All published articles of this journal are available on ScienceDirect.
Application of Spray-dried Microcapsules of Purslane (Portulaca oleracea L.) Seed Oil Enhances Quality of Mango Juice
Abstract
Introduction:
Purslane (Portulaca oleracea L.) seeds oil are a non-traditional alpha-linolenic acid source (ALA), which is an omega-3 fatty acid. This study aimed to evaluate the physicochemical and sensory properties of mango juice fortified with purslane seed oil (PSO) microcapsules.
Materials and Methods:
Gum Arabic (GA) and maltodextrin, as wall-materials, were used in the microencapsulation of PSO by spray drying technique. The spray-dried microcapsules were added to the mango juice (200 mL) at the levels of 0.5, 1 and 1.5 g, ALA. Physicochemical properties such as viscosity, total soluble solids (TSS), pH and titratable acidity were measured, as well as sensory evaluation, during 28 days' storage at 4.0 ±0.5°C.
Results:
Our study indicated that the microencapsulation of PSO by spray drying resulted in the best microencapsulation yield (85.17%) as well as the microencapsulation efficiency (77.40%). The pH and TSS of four juice samples ranged from 3.0 to 3.6 and from 18.8 to 19.1 Brix°, respectively. In addition to that, storage periods had no significant effect on them.
Conclusion:
According to the findings presented in this paper, it has been concluded that the nutritional value of mango juices was enhanced by the addition of microencapsulated PSO as a source of ω-3 fatty acids.
1. INTRODUCTION
Purslane oil is composed of unsaturated fatty acids; it is considered one of the rich plant sources for ω-3 fatty acids. The unsaturated fatty acid content of the purslane makes it beneficial for the inhibition of cancer cell growth and reduction of cholesterol [1]. Purslane is high in α-linolenic acid, ß-carotene, volatile terpenoids and phenolic compounds; hence, it is considered a healthy food for patients with cardiovascular diseases [2-4].
The encapsulation process can be applied in the food industry. This process is a good technique for upgrading the delivery of bioactive compounds (essential fatty acids, antioxidant compounds and living cells “probiotics”). Furthermore, encapsulation can be used for the protection of volatile agents from radiation, light and oxygen [5]. Microencapsulation is defined as a method in which tiny droplets or particles are surrounded by a wall, embedded or coated in a homogeneous or heterogeneous matrix to give small capsules, effectively building a barrier between the environment and the component in the capsule [6-8].
Many methods have been developed to microencapsulate food ingredients. Spray drying is a method widely used for microencapsulation of flavours and oils. It results in good quality powders with low water activity, facilitating handling and storage, and protection of the active components against undesirable reactions. Both wall material selection and emulsion properties (droplet size, stability and viscosity) can affect the method efficiency and the microencapsulated final product stability. Minimum surface oil, maximum retention of the active material and powder form are the most important measures of a successful oil microencapsulation process [9-11].
The world is suffering from the spread of many diseases because of immunodeficiency, especially in developing countries. Therefore, it was necessary to try to increase food immunity, using elements such as fortified food enriched with ω-3 from natural sources, especially for individuals most vulnerable to diseases, elderly and children [12]. In 2009, the European Food Safety Authority [13] published its recommendations for polyunsaturated fatty acids PUFA: a) An ω-3 fatty acid intake of 2 g per day ALA and 250 mg per day long-chain ω-3 fatty acids; eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). b) An ω-6 fatty acid intake of 10 g per day linoleic acid (LA).
The growth of the juice industry growth rate is about 2.6% per year. This rapid growth reflects the growing trend of healthy living. These juices are supplied with enough fruit to supersede a meal. With busy work and school schedules, people can make a healthy decision to grab a bottle of juice instead of consuming junk food [14]. In this study, the characteristics of the purslane seed oil microcapsules were studied to evaluate their possible uses as a natural additive source of ω-3 fatty acids in mango juice; physicochemical characteristics and sensory properties of juice were also evaluated.
2. MATERIALS AND METHODS
2.1. Materials
Purified purslane seeds were obtained from a local market in Cairo, Egypt. Ripe mango fruits and sugar were purchased from a local market in Zagazig city, Sharqia Governorate, Egypt.
2.2. Methods
2.2.1. Extraction of PSO
The PSO was extracted according to the method reported by Zahran and Soliman [15] with some modifications. A total of 200 g of purslane seeds were arranged as a uniform layer in a Pyrex petri dish and placed in a home microwave oven (Samsung, Convection Microwave Oven with HotBlast™, 28L model MC28M6035KW, 900 W output) for 120 sec. The extraction was carried out using a hydraulic cold pressing machine at room temperature (25 ±1.0°C) with pressure at 10 MPs. Extracted PSO was then filtered using a filter paper Whatman No. 1, 10 cm; it was centrifuged at 3500 rpm for 10 min in order to separate those components settled during storage. The oil was stored in dark bottles at refrigerator temperature until used.
2.2.2. Physicochemical Characteristics of PSO
Physicochemical analyses of PSO samples, such as refractive index, peroxide value, acid value, saponification value, and iodine value, were examined according to the standard official methods of AOCS [16].
2.2.3. Total Phenol Content (TPC) of PSO
Phenolic compounds of PSO were extracted by methanol/water solution at 80:20 ratios according to Naeem et al. [17]. The Folin-Ciocalteu method was used for the determination. 2.5 g of extracted PSO were dissolved in 5 mL hexane, and extraction was carried out by methanol/water solution (80:20, v/v). The aqueous phase was collected by centrifugation at 3500 rpm for 10 min, followed by vacuum drying at room temperature. The dried sample was dehydrated in 5 mL of the methanol solution, mixed with 2.5 mL of Folin reagent and 10 mL of sodium carbonate (7%) solution in 50 mL volumetric flask, and was adjusted to volume with deionized water. The absorbance was measured at 765 nm after 30 min. Gallic acid was used for calibration, and the results were expressed as mg gallic acid equivalent (GAE) per 100 g of oil samples. A triplicate test was performed for each sample.
2.2.4. Antioxidant Activity of PSO
Antioxidant activity was evaluated using the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity [18] with slight modifications. Diluted purslane seed oil (0.1 mL) was added to 3.9 ml of methanolic DPPH solution (0.1 mM). The decrease in absorbance was detected at 517 nm after 30 min incubation in darkness at room temperature. A DPPH solution devoid of a sample was used as a control. The DPPH radical scavenging activity (%) was determined using the following equation:
| DPPH scavenging activity (%) = |
| (Absorbancecontrol - Absorbancesample)/Absorbancecontrol * 100 | (1) |
2.2.5. Fatty Acid Composition of PSO
The fatty acid composition was determined by the conversion of oil to Fatty Acid Methyl Esters (FAMEs) according to the modified method of Zahran and Tawfeuk [19]. FAMEs were analyzed using an HP 6890 plus Gas chromatography (GC) (Hewlett Packard, USA), a capillary column Supelco™ SP-2380 (60 m×0.25 mm×0.20 μm), (Sigma-Aldrich, USA), Detector (FID) while the injector and detector temperature were 250°C. The column temperature was 140°C (hold for 5 min) and rise to 240°C at 4°C/min., and hold at 240°C for 10 min. The carrier gas was helium at a flow rate of 1.2 mL min-1. FAMEs were identified by comparing their relative and absolute retention times to those authentic standards of FAMEs (SupelcoTM 37component FAME mix). The fatty acid composition was reported as a relative percentage of the total peak area.
2.2.6. Preparation of PSO Microcapsules by Spray Drying
The wall materials (gum Arabic and maltodextrin) were added to distilled water at 50°C and the mixture was stirred until completely dissolved. The total solid concentration (wall material + oil) was fixed at 30%. Purslane seed oil was then added to the wall material solution at a concentration of 20% with respect to total solids. Emulsions were formed using an Ultra-Turrax homogenizer MA-102 (Marconi, Piracicaba, Brazil) operating at 18,000 rpm for 5 min. After homogenization, the sample was kept for stabilization at room temperature for 1 h, and spray dried using a tabletop spray dryer (S. M. Scientech, Kolkata, India) under the following experimental conditions: inlet temperature 160°C; outlet temperature 80°C; spray flow feed rate 5 mL/min; nozzle diameter 3 mm; drying airflow 0.5 L/h, air pressure 4 bar. The encapsulated powders obtained by spray drying were used for juice preparation.
2.2.7. Encapsulation Characteristics
2.2.7.1. Encapsulation Yield and Efficiency
The encapsulation yield (EY) was expressed as the ratio of the mass of the final product to the mass of the initial material used, considering the masses of the wall and core [20].
Encapsulation efficiency (EE) was determined according to the method described by Bae and Lee [10]. n-hexane (15 mL) was added to 1.5 g of powder in a glass jar with a lid, which was shaken by hand for the extraction of free oil for 2 min at room temperature. The solvent mixture was filtered through a Whatman filter paper N° 1, and the powder collected on the filter was rinsed three times with 20 mL of hexane. Then, the solvent was left to evaporate at room temperature and after at 60°C, until constant weight. The non-encapsulated oil (surface oil) was determined by the mass difference between the initial clean flask and that containing the extracted oil residue [21]. Total oil was assumed to be equal to the initial oil since preliminary tests revealed that all the initial oil was retained, which was expected since flaxseed oil is not volatile. Encapsulation efficiency (EE) was calculated from the following:
| EE = (TO-SO)/ TO×100 | (2) |
Where TO is the total oil content and SO is the surface oil content.
2.2.7.2. Scanning Electron Microscope (SEM)
Scanning electron microscopy (SEM) was conducted on a Hitachi S-4800 microscope (Hitachi High-Technologies, Tokyo, Japan) at an accelerating voltage of 10 kV and a working distance of 8 mm. Samples were sputter-coated with a gold-palladium mixture under vacuum prior to the examination. Particle diameters were measured from the SEM micrographs in their original magnification using the Image software. Size distributions were obtained from a minimum of 200 measurements.
2.2.8. Preparation of Mango Juice Enchanted with ω-3 Microencapsules
Mango fruit was washed, peels and seeds were removed, and then the fruit was cut into small pieces. The juice of the fruit was extracted with distilled water using a kitchen mixer (Moulinex, France), and sugar was added to each drink (100 g); the mixture was homogenized using a kitchen mixer. Microcapsules powder was mixed with mango juice (200 mL) at the levels of 0.5, 1 and 1.5 g ALA, control sample (without addition) was carried, based on European Food Safety Authority [13] recommendation. The juices were filled in 200 mL glass bottles, then pasteurized at 80°C for 10 min, cooled and stored at 4.0 ±0.5°C for 28 days.
2.2.8.1. Physicochemical Analysis of Prepared Juice
The viscosity was determined using a Brookfield Viscometer (Model DV-I, USA) at room temperature. Total soluble solids were determined using Abbe refracted meter and expressed as (°Brix). The pH was determined using a glass electrode pH meter. Titratable acidity was measured by titration with 0.1 N NaOH, and the results expressed as grams of citric acid per liter AOAC [22]. Determination of lipid oxidation was assessed in triplicates by the 2-thiobarbituric acid (TBA) method of Fernandez-Lopez et al. [23].
2.2.8.2. Sensory Analysis
Sensory evaluation of mango juices was evaluated according to Habibi et al. [24], with slight modifications. Mango juices were sensorial evaluated for general appearance, taste, flavor, texture and overall acceptability by trained panelists from the Food Science Department, Faculty of Agriculture, Zagazig University. The panelists were asked to rate the different juice samples presented to them. Water was provided to panel members to cleanse their palates between samples. In this case, A 1-9 point hedonic rating test was performed to assess the degree of acceptability of these juices ranging from “like extremely” (9) to “dislike extremely” (1).
2.3. Statistical Analysis
The obtained results were statistically analyzed using the SPSS statistical package (Version 18). An analysis of variance (ANOVA), Duncan’s multiple range test and least significant difference (LSD) were chosen to determine any significant difference among various treatments at p<0.05.
3. RESULTS AND DISCUSSION
3.1. Physicochemical Characteristics of PSO
Some important physicochemical properties of PSO, such as refractive index (RI), the percentage of acidity, peroxide value (PV), saponification value (SV) and Iodine value (IV), were performed and are given in Table 1. The refractive index of PSO samples was similar to the values for PSO (1.4801 to 1.4819), linseed oil (1.482) and flaxseed oils (1.479 to1.480) reported by authors [25-27]. The percent of free fatty acids was measured to be 0.87% of the acid value (as oleic acid). The high content of free fatty acids in cold-press extraction oil may be due to the hydrolysis of triglycerides during the extraction process [28]. The PV of PSO samples was 1.02 mEq.O2/kg. However, the degree of unsaturation of oils was measured by the IV, which was 155.24 g I/100 g oil. This value was agreed with the value found by Zahran and Soliman [15] for PSO (158.39 g I2/100 g oil). The SV of the PSO was 189.20 mg KOH/g oil, which was within the range reported by Delfan-Hosseini et al. [25].
The value of TPC was 0.135 mg GAE/g oil. Jalali-Mousavi et al. [29] reported 0.121 mg GAE/g for TPC of PSO, which was almost similar to our result. Moreover, Delfan-Hosseini et al. [25], reported 0.066 to 0.156 mg GAE/g oil related to the different extraction methods, which was also in agreement with our result. On the contrary, other researchers have reported different total phenolic content mean values for PSO, possibly due to the variation of environmental conditions, extraction methods, and/or time of harvest.
The antioxidant properties of PSO have a major effect on its oxidative stability behavior. Antioxidants may be scavenging the free radicals of oil and prevent the chain reaction, and therefore prohibit the lipid oxidation [30]. The antioxidant capacity of PSO samples against DPPH was assayed in order to determine its antioxidant activity. The antioxidant activity for PSO was 67.43%, which was slightly higher than the results (53.90 to 63.01%) obtained by Delfan-Hosseini et al. [25].
3.2. Fatty Acid Composition of PSO
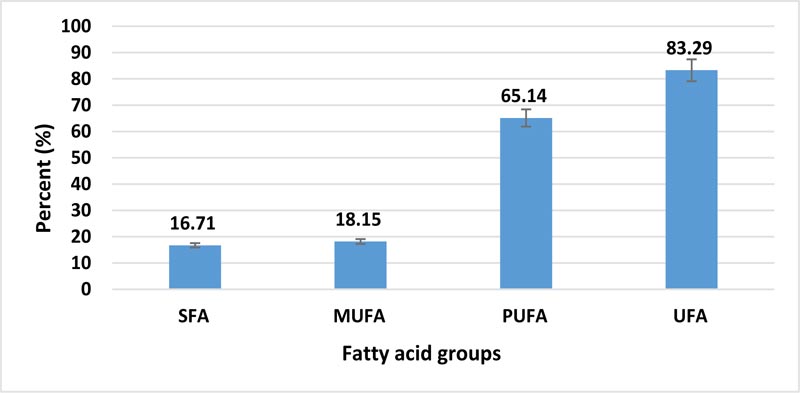
As indicated above, purslane is considered the richest vegetable source of essential fatty acids, particularly ω-3 fatty acids [15, 25]. Therefore, the analysis of the fatty acid profile of purslane seeds oil is essential. The fatty acid composition of extracted PSO is presented in Table 2 and Fig. (1). The total UFA was in the highest amount (83.29%) compared to SFA (16.71%), (Fig. 1). These values are in harmony with the results of iodine value. The major fatty acids revealed in PSO were similar to those reported by Delfan-Hosseini et al. [25] and Stroescu et al. [31], but the amounts of these fatty acids were varied. These differences may be due to many factors, like growing condition, method of extraction, genotype, etc [32]. Linolenic acid (C18:3), as ω-3 fatty acids, followed by linoleic acid (C18:2), were the most numerous fatty acids in PSO. The proportions of linolenic and linoleic acids in oil samples were 37.71% and 27.43%, respectively. Polyunsaturated fatty acids (PUFA) percent was an essential and important remarkable component of PSO (65.14%), however, the mono-unsaturated fatty acids (MUFA) were in low percent (18.15%) compared to PUFA.
| Parameters | Value |
|---|---|
| Refractive index | 1.4799 ±0.0001 |
| Acidity (%) as oleic acid | 0.87 ±0.05 |
| Peroxide value (mEq/kg) | 1.02 ±0.10 |
| Saponification value (mg/g) | 189.20 ±1.16 |
| Iodine value (g/100g) | 155.24 ±1.33 |
| Total phenolic content (mg GAE/g) | 0.135 ±0.011 |
| Antioxidant activity (%) | 67.43 ±1.24 |
| Fatty acid | Relative area percent (%) |
|---|---|
| Myristic acid (C14:0) | 0.07 ±0.01 |
| Palmitic acid (C16:0) | 11.52 ±0.31 |
| Palmitoleic acid (C16:1) | 0.34 ±0.05 |
| Margaric acid (C17:0) | 0.25 ±0.02 |
| Stearic acid (C18:0) | 4.37 ±0.22 |
| Oleic acid (C18:1) | 17.81 ±0.64 |
| Linoleic acid (C18:2) | 27.43 ±1.06 |
| Linolenic acid (C18:3) | 37.71 ±0.92 |
| Arachidic acid (C20:0) | 0.33 ±0.07 |
| Behenic acid (C22:0) | 0.17 ±0.03 |
3.3. Encapsulation Characteristics
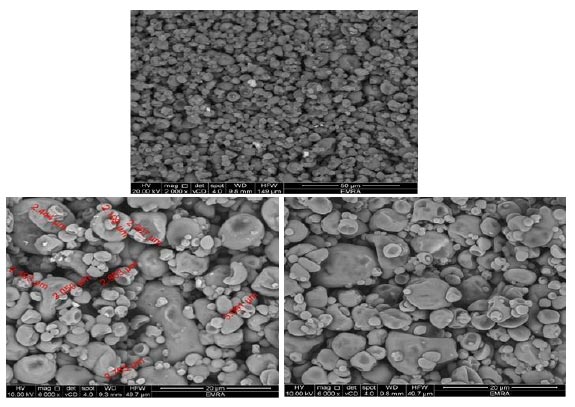
Microencapsulation yield is defined as a ratio of the mass of the total oil of the spray-dried powder to the mass of the total oil of the emulsion before spray drying. Gum Arabic and maltodextrin wall materials showed the highest lipid microencapsulation yield (85.17%) by the spray drying process. The encapsulation efficiency of oil powder is a measure for the completion of coating material in covering the oil droplets. When the Free oil (surface oil) is minimal, it expresses high encapsulation efficiency because the surface oil will rapidly deteriorate due to exposure to light, oxygen and other environmental factors. Kaushik et al. [33] stated that a successful microencapsulation process was measured based on the encapsulation efficiency of ω-3 oil microcapsules. The wall materials (gum Arabic and maltodextrin) recorded microencapsulation efficiency was 77.40%. Partanen et al. [11] stated that the spray-drying method was mostly used for microencapsulation of oils where results in a powder with good quality, leads to higher encapsulation efficiency, protects the oils against undesirable reactions, in addition to easier handling and storage. The scanning electron microscopy (SEM) images of microcapsules gum Arabic and maltodextrin wall materials are presented in Fig. (2). Observing the external morphology, the particles show a spherical shape without obvious cracks or cracks, which is an advantage.
3.4. Physicochemical Analysis of the Fortified Mango Juices
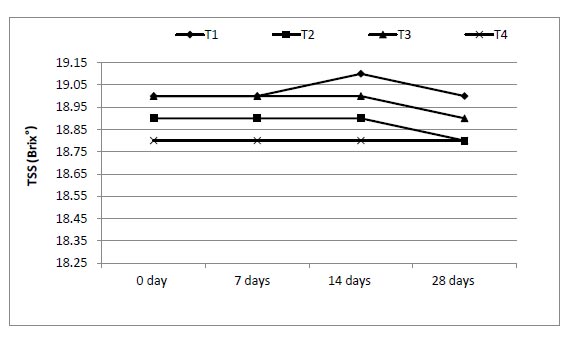
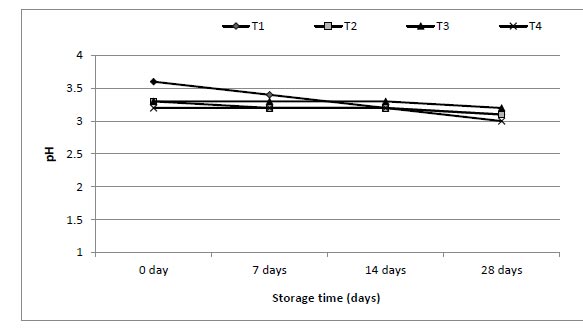
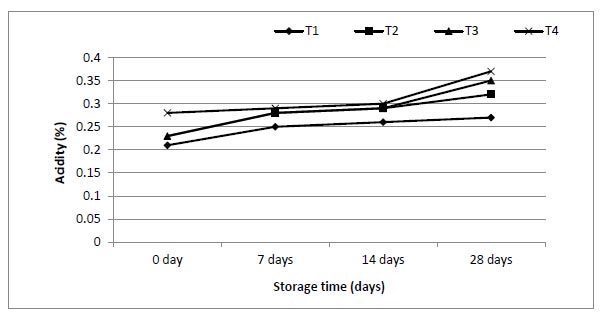
The effect of enriched 0.5, 1 and 1.5 g of mango juices with ALA on physicochemical properties of juices during 28 d was studied, and the data are shown in Figs. (3-7). The ranges of viscosity values at the beginning and end of storage periods (zero time and 28 days) were 159-200, 200-212, 205-216 and 210-220 cP from T1, T2, T3 and T4, respectively. The addition of ω-3 and period of storage were the main factors responsible for slight changes in TSS (Brix°) for each period of storage (Fig. 4). Also, the addition of ω-3 led to a small decrease in pH (Fig. 5), but the acidity of samples increased gradually over the storage periods (Fig. 6).

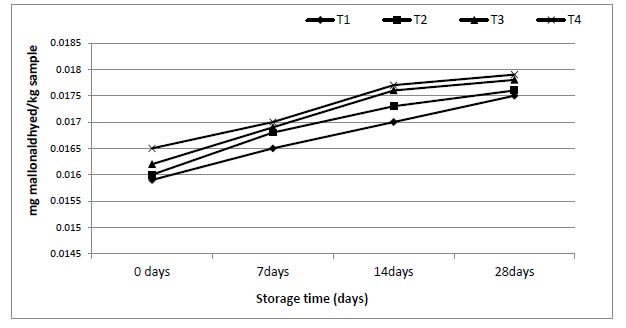
In recent years, the thiobarbituric acid test has been used in the field of food science to detect lipid peroxides. The results in Fig. (7) regard TBA values (mg malondialdehyde/kg sample) of mango juices treatments and storage. At storage initiation, TBA in various treatments T1, T2, T3 and T4 were 0.0159, 0.0160, 0.0162, and 0.165, respectively, that subsequently slight increased to 0.0175 (T1), 0.0176 (T2), 0.0178 (T3) and 0.0179 (T4) mg malondialdehyde/kg sample correspondingly at storage termination. This study showed that the antioxidants of POS have the ability to enhance the oxidative stability of mango juices enriched products
3.5. Sensory Evaluation
The panelists evaluated the sensory attributes of mango juices enriched with the microcapsules powder, as displayed in Table 3. The microcapsules-free mango juices (control) obtained maximum sensory attribute scores, but the judges accepted all juices. The enriched with 1 and 1.5 g of ALA in mango juices caused the relatively low score in appearance (7.3, 6.7) and taste (7.2, 6.5), respectively, when compared to control juices, which recorded the highest scores in appearance 8.4 and taste 8.4. These changes may be due to the changes in acid values because of acidity development by added oil microcapsules. Data also indicated that there was no significant difference in flavor between different samples, the results displayed that POS did not have any adverse effects on the panel members' evaluation, and the samples rich in this ingredient were similar to the control samples. The textures of mango juices containing 0.5, 1 and 1.5 g of ALA had gradually fewer scores 7.9, 7.7 and 7.2, respectively, compared to control juices with 8.5. At the same time, we found no significant variations in overall acceptability among control juices (8.4) and juices enriched with 0.5 g ALA (8.0). However, all juices were sensory accepted. Generally, it could be concluded that additional ω-3 fatty acids provide better nutritional quality of mango juices. Similar results have been reported by Habibi et al. [24], who stated that the fish oil microcapsules powered up to 0.07% could be added to pomegranate juice without affecting sensory properties.
| Treatment | Sensory properties | ||||
|---|---|---|---|---|---|
| Appearance | Taste | Flavor | Texture | Over all | |
| T1 | 8.4 a | 8.4 a | 8.3 a | 8.5 a | 8.4a |
| T2 | 7.7 b | 7.7 b | 8 a | 7.9 a | 8.0 a |
| T3 | 7.3 c | 7.2 c | 8 a | 7.7b | 7.5 b |
| T4 | 6.7 c | 6.5 c | 8 a | 7.2 c | 7.0c |
| LSD | 0.456 | 0.396 | 0.205 | 0.343 | 0.434 |
CONCLUSION
The present study was designed to develop a PSO, ω-3 oil-enriched mango juice and to evaluate the effects of adding PSO powder on the physicochemical and sensory properties of the mango juice and during storage. The results obtained for the fatty acid composition of PSO show that linolenic acid as ω-3 fatty acids (37.71%), followed by linoleic acid (27.43%), were the most numerous fatty acids in PSO. Results obtained for physicochemical properties of mango juice show that the viscosity of the juice was increased gradually by the addition of capsules of PSO ω-3 during the storage period. Sensory analyses indicate that a low concentration of PSO powder could be applied in the development of mango juice enriched with ω-3 oil. The mango juice that incorporates PSO ω-3 could be regarded as a possible health-promoting nutraceutical product.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to express their gratitude to the National Research Centre (NRC) and Zagazig University for providing all facilities to conduct the experiment in their labs.


