All published articles of this journal are available on ScienceDirect.
Isolation, Identification and In Vitro Test for the Biocontrol Potential of Trichoderma viride on Fusarium oxysporum f. sp. Lycopersici
Abstract
Introduction:
Tomato production in Ethiopia is challenged by many pests and diseases. Fusarium wilt is one of the most important diseases of tomato affecting its productivity.
Methods:
Tomato tissue and soil samples were collected from tomato farmlands around Aksum town to isolate and identify pathogenic Fusarium species and Trichoderma species with biocontrol efficacy. Samples were processed in the Aksum University Biotechnology laboratory following standard procedures.
Results and Discussion:
Eight Fusarium and five Trichoderma isolates were obtained. Six of the Fusarium isolates were identified as Fusarium oxysporum, whereas the remaining two were Fusarium equiseti and Fusarium circinatum. Detached leaf bioassay of the F. oxysporum on tomato leaves showed leaf lesion on the tomato variety, Melka oda. The isolated Trichoderma strains were screened for biocontrol potential against virulent F. oxysporum in vitro. The Trichoderma isolate showing the highest biocontrol efficacy against the virulent Fusarium was morphologically identified as Trichoderma viride. in vitro F. oxysporum-T. viride dual culture assay demonstrated that T. viride inhibits the growth of F. oxysporum f.sp. lycopersici with 76.94% growth inhibition.
Conclusion:
Fusarium oxysporum is prevalent in tomato growing farmlands covered in this study. T. viride identified in this study is an effective biocontrol agent for the identified F. oxysporum fsp. lycopersici in vitro.
1. INTRODUCTION
Tomato is one of the major economically important vegetables worldwide [1, 2]. In particular, it is a widely grown vegetable cash crop in Ethiopia [3]. It has been found to be the most profitable crop to small farmers in the country [4]. While the productivity of tomato at demonstration sites and research plots in the country reached levels of 250 and 400 qt/ha, respectively, productivity in the agricultural farms is very low (90 qt/ha,) [4] as compared to the productivity level in the rest of the world, which is between 600-1000 qt/ha [5]. This is because tomato production in Ethiopia faces a variety of constraints, among which are pests and diseases.
Fusarium wilt is one of the most important diseases of tomatoes worldwide. It is a devastating disease that affects many important food and vegetable crops and a major source of loss to farmers worldwide [6]. The disease is characterized by wilted plants, yellowed leaves and reduced or even total loss of crop yield [7]. There are more than 100 Fusarium vascular wilt diseases worldwide [8, 9]. Fusarium wilt of tomato is commonly caused by the fungal pathogens, F. oxysporum or Fusarium solani. Fusarium equiseti is also becoming an emerging tomato wilt pathogen [10]. The most familiar formae species to tomato is Fusarium oxysporum f.sp. lycopersici [2, 10]. The pathogen penetrates the plant, colonizing and leaving the vascular tissue dark brown and the discolouration extends to the apex, leading to the plants wilting, collapsing and dying. The initial symptoms of the disease appear in the lower leaves gradually, trailing to wilting of the plants.
Ethiopian farmers apply chemical spray to protect their crops from pests and diseases. In Axum areas, farmers apply fungicides; Ridomil and Mancozeb. But the use of chemical sprays on edible crops is unacceptable for human health concern and from an environmental point of view [11] and more importantly, as Fusarium wilt disease is soil-borne in nature, application of fungicides to control this pathogen is not practical [12, 13]. Ethiopian GTP II has planned to establish stringent testing and certification services regarding chemical use and resulting residues on agricultural commodities with a view to providing chemical free organic products for the market [13]. As a result, there is a need for some eco-friendly biocontrol systems that help to mitigate these problems. Biological control of plant pathogens by microorganisms has been considered a more natural and environmentally acceptable alternative to the chemical treatment methods [14, 15]. Various types of biological control agents such as bacteria and fungi are involved in biocontrol activity [16, 17]. Among them, the fungal genus Trichoderma plays a major role in controlling plant diseases [18]. It is one of the best known and well described biocontrol fungi for its antagonistic properties against phytopathogens [19]. Several strains of Trichoderma have been developed as biocontrol agents against fungal diseases of plants [20, 21]. Most biocontrol agents are from the species of T. harzianum, T. viride and T. hamatum [20, 15].
Farmers in the surroundings of Axum town cultivate tomato. But their tomato cultivation is affected by fungal infections both on the field and after harvesting, the consequences of which are loss of a significant quantity of the product (Pers. Com, local agricultural expert). Fusarium species alone accounted for 25% postharvest deterioration of tomato fruits [unpublished]. Therefore, the current research presented the isolation, identification and development of the fungal biocontrol agent, Trichoderma species against the fungal pathogen of tomato, Fusarium oxysporum f.sp. lycopersici. The objective sought to evaluate the biocontrol efficacy of Trichoderma species against F. oxysporum in vitro by dual culture assay and in vivo by detached leaf bioassay.
2. MATERIALS AND METHODS
2.1. Study Area and Study Period
The areas considered in this study were the farmlands within a 5 km radius from Axum town; Hatsebo in the east, Dura in the west and Medoge in the south through the altitudinal range of 1834-2096 m.a.s.l. Plots of lands cultivated with tomato were investigated for the occurrence of Fusarium wilt disease and 20 tomato and 26 soil samples were collected from the plots using sterile polyethylene plastic sampling bags. The collected samples were transported to Aksum University, Biotechnology Laboratory for isolation and identification of plant pathogenic microfungi and fungi with biocontrol potential. The study was conducted through the period between March 2018 to April 2019.
2.2. Isolation of Fusarium Pathogens from Tomato Vegetative Parts
The Fusarium wilt pathogens were isolated following the tissue transplanting technique [22]. The tomato plant parts were washed with running tap water to remove soil and dust from the tissues; followed by the stem and root samples cut into 5mm-10mm sized slices using sterile scissors and surface sterilised using 96% ethanol for 1 minute. The surface disinfected tissues are then washed with three successive changes of sterile distilled water to washout the ethanol and were blot dried using Whatman Number 1 filter papers. Blot dried tissues were placed onto 10cm glass Petri dishes containing the solidified Potato Dextrose Agar (PDA) medium. The plates were then incubated at 25+2 °C for seven days with periodic checks for the emergence of fungal mycelia from the transplanted sample tomato tissues. Fungal hyphae emerging on the transplanted tissues were transferred onto the fresh PDA medium by means of the hyphal tip transfer technique [23]. The cultured fungal isolates were purified by three subsequent sub-culturing on to PDA. Eight pure fungal cultures inspected to be Fusarium were maintained in the form of slant culture at 4°C for further study.
2.3. Cultural Characters of Fusarium Isolates
The cultural characters of the isolates on the PDA medium were studied by culturing each isolate in three replicates at 25+2°C. Cultural characteristics (colony appearance, growth pattern, marginal shape, surface colony colour, reverse colony colour/pigmentation, radial growth) were examined seven days after inoculation (7DAI) and the growth rate was measured. Radial growth was evaluated by measuring the radius of the petri dish covered by the colony culture using an mm-calibrated glass ruler. Growth rate data was recorded by measuring the radius that the colony covered 24-hour intervals for seven days. The growth rate value was then calculated by subtracting the radial growth value on the first day from the radial growth value of the second day and so on for the seven days; finally tabulating the mean of all the differences thereby giving the growth rate of that specific isolate [23].
2.4. Morphological Identification of Fusarium Isolates
A slide culture was prepared for each of the eight isolates by taking a 5mm bit from a seven-day old pure culture and inoculating on to a rectangular cut of the PDA medium. It was then placed on sterilized glass slides and covered with coverslips. The slides were then placed inside a sterilized Petri dish underlay with moistened filter paper to provide moisture for the fungi, and incubated at 25± 2° C [22]. The mycelia developed on the slide culture are then subjected to microscopic examination to morphologically differentiate the identity of the isolates to species level at 40X magnification [23] using an Olympus Microscope (Carl Zeiss Microscopy, Germany).
2.5. Isolation of Trichoderma Species
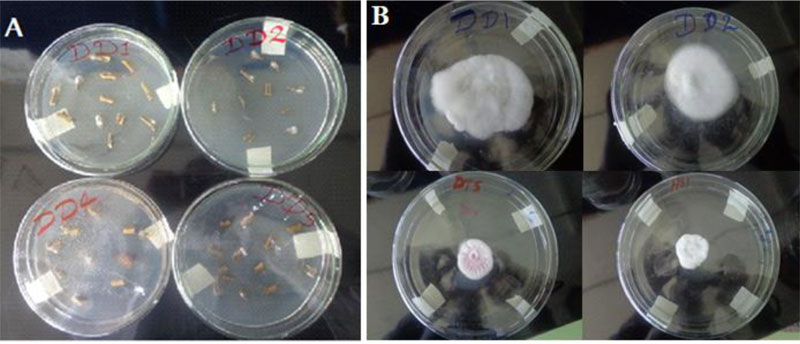
Soil samples were collected from rhizosphere areas of referenced garden trees (Mango, Lemon, Orange, Guava and Avocado) within 100cm cardinal points and 10-15cm deep [24]. Trichoderma species were isolated by a soil serial dilution- agar plating method and stored at 4oC for later use (Fig. 1). Culture purification was attained by repeated sub-culturing through the hyphal tip transfer technique [23].
2.6. Identification of the Trichoderma Isolate
The identification of the Trichoderma was made based on in-depth observation of cultural characters and Microscopic examination of morphological features with reference to the literature [24]. To capture a photo of the conidia, a digital camera (SONY Cyber-shot, 16.1 Mega pixels) was used by attaching to the eyepiece of the microscope. The photograph was captured by focussing the zoom adjustment to obtain a magnified image.
2.7. Radial growth and Growth Rate of Isolates
The radial growth and growth rate evaluation was done for both Fusarium and Trichoderma isolates. Radial growth is recorded by measuring growth seven-days post-inoculation using an mm calibrated glass ruler. The growth rate is also recorded by measurements taken every 24 hrs and calculating the difference. The tabular value was obtained by subtracting the radial growth of the isolate on day one from the radial growth of the isolate on day two. This is taken as the growth rate value, i.e. how fast does it grow within 24 hours of duration [25].
2.8. Pathogenicity Test for Fusarium Isolates
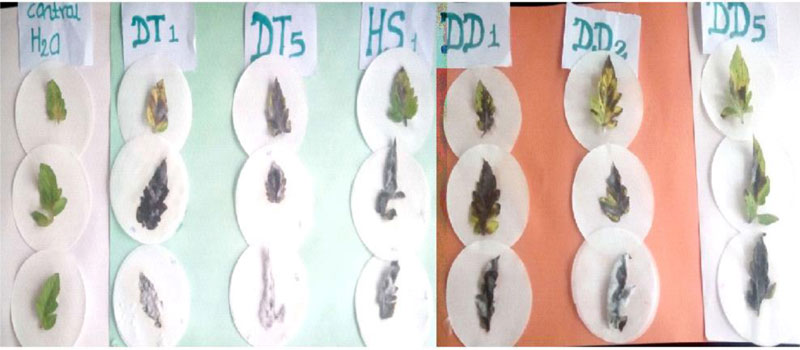
The pathogenicity of the isolated Fusarium species was tested through bioassay on detached leaves of the Tomato cultivar-Melka oda grown on pots following standard procedure [26]. Healthy looking vigorous tomato leaves cultivated on pots with autoclave sterilized soil were used for the bioassay. Sixty (60) days old leaves were cut from the parent plant and transported to the laboratory. The leaves were surface sterilised with 70% ethanol for 30 seconds, rinsed with three successive changes of sterile distilled water and blot dried. The leaves were placed in sterile plates and were inoculated with 500µl spore suspension of Fusarium oxysporum. The control leaves were inoculated with the same amount of sterile distilled water and the treatments were in three replicates. The plates were incubated at 25± 2° C until the emergence of mould growth was observed on the surface of the leaves. Observations were taken starting from the second day where the lesion diameter was recorded.
2.9. Biocontrol Potential of T. Viride against F. Oxysporum by in vitro Biocontrol Test
Five Trichoderma isolates initially obtained from soil samples taken from garden areas of Hatsebo and Medoge kebeles were screened through preliminary in vitro bioassay of dual culture with one of the pathogenic Fusarium isolates. Trichoderma with the better biocontrol potential was used for the main experiment. The biocontrol potential of T. viride was evaluated using a dual culture assay. The percentage growth inhibition (PGI) was calculated using the following formula.

 |
3. RESULTS AND DISCUSSION
3.1. Isolation
Upon incubation at 25± 2° C for three to seven days, mycelial masses of fungi emerged on top of the inoculated specimen (Fig. 2). After three subsequent sub-culturing and microscopic examinations, eight pure cultures of Fusarium were identified. 87.5% (7 isolates) of the Fusarium were found from the tomato samples collected from Dura plots.12.5% (1 isolate) was found from the tomato samples collected from the Hatsebo plots. Five Trichoderma isolates are found from soil samples taken from garden areas of Hatsebo and Medoge kebeles. To the best of our knowledge, this is the first investigation conducted on Fusarium and Trichoderma species from the farmlands around Axum town, the centre of ancient Axumite civilization, where scientific studies are emerging following the establishment of Aksum University as a governmental higher learning institution. It also begins to fill gaps at the country level with information on the importance of Fusarium species. That hitherto, has largely been lacking [27].
3.2. Detached Leaf Bioassay Test of Fusarium Species on Tomato Leaves
Pathogenicity tests of the isolated Fusarium species on detached leaves of tomato are presented in Table 1. As indicated in the table, two of the Fusarium isolates (DT1 and DT2) caused 100% leaf damage on the tomato leaves. The control leaves that received sterile distilled water were free from any mould development, whereas the leaves that received Fusarium spore suspension showed mould growth on their surface and finally became wounded (Fig. 3). This implies that the Fusarium species isolated from the tomato tissues are pathogenic to the tomato variety, Melka oda used for this test. F. oxysporum, F. solani, F. equiseti and F. striatum have been reported to affect tomato [10]. But the finding that F. circinatum causes injury on tomato leaves is a first-time report by the current investigation. This also strengthens reports that indicate new pathogenic Fusarium species are continually evolving [10].
| No. | Treatments | Isolate code/species | Percent leaf damage value |
|---|---|---|---|
| 1 | Sterile distilled water | control | 0% |
| 2 | Fusarium spore suspension-1 | DD1(F. circinatum) | 56.56% |
| 3 | Fusarium spore suspension-2 | DD2w (F. equiseti) | 60.46% |
| 4 | Fusarium spore suspension-3 | DD2 (F. oxysporum) | 70.27% |
| 5 | Fusarium spore suspension-4 | DD5 (F. oxysporum) | 68.48% |
| 6 | Fusarium spore suspension-5 | DT1 (F. oxysporum) | 100% |
| 7 | Fusarium spore suspension-6 | DT2 (F. oxysporum) | 100% |
| 8 | Fusarium spore suspension-7 | DT5 (F. oxysporum) | 90.5% |
| 9 | Fusarium spore suspension-8 | HS1 (F. oxysporum) | 92.5% |
3.3. Cultural Characteristics of Fusarium Isolates on PDA
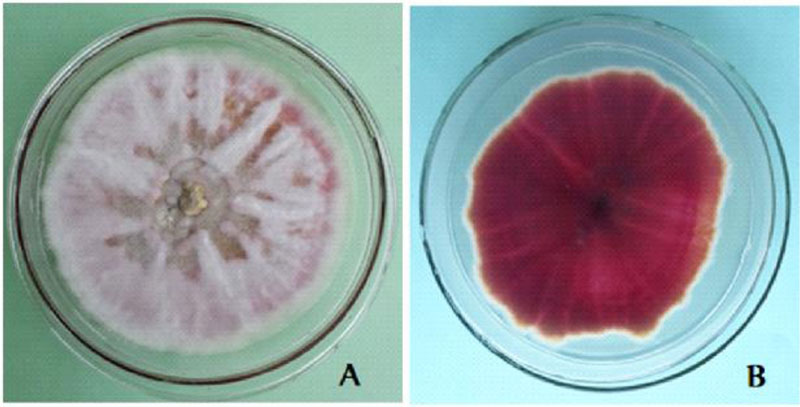
The cultural characters of the eight Fusarium isolates are presented in Table 2. The isolates showed purple tinged white, white cottony and full pink colony colours (Table 3). On the reverse side, the isolates showed various pigmentations (light reddish purple, gray with a light purple tinge, yellow stained white, normal purple and intense dark reddish purple) (Fig. 4), as reported in a previous study [10]. The growth rate of the isolates ranged from 4.66mm to 7.28mm which is far less than that of FOL isolates from Mexico-7cm-12cm [10]. Two of the isolates are rated as fast growing (DD1 and DT1) and the remaining were medium growing (DD2, DD2w, DD5, DT2, DT5, HS1).
| No. | Isolate | Radial growth 7DAI (mm) | Mean Growth rate in 24 hrs (mm) | Ratings: Fast/medium/slow | Species identity | Percentage occurrence |
|---|---|---|---|---|---|---|
| 1 | DD1 | 52.13 | 7.28 | Fast growing | Fusarium circinatum | 12.5% |
| 3 | DD2w | 40.5 | 5.98 | medium | Fusarium equiseti | 12.5% |
| 2 | DD2 | 43.13 | 6.44 | medium | Fusarium oxysporum | 75% |
| 4 | DD5 | 46.16 | 6.83 | medium | Fusarium oxysporum | |
| 5 | DT1 | 54.25 | 8.27 | Fast growing | Fusarium oxysporum | |
| 6 | DT2 | 41.75 | 5.98 | medium | Fusarium oxysporum | |
| 7 | DT5 | 33.38 | 4.66 | medium | Fusarium oxysporum | |
| 8 | HS1 | 41.50 | 6.10 | medium | Fusarium oxysporum |
| Character | Character descriptor | Description | |
|---|---|---|---|
| Cultural characters |
colony color |
surface | Pink floccose mycelium -Regularly circular -flattened spreading with concentric circles |
| reverse | reddish purple | ||
| pigmentation | Produced Intense reddish purple pigment | ||
| Morphological characters | macroconidia | Single, short, hyaline, appeared in single on PDA medium. | |
| microconidia | Ellipsoidal shape and non septated | ||
| chlamydospore | Abundant pseudochlamydospores | ||
3.4. Morphological Characteristics of Fusarium Isolates Grown on PDA
The morphological character of the Fusarium isolates is presented in appendix 1. Generally, macroconidia are more or less abundant, 3-5 septate, straight and with more ventral curvature than dorsal in F. oxysporum (Fig. 5). In F. circinatum, macroconidia with 3 septa, thinner and slender occasionally found in bunch and chlamydospores are absent. In F. equiseti, macroconidia were with prominent foot shape, abundant on the aerial mycelium with the interwoven state. These morphological features are similar to the descriptions of the respective Fusarium species [23, 25]. Fusarium oxysporum and Fusarium equiseti are commonly isolated from soil and plant tissue samples in Ethiopia [27]. As to our information, the isolation of Fusarium circinatum from diseased tomato tissues in Ethiopia is the first-time report by this investigation. Similarly, researchers from Mexico also identified Fusarium circinatum from diseased tomato that is confirmed by a molecular method [10]. From the isolated Fusarium cultures, F. oxysporum accounted for 75% of occurrence, whereas the Fusarium equiseti and Fusarium circinatum occurred each with 12.5%. The occurrence rate is also similar to a previous report [10].
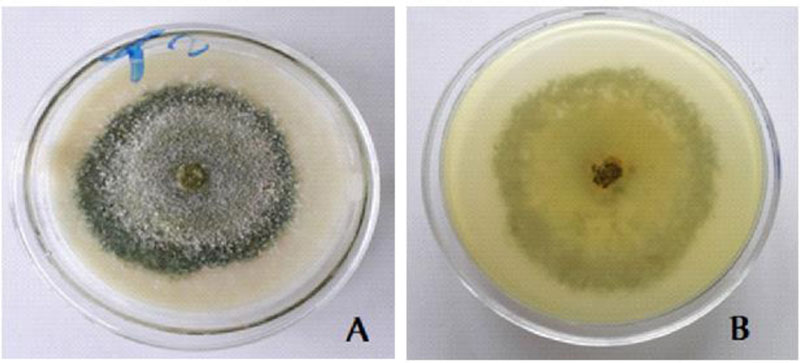
3.5. Cultural Characteristics of the Trichoderma Isolate with Biocontrol Potential
White scanty mycelia in the early 1-3 days; Warty green concentric rings in 4-5 days; whole warty green culture in 5-7 days and yellowish green pigment were observed on the reverse side [28] (Fig. 6).
3.6. Radial Growth and Growth Rate Data of the Trichoderma Isolate
The isolated Trichoderma species has a mean radial growth of 100mm 3days after inoculation and a mean growth rate of 31.70mm per 24 hours. The mean radial growth value of the current Trichoderma isolate is very high as compared to a previous report from Ethiopia, which was 57.67mm, 7DAI on PDA and with similar growth conditions [29]. The recorded growth rate is also higher than a previous report where the measurements were 28mm-30mm [30]. The implication of this is that the current Trichoderma isolate has better biocontrol potential (Table 4).
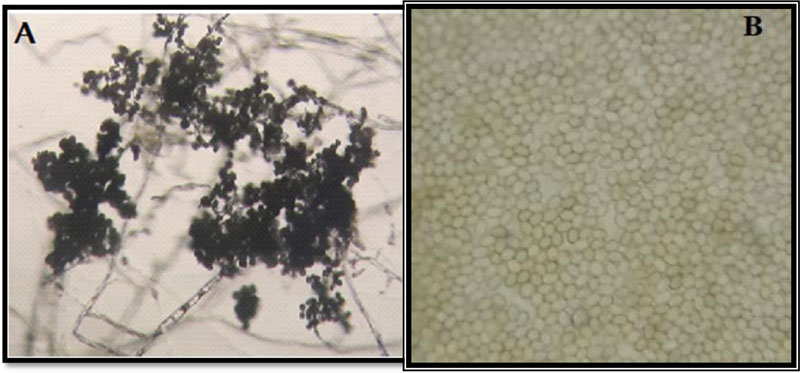
3.7. Microscopic Features of the Isolated Trichoderma
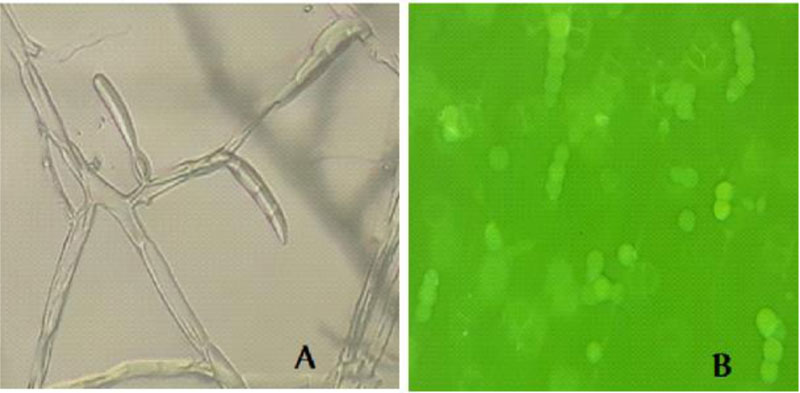
Microscopic examination of the morphology of Trichoderma isolates revealed dark green grossly warted conidia conspicuously observed with irregular ornamentations and oval conidia (Fig. 7). The microscopic features of this Trichoderma isolate as examined using the Olympus system microscope is similar to the description of Trichoderma viride [31]. Accordingly, the Trichoderma isolate is identified as Trichoderma viride.
| Isolate | Mean radial growth record (mm) | Mean growth rate/24 hrs (mm) | ||||
|---|---|---|---|---|---|---|
| Trichoderma | 1DAI | 2DAI | 3DAI | GR-1 | GR-2 | Mean GR |
| 36.70 | 65.90 | 100 | 29.20 | 34.10 | 31.70 | |
| Mean radial growth of Trichoderma species in control plate (mm) | Mean radial growth of F. oxysporum in control plate (mm) | Mean radial growth of F. oxysporum in dual culture (mm) | Mean %GI |
|---|---|---|---|
| 100 | 100 | 22.00 | 76.94% |
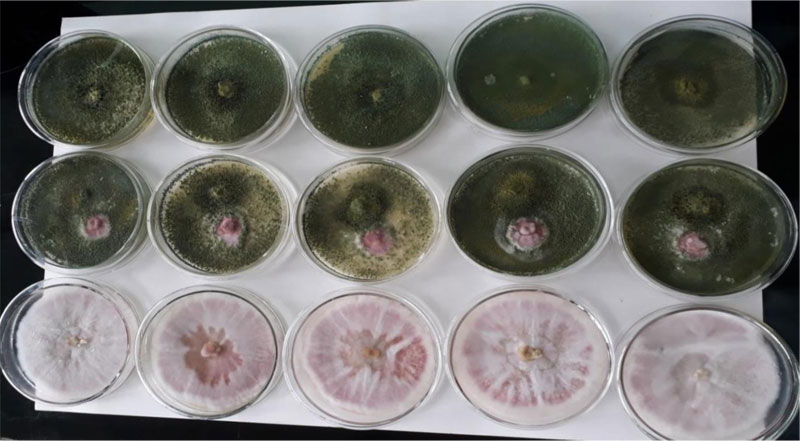
3.8. In Vitro Biocontrol Experiment
in vitro dual culture assay of the pathogenic F. oxysporum and the biological control agent, Trichoderma species, is presented (Table 5). In the in vitro dual culture experiment, the Trichoderma isolate showed high biocontrol efficacy (76.94%). This PGI value is higher than a previous report (61.80% - 72.05%) [32]. The Trichoderma showed a very fast spread to cover the entire space on the medium during the first three days, restricting the growth of the F. oxysporum fsp. lycopersici within a narrow cleft. Later, the Trichoderma started shuttering its conidia on top of its own colonized space. Finally, the Trichoderma species also shuttered its conidia on top of the encapsulated F. oxysporum. F. oxysporum showed colony expansion until the Trichoderma approached near its vicinity; then, the Fusarium was totally under control and colonized by the shots of T. viride conidia resting and germinating on its colony, leading to a mycoparasitic activity on the Fusarium oxysporum. f. sp. lycopersici (Fig. 8).
CONCLUSION
Isolation and morphological identification revealed Fusarium oxyporum, Fusarium equiseti, and Fusarium circinatum in the tomato growing farmlands around Axum town. Fusarium oxysporum fsp. lycopersici is the dominant isolate in its occurrence. The isolates were consistent in their cultural characters across different batch cultures on the PDA medium. Detached leaves bioassay is a good indicator for in vivo pathogenicity of Fusarium oxysporum on tomato. The Trichoderma viride identified in this study is an effective antagonist for identified F. oxysporum fsp. lycopersici in vitro. It showed competition for space and nutrients as well as mycoparasitism against the phytopathogen, Fusarium oxysporum.
ETHICS APPROVAL AND CONSENT TO PARTI-CIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No human or animal were used in the study.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
All the data and materials pertaining to this research article are within the manuscript.
FUNDING
This study was financially supported by Aksum University, Axum, Ethiopia with grant number ‘AKU-INHOUSE PROJECT- 2018’.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors have agreed upon the publication of this research article in The Open Agriculture Journal. The authors duly acknowledge Aksum University for funding the research project.
APPENDIX
| No. | Cultural characters of isolates on PDA | Morphological characters on PDA | Species identity | |||||
|---|---|---|---|---|---|---|---|---|
| Isolate code | Colony color | Pigmentation | macroconidia | microconidia | chlamydospores | identified as | ||
| surface color | reverse color | |||||||
| 1 | DD1 | Purple tinged white with furry aerial mycelium. Regularly circular radial growth |
Light reddish purple | Light reddish purple | Mostly 3-septated, slender narrow Arranged in bunch formed on the aerial mycelium. Not abundant |
Berry looking phialides arranged in clusters, chains and in single are abundantly observed | Not seen | Fusarium circinatum |
| 2 | DD2 | -Purple tinged white aerial mycelium -Regularly circular radial growth -no concentric circles |
Gray with light purple tinge | light red tinged pigment | Short stout macroconidia with 3-5 septa arranged in bunch were Abundant |
Short monophialides were more frequently observed. Ellipsoidal Microconidia |
Pseudo chlamydospores were abundantly observed | Fusarium oxysporum |
| 3 | DD2w | -White cottony aerial mycelium -irregular in marginal growth with in and out curvatures on the outside -no concentric circles |
Yellow stained white | light | Highly abundant macroconidia formed on the aerial mycelium in bunch form. Highly twisted and interwoven | absent | Chlamydospores rarely observed | Fusarium equiseti |
| 4 | DD5 | -Purple tinged white colony with furry mycelium _regularly circular radial growth pattern |
Gray with light purple tinge | -No pigmentation | Abundant macroconidia in bunch; 3-5 septated, shorter and straight | Elliptical microconidia in rare presence Short phialides |
present | Fusarium oxysporum |
| 5 | DT1 | Whitish purple, furry aerial mycelium Dense and spreading growth -Regularly circular Radial growth With no concentric circles |
normal purple | Pigment: light red tinged purple | Long non septated hyaline; straight and pointed on both ends | Large ball looking monophialides formed on the aerial mycelium | present | Fusarium oxysporum |
| 6 | DT2 | -Purple tinged white aerial mycelium with furry appearance -Regularly circular growth Spreading growth |
Gray with light purple tinge | No | macroconidia with 3-5 septa arranged in bunch abundantly seen | Ellipsoidal microconidia | Abundant pseudo chlamydospores | Fusarium oxysporum |
| 7 | DT5 | -Pink floccose mycelium -Regularly circular -flattened spreading with concentric circles |
Intense dark reddish purple; yellowish bright at the margin Golden bright on the margin |
Intense reddish purple pigment | macroconidia with 3-5 septa arranged in bunch abundantly seen | Mostly ellipsoidal in shape; non septated, hyaline; inside the agar. Dark ballus and branched short phialides. | Abundant pseudo chlamydospores | Fusarium oxysporum |
| 8 | HS1 | White fluffy aerial mycelium Irregular lobed radial growth No concentric circles |
Yellow tinged white | light yellowish pigment | Septated macroconidia curved from both ends. Narrow in the middle. Formed on the mycelium and also detached. Mostly in single; fusiform. |
Spherical to elliptical microconidia | Abundantly seen in pairs, in chains and in cluster at intercalary and apex position. | Fusarium oxysporum |


