All published articles of this journal are available on ScienceDirect.
Erosion of Quantitative Resistance in Wheat and Barley to fusarium Head Blight: Gene Pyramiding Achieves and Durability Study
Abstract
Background:
Fusarium head blight (FHB), caused by several fusarium species, is one of the diseases causing the greatest worldwide damage to small grain cereals, especially wheat and barley. FHB outbreaks can substantially diminish grain yield and end-use quality due to sterile florets and withered mycotoxin-contaminated grain kernels. Great effort has been accomplished to combat FHB in the past decades; however, solutions to prevent FHB damage are limited. The development of quantitative resistant cultivars is considered a sustainable and highly desired approach to reducing FHB damage.
Objective:
This review aims to combine novel data related to the potential ability of pathogens to evolve aggressiveness, erosion of quantitative head blight resistance in wheat and barley, and applying gene pyramiding which enhances host resistance to FHB infection to achieve durable head blight resistance.
Results:
Although FHB-resistance resources have been successfully utilized by resistant parents in wheat and barley breeding programs globally, this policy does not ensure high resistance to FHB since resistance will erode due to aggressiveness shifts of the head blight population. The increasing practice of monoculture wheat and barley cultivation has perhaps raised the rate of head blight pathogen evolution and obligated the natural balance shifting in favor of the pathogen, creating more repeated and grave epidemics, even in provinces where FHB has not been earlier recorded. More aggressive FHB populations have emerged in the field and under experimental laboratory conditions. It suggests adaptation followed by a spread of some strains in their environment, including adaptation to FHB-resistant breeds and possible erosion of wheat and barely resistance. Therefore, the pyramiding of several QTLs with high impact in one cereal cultivar may extend durability.
Conclusion:
If a pyramiding of multiple resistances improving QTL combined with selection against suspected susceptibility factors is achieved in novel cultivars, the evolution of FHB pathogens might be slowed owing to reduced exposure to the pathogen, disruptive selection on FHB populations and subsequently reduced fitness of fusarium fungi. This would stabilize the pathogen population and contribute to the durability of FHB resistance.
1. INTRODUCTION
Wheat, including bread (Triticum aestivum) and durum (T. durum), is the second most important cereal in terms of production in the world, after rice. Wheat grown on about 219 million hectares provides a vital food source for 36% of the world’s population, 20% of their diurnal intake of protein and 18% of their calories [1]. Barley (Hordeum vulgare) is the fourth most-produced cereal crop globally and is cultivated in temperate climate zones. Nearly 140 million tones per year are produced worldwide, which are principally used as animal feed (70%) and for beer production (27%) [1]. Wheat and barley are susceptible to a wide array of harmful fungal diseases. fusarium head blight (FHB) is one of the most destructive diseases representing the enormous economic impacts of bread, durum wheat, and barley production [2, 3]. While FHB is common in most cereal-producing regions worldwide, it has proven extremely challenging to control [4]. Despite global-scale research, international cooperation and continuous efforts to curb FHB, the disease reemerged in the 1990s and devastated cereals [5]. Yield losses due to FHB ranked second after leaf rust and were particularly high in China, the US Midwest, Canada, South Brazil, Paraguay, Uruguay and Argentina [6]. In the United States, economic losses caused by FHB in wheat have barely been over $ 4.8 billion since 1990 [7]. In China, FHB has affected more than 1 million tons in severe epidemics [8]. The estimated barley yield losses were 70 million tons, with a raw commodity value of $ 122 million in the 1993 epidemic alone [9].
Increased demand for wheat and barley products has led to expanding cereal production zones from traditional warm and dry cropping areas to more humid regions with climatic conditions conducive to the disease [10]. Head blight symptoms in wheat and barley include necrosis and bleaching of infected spikelets or the entire spikes with shriveled or deformed grains resulting in floret sterility [2], reduction of yield and contamination of grains with harmful mycotoxins [3]. The trichothecene-deoxynivalenol (DON) produced by the pathogen remains in processed foods causing health hazards in humans and animals [11]. In Europe, 15–55% of barley products are contaminated with DON [12].
The infection is caused by more than 17 fusarium pathogens, including F. graminearum and F. culmorum [10, 13]. All these species can infect wheat and barley when spikes are inoculated but with varying levels of aggressiveness [14] - defined herein as the quantitative variation of the pathogenicity [15]. F. graminearum has been associated with cool, wet, and humid conditions, whereas F. culmorum has been found to dominate in regions with warm and humid conditions [6]. Pathogen strains may vary in cultural characteristics, toxigenicity, and vegetative compatibility groups [16]. FHB Strains from regions where epidemics are severe and frequent may be more aggressive than those where disease pressure is less. Highly aggressive strains may cause more severe symptoms in moderately resistant cultivars than less aggressive strains [17]. FHB populations show important levels of diversity and gene flow within populations [18-21]. Also, head blight pathogens reproduce through both clonal reproduction and sexual outcrosses, and spores can be dispersed from short to long-range scales [22]; these patterns are often associated with fungal pathogens of strong evolutionary potential [23].
Severe epidemics of FHB occur when warm and humid weather coincides with an abundance of spores during wheat and barley anthesis [6]. Genetic resistance can potentially provide economical and effective disease control [16]. Disease control is achieved by the deployment of resistant cultivars [9]. Considerable progress in the search for host resistance has been made in China, Japan, and some other countries in the past decades [5]. Improving cultivar resistance has become a major breeding objective worldwide [10]. Recent developments in genomic research and biotechnology hold promise for understanding the genetic mechanisms of FHB resistance and allow more effective utilization of FHB resistance genes to develop new resistant wheat and barley cultivars [6]. FHB-resistance QTL (quantitative trait loci) Fhb1 is the most effective and widely deployed durable resistance source against FHB [4]. This quantitative and broad-spectrum resistance was originally described in the Chinese wheat cultivar Sumai 3 (released in 1972) and its derivatives [8]. Failure of resistance in the Sumai 3 source has not been reported, and it is still the best source worldwide for resistance to spared symptoms in the spike [10]. Novel findings show that the higher genome plasticity provided by the wheat and barley genome serves as a reservoir for the evolution of unique alleles that confer durable disease resistance [4, 5]. However, breeding for FHB resistance has proven difficult due to the complex inheritance of resistance genes and the strong genotype-by-environment interaction [9]. While QTLs have been identified and incorporated into elite backgrounds, a large portion of the genetic variance for resistance is controlled by numerous genes that present small effects that may also be environmentally specific [6]. Plant breeders focusing on FHB resistance will be challenged to continue advancing in breeding cultivars with enhanced resistance [4].
The complex interactions between FHB pathogens and their cereal hosts involve several genetic factors from both partners, the outcomes being quantitative resistance by cereals [24] and aggressiveness [21, 25, 26]. This interaction is dynamic, and more aggressive FHB populations have emerged in the fields [27-29] and under in vitro conditions [30, 31]. It suggests adaptation followed by a spread of some strains in their environment, including adaptation to FHB-resistant breeds and rendering resistance lacks durability, which leads to resisting erosion. Therefore, the pyramiding of several QTLs with high impact in one cereal cultivar may extend durability [32]. This review analyzes novel data related to the potential ability of pathogens to evolve aggressiveness and erosion of quantitative head blight resistance in wheat and barley and applies gene pyramiding enhancing host resistance to FHB infection to achieve durable head blight resistance.
2. HEAD BLIGHT PATHOGENS
More than 17 fusarium species have been strained from naturally infected wheat or barley spikes [2, 10]. The dominance and severity of a species or a few others are affected by weather conditions, geography and crop planted [13]. Temperature, a major weather parameter, affects the predominance of the species [6, 10], therefore in warm moist seasons, F. graminearum is the most dominant species that coincide with Canadian and American weather conditions, and F. moniliforme was also recorded highly under such conditions [2]. However, in cooler maritime regions, as in northwest Europe, F. culmorum tends to predominate, and F. poae and M. nivale assume greater importance [2].
FHB severity on farmers' fields may either reach epidemic proportions, develop moderately severe or remain low and largely undetected [23]. Central to FHB infection and development are (a) the abundance and aggressiveness of inoculum during the vulnerable plant stage, which essentially spans several days around anthesis, (b) the environmental conditions during this critical period and (c) the susceptibility or resistance status of the plant [5]. When temperature and moisture are favorable, an infection can occur any time after the commencement of flowering in wheat and barley, but anthesis is the growth stage most vulnerable to infection. Because of this brief period of vulnerability, fusarium pathogens are generally limited to one infection cycle per season [4-6, 10]. For natural infection, ascospores of fusarium pathogens released from perithecia are usually the primary inoculum to initiate disease epidemics [6]. Macroconidia are equally infective and commonly used to inoculate wheat and barley [10].
3. POTENTIAL ABILITY OF PATHOGENS TO EVOLVE AGGRESSIVENESS
FHB species are pathogens of heightened concern for the wheat and barley industries due to their high aggressiveness and ability to cause severe disease under epidemic conditions [33, 34]. The species are evolving means to overcome host resistance genes, enabled by its mixed reproduction system, encompassing both sexual and asexual reproductive states and allowing for genetic recombination and the propagation of clones [3, 6]. During infection, FHB pathogens produce cell wall degrading enzymes (CWDEs), such as pectinases, xylanases and cellulases, to degrade cell wall polysaccharides to penetrate and colonize the host tissues [3]. The content and composition of cell wall polymers affect the susceptibility of the cell walls to CWDEs and can play a role in the outcome of host-pathogen interactions. Thus, these secreted proteins give the fungi an arsenal of aggressiveness effectors to enter and colonize hosts [4].
The genome of head blight pathogens shows low levels of repetitive elements due to the targeting mechanisms associated with repeat-induced point mutation [35], thereby promoting rapid adaptation to selection pressures [36]. fusarium genome studies have demonstrated the existence of specialized pathogenicity chromosomes with evidence of horizontal acquisition [37]. The pan-genomic analysis of North American strains of FHB populations has identified abundant signatures of selection within genomic regions of dispensable accessory genes associated with pathogen specialization [29]. Advancements in fusarium genomics have elucidated the fungi's genes for invasion, which may be host defense response elicitors. Cuomo et al. [35] discovered high degrees of polymorphism in telomeric regions, which contained an abundance of pathogenicity genes, and these genes are commonly expressed under host-pathogen interaction. Head blight strains of varying aggressiveness express the same genetic programs and show similar effector protein profiles in cereal infections, but more aggressive strains accumulate these substances with higher abundance [26]; thus, specific effectors do not determine aggressiveness but rather by their general rate of production.
A detailed description of aggressiveness and its genetic basis, not to mention other parts of the FHB species' life cycles, such as dissemination or overwintering survival, is, therefore, necessary to understand and anticipate its evolution and consequences. As a whole, every life-related trait during the life cycle of head blight pathogens that will modulate its level of pathogenicity over time participates in its aggressiveness [15]. Hence, aggressiveness is hard to fully estimate, and proxies like disease severity measurement are often used to assess it. For example, several studies have highlighted important variations of disease severity between strains. For some fungi, mycotoxins can be an important component of aggressiveness by killing plant cells and helping their conversion into resources for fungal growth [15]. fusarium strains can produce mycotoxins of the type B trichothecene family, either DON and derivatives or nivalenol and derivatives, depending on the strain's chemotype [38-40]. Some mycotoxins are considered to be aggressiveness factors since their nature and quantities produced have been associated with severity variation [28, 41, 42]. But this relationship is not always straightforward, and some correlation is not always observed [25, 26, 43]. In addition to toxin biosynthetic gene clusters, gapless whole-genome sequencing of the four chromosomes of the species associated with extensive genome-mining efforts has highlighted a large repertoire of genes that could be involved in pathogenicity [35, 44-46]. Re-sequencing analyses highlighted high levels of intra-species polymorphism at these loci, further suggesting that putative pathogenicity genes are easily affected by mutations, which could play a role in aggressiveness variation and fungal adaptation [29, 47, 48].
Several genetic loci in the FHB pathogen’s genome have been associated with aggressiveness variation. For example, 50 single nucleotide polymorphisms affecting 26 different genes sparsely located on the genomes of 119 German field strains have been associated with disease severity variation and suggest a complex genetic control [49]. The possible roles of each of these genes in aggressiveness have been functionally validated by Laurent et al. [50]. One major and single QTL was mapped for all the traits measured on chromosome I, which explained up to 90% of the variance for disease severity. Four candidates were selected based on the postulate that a non-synonymous mutation affecting protein function may be responsible for phenotypic differences. A new mutation was identified and functionally validated in the gene FgVe1, coding for a velvet protein known to be involved in pathogenicity and secondary metabolism production in several fungi [50]. The high-gene flow suggests the potential to create pathogen populations that can rapidly adapt to management strategies like fungicide applications and resistant cultivars [51].
The extreme level of diversity and the significant correlation with genotypic variation in aggressiveness observed within fusarium pathogens for wheat, and barley [52-54] should be considered in developing breeding strategies. The existence of more aggressive forms of pathogens imposes the necessity to select the strains that best represent the pathogen population, also requiring continuous updating when screening breeding lines for resistance [4]. The pathogen carries numerous pathogenicity factors that can be employed under various circumstances. The alternative ways these pathogens can invade a host may be responsible for the quantitative levels of resistance achieved in wheat and barley [26].
4. FHB RESISTANCE IN WHEAT AND BARLEY
Genetic resistance based on monogenic inheritance follows the gene-for-gene interaction model and is more suitable for manipulating plant breeding. In contrast, genetic resistance against necrotrophs and hemibiotrophic shows complex multigenic inheritance, challenging breeding efforts [55]. Comparing the resistance of winter wheat cultivars released from 2002 to 2017, Schweizer [56] concluded that resistance to biotrophic pathogens such as rusts and powdery mildews have been improved significantly, while resistance to the tan spot disease caused by the necrotrophic pathogen Pyrenophora tritici-repentis, and FHB caused by the hemibiotrophic head blight pathogens remained almost the same in Germany. Furthermore, Mesterhazy [57] proposed that wheat resistance against rusts and powdery mildew is easier to improve than against Septoria tritici leaf spot and FHB caused by hemibiotrophic pathogens. Nevertheless, developing highly resistant commercial wheat and cultivars serving as the most promising approach to control FHB remains an elusive goal [32].
Durum wheat suffers from susceptibility to FHB than bread wheat. The lack of resistance in durum wheat may be attributed to historically low exposure to head blight and to the limited breeding efforts put into this relatively modern crop, leading to a narrow genetic base compared to other wheat species [3]. It also speculated that durum carries susceptibility factors and/or suppressor genes that comprise FHB resistance [4, 5]. However, barley is less susceptible than wheat [6, 10]. Current commercial durum, bread and barley cultivars are generally susceptible to FHB with the only exception of some cultivars, which proved to be moderately susceptible [5], indicating that no commercial cultivars exist with full resistance to FHB [4]. Host resistance in wheat and barley against FHB has been studied exhaustively and is mostly quantitative in nature [58]. Two major types of FHB resistance are widely accepted: resistance to the initial infection (Type I) and resistance to the spread of infection in the spike (Type II) [10]. Type I resistance is common in barley but rare in wheat, which is most likely contributed by spike morphology [59] and activation of systemic innate immune responses [60]. In contrast, Type II resistance is attributed to different resistant genes and has been more extensively studied and utilized in wheat [4]. These factors pose great difficulties in phenotype evaluation because of the requirement for suitable facilities, different inoculation methods and assessments, repeated trials, and considerable labor and time investment, and thus limit the efficiency of FHB resistance improvement through conventional breeding [32]. The advent of marker-assisted selection (MAS) provides a promising option to overcome these problems [61]. Effective disease-resistance genes should be durable [4-6, 10].
Until now, more than 432 QTLs for FHB resistance have been mapped in wheat [62], of which many for Type I and Type II resistances overlap with QTLs for other types of resistance, indicating the principal roles of Type I and Type II resistances in controlling FHB. Some of these QTLs have been applied to MAS-based FHB resistance improvement with success [58]; however, most still require verification due to small effects and large confidence intervals. No accessions or lines showing immunity to FHB have been found among wheat germplasm. In wheat breeding programs worldwide, FHB-resistant Sumai 3, a wheat cultivar developed from the cross of Funo with Taiwanxiaomai by Suzhou Institute of Agricultural Sciences, China, and its derivatives are the main sources of FHB resistance [63, 64]. The utilization of Sumai 3-derived resistance genes has only been partially successful so far because of the difficulty in simultaneously improving the resistance and agronomic traits. Moreover, using a single resistant source could potentially diminish genetic diversity. Wangshuibai (WSB), an indigenous wheat accession in Jiangsu, China, is highly resistant to FHB and carries QTL for different types of FHB resistance [65]. Using a recombinant inbred line population, WSB was found to possess Type I resistance QTL on chromosomes 3A, 4B (Fhb4), and 5A (Fhb5), Type II resistance QTL on chromosomes 2A, 3B (Fhb1), and 6B (Fhb2) [66]. To speed up utilization of the WSB QTL, Fhb1 has been cloned, and Fhb2, Fhb4, and Fhb5 have been mapped to small intervals [67]. Recently, Zhang et al. [32] conducted a study investigating the effects of Fhb1, Fhb4, and Fhb5 pyramiding in five modern Chinese wheat cultivars or lines on FHB resistance. Introgression lines showed significantly improved resistance to the fungal infection and disease spread in 2-year field trials after artificial inoculation. Compared to recipient lines, the Fhb1, Fhb4, and Fhb5 pyramiding could reduce the disease severity by 95% and did not systematically affect plant height, productive tiller number, kernel number per spike, thousand-grain weight, flowering time, and unit yield [32]. Yet, attempts to transfer resistance into durum wheat have met limited success. One hypothetical explanation for the often disappointingly low effect of bread wheat QTL wheat alleles when transferred into durum wheat is that the D-genome, absent in durum wheat, contributes resistance-stimulating factors [4, 5]. Resistance to fusarium was transferred from bread to durum wheat, with five out of six QTLs including the resistance derived from the bread wheat parent. However, the major Fhb1 QTL from ‘Sumai-3’ was not efficient in this interspecific wheat population [6].
Resistance to FHB has been mapped to all seven barley chromosomes, and the most common regions related to FHB resistance have been previously reported to be located on chromosomes 2H and 6H [68]. The number of detected QTLs varies in different reports, ranging from only one in the study by Mesfin et al. [69] to two [70] and up to 10 [71]. So far, few sources of FHB resistance have been found in barley, and their resistance level is modest [6, 10]. CI 4196 is one of the best sources of FHB resistance identified to date. This resistant cultivar is two-rowed barley. Six-rowed types are preferred for malting, but they are generally more susceptible to FHB than two-rowed barley [9]. Chevron, an old cultivar from Switzerland, is six-rowed malting barley and a popular parent in barley breeding programs. It has high resistance to kernel discoloration, a disease complex caused by several fungi, including fusarium. It is the best source of FHB resistance yet identified from six-rowed barley [72]. A recent study by Ogrodowicz et al. [73] detected a set of 70 QTLs through phenotyping the mapping population in field conditions and genotyping. Six loci were detected for the FHB index on chromosomes 2H, 3H, 5H, and 7H. A region on the short arm of chromosome 2H was detected in which many QTLs associated with FHB- and yield-related traits were found [73].
5. EROSION OF QUANTITATIVE HEAD BLIGHT RESISTANCE IN WHEAT AND BARLEY
Discernment on the adaptive potential of plant pathogen species is essential to designing a sustainable integrated pathogen management framework in which the durability of crop resistance is one crucial point [55]. The adaptive potential of plant pathogen species highly depends on the forces shaping the pathogen evolution. Among these forces, migration (spatial dispersal), recombination (reproductive mode), genetic drift and, above all, the selection by the host-plant resistances are of particular importance [23]. Pathogen populations can either adapt to the most common host (general adaptation) or be divided into several entities, each adapted to one particular host genotype (local adaptation [74],). The theory of polygenic traits’ evolution under divergent selection has not been investigated much, especially while investigating host-pathogen interactions [74]. Few studies focusing on the host quantitative resistance impact on pathogen populations reported either the local adaptation of pathogen populations or a directional selection toward an increase in pathogen aggressiveness on all host cultivars [23, 55, 74, 75].
The growing practice of monoculture wheat and barley cultivation has probably increased the rate of head blight pathogen evolution and forced the natural balance shifting in favor of the pathogen, resulting in more frequent and severe epidemics [6, 10], even in regions where FHB has not been previously reported [4]. The cereal–FHB association is ideal for analyzing the aggressiveness trait evolution in response to quantitative resistance selection pressure. FHB populations possess high levels of aggressiveness variation [76-84] and thus the basis for rapid adaptation. FHB disease severity varies quantitatively depending on the resistance of the wheat and barley cultivars [5]. However, in the wheat-FHB pathosystem, only one research showed that a newly emerging collection of F. graminearum isolates (since 2000) were significantly more aggressive by causing more disease in wheat cultivars than old pathogen isolates (before 2000); this difference in aggressiveness could vary with wheat genotypes released [85]. The authors suggested investigating whether FHB wheat resistance plays any role in the aggressiveness evolution of the new F. graminearum population [85].
Recently, Sakr [30, 31] explored how the deployment of quantitative wheat and barley resistance affects the aggressiveness changes under in vitro conditions, leading to potential resistance erosion. Differences due to the selective effect of a cultivar among non-selected and selected FHB strains were quantified for traits participating to parasitic (latent period (LP) and area under the disease progress curve (AUDPC)) fitness. LP (time from inoculation to sporulation) and AUDPC [76-84] have been regarded as the most important in vitro components for analyzing both aggressiveness and quantitative resistance in wheat and barley–FHB pathosystem. FHB strains with shorter LPs and greater AUDPCs are considered more aggressive on wheat barley plants than strains with longer LPs and lesser AUDPCs. These in vitro aggressiveness components measured on the scale of a given wheat and barley plant might largely determine the rate of epidemic development [76-84]. As long as LP and AUDPC are in vitro indicators of mechanisms of aggressiveness and quantitative resistance occurring in adult wheat and barely plants during FHB infection; the measured changes of evolutionary response of FHB populations faced with resistance selection pressure can be considered to be largely the same as pathogenic responses in cereal plants grown under field conditions [76-82]. Sakr [30, 31] found that pathogen populations adapted more quickly to the moderately resistant “MR” cultivar than the susceptible “S” cultivar. Selected pathogens were significantly more aggressive than non-selected strains for LP and AUDPC, while no increase in aggressiveness was detected in those obtained on potato dextrose agar, suggesting that the evolution of aggressiveness in FHB pathogens is associated with the presence of wheat and barley plants with varying resistance levels. The results indicate that selected strains from the “MR” cultivar presented a higher level of aggressiveness than those from the “S” cultivar, as they had a shorter LP and a higher level of AUDPC. These findings provide the first direct evidence that FHB pathogens evolve rapidly to adapt to the increasing aggressiveness of wheat and barley, indicating a risk of directional selection and possible erosion of FHB resistance [30, 31].
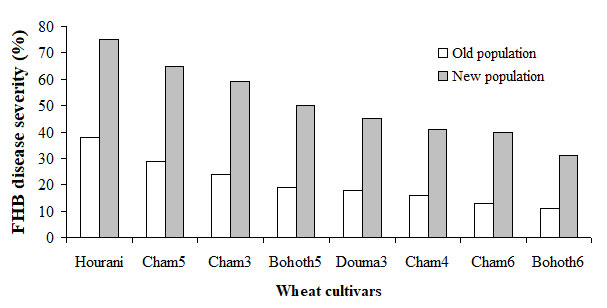
In another study conducted by Sakr [unpublished data], FHB populations were sampled in 2005 (old population) and 2015 (new population) from one of the major Syrian wheat production regions, chosen as a location where head blight occurs regularly. Single-floret inoculation was used to determine the aggressiveness of the two populations of eight durum and bread wheat cultivars of contrasting susceptibility to FHB, indicating that the new population caused a higher disease severity at a significant level than the old population (Fig. 1). Their aggressiveness increased between early and late samplings, suggesting that wheat plants cultivated over 10 years were selected for increased aggressiveness during epidemics. The information obtained in this study indicated that FHB populations adapt to prevailing wheat cultivars, irrespective of their resistance levels, and can therefore overcome polygenic quantitative resistance [Sakr, unpublished data]. Adaptation to wheat and barley resulting in increased pathogen aggressiveness that was not specific may render quantitative resistance nondurable if not properly managed. Therefore, the pyramiding of several QTLs with high impact in one cereal cultivar may extend durability [32].
6. GENE PYRAMIDING ENHANCES HOST RESISTANCE TO FHB INFECTION
There is a broad agreement that combining genes for resistance (gene pyramids) is useful for increasing durability [55], with many known successes. Perhaps the best success story, and certainly the best documented one, is for the control of stem and leaf rusts of wheat [86]. Breeding schemes for pyramiding resistance QTL were also applied to increase resistance levels in cultivated varieties, such as barley, wheat, bean, and pepper [87]. There are few reports of studies aiming to integrate disease resistance QTL in breeding strategies, in contrast to major R genes that have been widely used in plant breeding. The durability of QTL pyramids was rarely evaluated [55]. Nevertheless, QTL combinations are expected to increase durability for different reasons. Pyramiding resistance QTL showing a varying spectrum of action on pathogen strains may generate contradictory selection pressures on pathogen evolution [88, 89]. Pyramiding resistance QTL associated with different resistance mechanisms may affect pathogen life-history traits such as latency, infection efficiency, plant colonization, and pathogen multiplication [90]. Regardless of mechanisms, it is reasonable to assume that resistance gene combinations will be more durable than a single gene [55]. Developing high-throughput marker systems allows for the integration of genotypic information in cultivar development [87]. Molecular breeding methods, comprising MAS, deploy marker-trait associations to predict the phenotype and select the desirable genotypes demonstrating great potential to accelerate the increase in genetic gain [4-6].
Fhb1 improves only Type II resistance, and Fhb4 and Fhb5 enhance only Type I resistance [91]. Compared with the introduction of a single QTL, a pyramiding of QTLs for different types of FHB resistance is more effective against the disease, as illustrated in FHB resistance improvement, and should be promoted in breeding programs due to the lack of genes conferring immunity to FHB [92]. It was noted that the lines without gene pyramids had longer diseased rachides after point inoculation than lines carrying Fhb2 and Fhb1, Fhb4, and Fhb5, implying that the introgression of Fhb2 could further improve the FHB resistance [32]. Based on the number of diseased spikelets obtained after single floret inoculation and the percentage of infected spikes obtained after spraying inoculation, the Fhb1, Fhb4, and Fhb5 pyramiding raised the FHB resistance level by 95% and made the introgression lines highly resistant to FHB [32]. The QTL pyramiding effects are, however, still in dispute, as shown by Brar et al. [58], who introduced Fhb1, Fhb2, and Fhb5 from Sumai 3 into two hard red spring wheat cultivars from Canada, and by Salameh et al. [93], who made a similar attempt in European winter wheat. The discrepancy could be due to the small effect of Fhb2, different trial conditions and resistance evaluation methods, and genetic backgrounds [32].
Wheat breeders often find it difficult to obtain plants with satisfied agronomic performance and a high level of FHB resistance in conventional breeding using Sumai 3 as a parent, which prompts deliberation on whether the FHB resistance genes have deleterious effects on agronomic traits. Indeed, the Fhb4 interval was associated with plant height [94], and the Fhb5 interval was related to plant height and grain weight [95]. In a few studies, the introduction of the Fhb4 interval resulted in a plant height increase [91], and the introduction of the Fhb5 interval led to lower disease severity and a slight increase in plant height [58]. In terms of yield performance, the Fhb1, Fhb4, and Fhb5 introgression lines were as good as the recipient parents, showing that the Fhb1, Fhb4, and Fhb5 pyramiding was not in conflict with agronomic trait improvement [32].
Recently, Wang et al. [96] reported the molecular identity of another gene (Fhb7). This gene encodes a glutathione S-transferase that detoxifies DON, conferring semi-dominant resistance. It was shown that it was acquired through a ‘natural’ fungus-to-plant gene transfer from Epichloë, a widely distributed ascomycete fungal genus that colonizes many types of grass, to the wild wheat grass relative Thinopyrum ponticum. The new in-depth knowledge of Fhb7, along with the genes reported earlier, provides breeders with the opportunity for gene pyramiding, which might confer optimal control for FHB in wheat. Also, the engineering of Fhb7 to increase resistance to FHB in other cereals (such as barley and rye) or crown rot in wheat and ear rot in maize can now be considered [97]. Gene pyramiding also has yielded very promising results in wheat breeding. Native FHB resistance genes from local winter wheat varieties in the USA have been introduced through identity-by-descent-based linkage mapping into the winter wheat cultivar ‘Wesley’ with the Fhb1 background [98]. Similar approaches to introducing native resistance into spring wheat have resulted in the identification of a novel QTL on chromosome 2A [99].
The quick improvement of resistance was mainly due to the introduction and pyramiding of new resistance QTL not present in the CIMMYTs, the International Maize and Wheat Improvement Center, and the germplasm pool. However, the introduced exotic resistance was not adapted outside Chinese environments or for intensive agricultural practices due to low yield performance and undesirable agronomic traits. Therefore, time-consuming backcrossing programs were required to eliminate the traits negatively affecting agronomic performance [3-100].
7. A FUTURE PROJECTION ON THE WIDESPREAD EFFECTS OF fusarium ON THE GLOBAL POPULATION OF GRAINS AND ITS ECONOMIC IMPORTANCE AND SPORADIC MEASURES FOR THEIR CONTROL
The combination of reduced yield, poor grain quality, and mycotoxin contamination makes FHB a serious threat to the economics of cereal production worldwide [2-6]. Its severity is increasing due to climate change caused by weather fluctuations; depending on diverse geographical and meteorological factors, the predominance of several fusarium species is highly variable from global regions to a field scale, between seasons and over longer time periods [101, 102]. Variable weather conditions over seasons provoke the presence of distinct fusarium species with their agroecological niche, and consequently, fusarium infestations and mycotoxin contaminations are highly variable and challenging to control [4, 103]. However, as recently reported, shifts in the composition of populations lead to the emergence of new chemotypes with changes in toxicity and aggressiveness worldwide [103], even with a replacement of one chemotype by a more toxigenic one within the same species [27]. Several studies suggest alterations towards the dominance of F. graminearum, replacing other fusarium species in recent years in Central Europe [101, 104] and Northern America [33]. A general increase in fusarium incidence and strong dynamics within the species complex was observed in barley grain over the last decades in Bavaria [102]. In the same time period, rising annual mean temperatures, air humidity and changes in management practices, e.g., expanded maize cultivation, may have enhanced FHB incidence. Other studies suggest similar trends and changes within the species composition towards 2050 in Northern Europe [105, 106], in the UK [107] or even the entire Europe [108], where rising temperatures and atmospheric CO2 levels will likely increase favorable conditions for FHB infections. It is suggested that warmer conditions will likely increase the proportions of soil-borne pathogens and inocula worldwide [109]. Moreover, extreme weather events, application of no-tillage practices and intensified cereal production with narrowed crop rotations could drive FHB outbreaks and mycotoxin contaminations, which will likely become a challenge throughout the future whole cereal value chain [110].
Fig. (1): Disease severity (%) on each of the eight durum and bread wheat cultivars with different levels of FHB resistance averaged based on old and new FHB populations collected at 2005 and 2015, respectively from Ghab Plain, a location where head blight occurs regularly and one of the major Syrian agricultural production regions consisting of durum and bread wheat cultivars grown over several years and displayed varying levels of resistance to FHB pathogens.
Effective management of FHB cannot be achieved through a single control strategy because each has its limitations. Different management strategies, including cultural, biological, chemical and host plant resistance, are all powerful tools for FHB control [3-6]. The management of FHB is facilitated by tools that can be applied in the field, i.e., forecasting tools [4]. Due to the close association with favorable weather conditions, disease or mycotoxin risk/intensity can be predicted with reasonable accuracy (>70%) using forecasting models using within-season weather around flowering and, in some cases, combined with agronomic factors [111]. Some of these models have been linked to projected climate scenarios for future estimates of FHB risk. In most studies targeting specific locations or geographic regions in the UK, China, Brazil and Argentina, FHB incidence is predicted to increase mainly due to warmer temperatures in the early season causing wheat to flower earlier, coinciding with projected more wet conditions [107, 112]. In contrast, FHB risk is predicted to decrease in Scotland, where earlier flowering coincides with projected drier conditions [113]. In wheat and barley, with respect to silicon absorption and accumulation [114], silicon fertilization is effective as part of the integrated management of FHB disease on wheat and barley. The application of silicon did increase wheat resistance to FHB infection measured in vitro; 1.7 mM silicon resulted in a significantly higher latent period and lesser area under the disease progress curve and coleoptile length reduction compared with controls [115]. Foliar spraying of silicon reduced FHB incidence and severity in spikes and spikelets in the growth chamber [116, 117]. Additionally, single and multiple silicon applications incorporated into the soil under controlled conditions [116, 117] and in the field [118, 119] led to a significant reduction of FHB on wheat and barley plants. Breaking the fungal disease cycle by adapting the sowing period [120] is also proven to be a good measure. However, the period when spraying of fungicides is effective might be very short and, depending on appropriate weather conditions, difficult to handle [121]. Additionally, the efficacy of the fungicide application determines if all fusarium species present and pathogenic under particular conditions can be controlled. Sometimes the application of a fungicide that works excellently against some specific pathogenic species might not be effective against other species, which subsequently become dominant [122]. The factors of inefficient treatment lead to variable success in controlling FHB by application of fungicides [122]. Application of bio-control agents is hampered by a lack of knowledge of the conditions under which fusarium species become dominant. For example, if F. graminearum is suppressed by the application of antagonistic bacteria that are used as bio-control agents and/or a species-specific fungicide, still large quantities of other fusarium species might occur, especially, if they had previously been suppressed by F. graminearum [121].
CONCLUSION
FHB constitutes a clearly quantitative, broad-spectrum and durable resistance making any investment in head blight resistance improvement long-lasting. However, this strategy does not guarantee high resistance to head blight since resistance broke down due to aggressiveness shifts of FHB populations. While FHB resistance acts primarily additive, gene pyramiding with relatively large and stable effects via direct phenotypic selection in combination with genomic-assisted selection methods such as MAS has proven to be an appropriate strategy for resistance breeding. If a pyramiding of multiple resistances improving QTL combined with selection against suspected susceptibility factors is achieved in novel cultivars, the evolution of FHB pathogens might be slowed owing to reduced exposure to the pathogen, disruptive selection on FHB populations and subsequently reduced fitness of fusarium fungi. This would stabilize the pathogen population and contribute to the durability of FHB resistance.
LIST OF ABBREVIATIONS
| FHB | = Fusarium Head Blight |
| DON | = Deoxynivalenol |
| CWDEs | = Cell Wall Degrading Enzymes |
| LP | = Latent Period |
| AUDPC | = Area Under the Disease Progress Curve |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The author would like to thank the Atomic Energy Commission of Syria for financial support.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The author would like to thank the Atomic Energy Commission of Syria for assisting with this research. The two unknown reviewers are also thanked for constructive comments on this manuscript.


