungal infection have been reviewed in the FHB-wheat pathosystem [15]. The genetic basis of FHB resistance is highly complex but is beginning to be understood. Quantitative trait loci (QTLs) for resistance have been linked with passive factors, such as plant height, spike architecture and flowering date [91], and with variability in specific genes. Some specific compounds of wheat anthers (choline, betaine) were thought to be responsible for fungal growth stimulation [92], but in a later study, a substantial role of floral structures in resistance to Fusarium graminearum was denied [93]. Multiple signaling pathways involved in spike response to F. graminearum infection [94] have been found to be similarly regulated in inoculated seedlings [95]. Moreover, some up-regulated genes during infection affect plant growth and stem cell division [95]. It is possible that lipid transfer and deoxynivalenol resistance genes associated with 5A (Qfhs.ifa-5A) and 3B (Qfhs.ndsu-3BS) QTLs in common wheat [96] are also components of resistance at the seedling stage. In addition, the observed correlations between parameters of coleoptiles and roots within each experiment could reflect concurrent gene expression in different tissues/organs. An improved understanding of these putative parallel mechanisms is expected as comparative gene expression studies across developmental stages increase in number.
The above-mentioned studies have generally concluded that the biological explanation for an association between the seedling and adult plant responses to infection remains unknown, but it can be hypothesized that similar genetic pathways become activated at both developmental stages.
7. OTHER APPLICATIONS OF IN VITRO TECHNIQUES IN THE HOST-FUNGAL PATHOGEN PATHOSYSTEMS
Reliable in vitro tests have been extensively used for studying plant-fungal interactions at the physiological or molecular levels, highlighting that the use of in vitro techniques is not only limited to evaluating QR components but extends to several fungal-plant life history traits.
7.1. Studying Plant-Fungal Interactions At The Physiological Level
Production of single-strain cultures of obligate pathogens for use in genetic studies is difficult. By using in vitro techniques, the ability to handle and propagate pathogens on the young plant materials helped to generate pure cultures and avoid cross-contamination for Podosphaera fusca, causing cucumber powdery mildew, Pseudoperonospora cubensis causing cucumber downy mildew, Plasmopara halstedii causing sunflower downy mildew, Puccinia coronata causing crown rust, Phakopsora pachyrhizi causing soybean rust, and Peronospora effuse causing spinach downy mildew [48, 86, 90, 97, 98]. As a pre-screening test, the effect of potential resistance-inducing chemicals, DL-3-aminobutyric acid, Bion (benzo-(1,2,3) thiadiazole-7-carbothioic acid S-methyl ester), and a foliar fertilizer containing potassium phosphite, on development of Microdochium majus causing Fusarium head blight was studied in detached leaves [99]. Reduced disease development of M. majus was also only observed in detached leaves pre-treated with the foliar fertilizer, indicating that the effect on disease development was at least partly due to a fungistatic effect [99]. The in vitro technique also performed well in testing the pathogenic behavior of Erysiphe necator on grapevine [41], Plasmopara halstedii on sunflower [100, 101], Mycosphaerella fijiensis on bananas [30], Phytophthora infestans on potato [102], Fusarium and Microdochium species causing a head blight on wheat and barley [16, 17, 103-105] and Phytophthora cactorum, P. citrophthora, Pythium dissotocum complex, Py. aphanidermatum, Globisporangium heterothallicum, G. ultimum, Phytopythium vexans, Phy. mercurial, and Phy. litorale on strawberries [106]. The in vitro screening method was effective in determining the pathotype of Pseudocercospora griseola isolates on a set of angular leaf spot differential common bean cultivars [107] and host-specialization in E. necator-V. vinifera interactions [41] and four Fusarium species causing a head blight on wheat and barley [108-110]. The in vitro laboratory assay was used efficiently to determine the sensitivity of P. infestans and two rust fungi to fungicides [111, 112]. Phytotoxins as selective agents under in vitro selection were utilized for improved disease resistance [113].
7.2. Studying Plant-Fungal Interactions At The Molecular Level
The detached leaf assay provided a means to enable fundamental studies on the defense mechanisms of Arabidopsis in response to F. graminearum/Fusarium head blight [114]. In vitro infection of detached leaves Puccinia substriata, the causal agent of rust disease, and Sclerospora graminicola, the causal agent of downy mildew, resulted in a significant reduction of disease symptoms in transgenic pearl millet cultivars in comparison to wild-type control plants; the disease resistance of pearl millet was increased by up to 90% when infected with two diverse, economically important pathogens [115]. On detached leaves, virus-induced gene silencing was adopted to dissect gene functions in cotton resistant to Verticillium wilt [116]. Using in vitro methods, transgenic lines of tomato harboring rice chitinase gene were evaluated for resistance to Fusarium oxysporum f. sp. lycopersici causing Fusarium wilt and Alternaria solani causing early blight. In vitro studies concluded that introducing a chitinase gene in a susceptible cultivar of tomato not only enhanced the resistance but was stably inherited in transgenic lines [117]. Using detached leaf assay, two novel powdery mildew resistance loci, Ren6 and Ren7, were identified from the wild Chinese grape species Vitis piasezkii [118]. Phenotyping of transformed potato cultivars developed through small interfering RNA (siRNA) and artificial micro RNA (amiRNA) techniques for late blight resistance was undertaken using in vitro assays. The siRNA and amiRNA-derived cultivars exhibited less lesion area as well as spore production and were categorized as moderately resistant [41].
7.3. Using In Vitro Techniques To Explore Defense Responses In Plants To Fungal Infection
Wheat endophytes, Phoma glomerata, Aureobasidium proteae and Sarocladium kiliense, were screened as biological control agents against Fusarium head blight using two different in vitro tests [119]. This study pointed out that the test on detached wheat spikelets provided information about the potential pathogenicity, growth capacity, and efficacy of the endophyte strains on the targeted plant before testing them on whole plants [119]. Detached leaves of the Arabidopsis lines were inoculated with Verticillium dahliae to evaluate the defense response under drought stress; leaves from transgenic plants showed increased callose deposition and reduced mycelia growth [120]. The effect of chemical treatments affecting the ethylene pathway was studied in detached head assays, and the results showed that the ethylene signaling could mediate wheat Fusarium head blight resistance to Fusarium graminearum [121]. A notable difference was found between non-amended and amended treatments with silicon on young plant parts in potato detached leaves infected with Phytophthora infestans (late blight) [122] and wheat detached leaves and seedlings challenged with four Fusarium head blight pathogens [123]. By in vitro techniques, the direct fungitoxicity was investigated for Fusarium head blight pathogens and Cochliobolus sativus causing spot blotch and common root rot [124]. Recently, Sakr [125, 126] explored how the deployment of QR in wheat and barley affects aggressiveness under in vitro conditions, leading to potential resistance erosion.
7.4. Using In Vitro Techniques In Plant Breeding
In breeding programs and during gene pyramiding, it will be challenging to evaluate plant symptoms using only conventional screening methods; however, this alternative rapid detached leave method is proposed for marker-assisted gene pyramiding in combination with molecular markers to aid the selection of resistant progenies during each backcross generation [107]. In addition, the proposed method can help when evaluating progenies for different pathotypes using leaves from the same plant, which could be difficult with the conventional evaluation method. Thus, this alternative screening technique could provide a great opportunity for breeders working in gene pyramiding and marker-assisted back-crossing programs to evaluate common bean genotypes and progenies for multiple disease resistance [107].
CONCLUSION
Besides tolerance to abiotic stresses like salinity and drought, plant breeders should also take into account the resistance to fungal pathogens. Therefore, further studies should be carried out on the pathogens, their life cycle, QR resistance components, and the environment by host-fungal interactions to develop new cultivars that resist the damage caused by fungal pathogens. The in vitro technique developed, optimized, and validated during several studies can be used to rapidly detect plant genotypes with superior resistance to fungal infection. The in vitro screening technique provides the plant breeders' community with highly informative parameters and allows them to establish diversified host germplasm and enhance breeding program efficiency. The in vitro assay can be conducted in a relatively short period of time, making it a convenient protocol for the evaluation of a large number of progeny at early breeding stages. Hence, evaluation of fungal resistance under greenhouse or field conditions could be restricted to a narrower sample of selected genotypes with the best performance in the seedling assay. The in vitro assay has a low-cost approach and can be carried out off-season. This assay may also constitute a model system for biochemical and genetic studies on plant defense responses.
LIST OF ABBREVIATIONS
| QR | = Quantitative Resistance |
| IE | = Infection Efficiency |
| LP | = Period |
| SR | = Sporulation Rate |
| LL | = Lesion Length |
| FHB | = Fusarium Head Blight |
| QTLs | = Quantitative Trait Loci |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This study is financially supported by the Atomic Energy Commission of Syria.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The author would like to thank the Atomic Energy Commission of Syria for providing assistance with this research. The author would also like to thank the reviewers for their constructive comments on this manuscript.


