All published articles of this journal are available on ScienceDirect.
Frequencies and Antimicrobial Susceptibility of Methicillin-Resistant Staphylococcus aureus (MRSA) Isolated from Horses in South Korea
Abstract
Background:
Methicillin-Resistant Staphylococcus Aureus (MRSA) has become one of the most prevalent pathogens in animals and humans giving rise to various diseases. MRSA infection in horses and transmission between horses and humans have dramatically increased recently.
Objective:
This study investigated the isolation frequency and antimicrobial susceptibility of MRSA isolated from horses in South Korea.
Method:
Screening of the MRSA isolates was conducted by conventional methods and multiplex Polymerase Chain Reaction (PCR). Minimum Inhibitory Concentration (MIC) of oxacillin was determined by the broth microdilution test method. Overall antibiogram was obtained by disk diffusion susceptibility test. All antimicrobial tests were conducted in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines.
Results:
S. aureus was isolated from 116 (19.46%) of 596 horses tested. Of the 116 S. aureus isolates, 52 (8.72%) strains were identified as MRSA by multiplex PCR. Among the 52 MRSA strains, 24 (46.15%) and 28 (53.85%) were oxacillin-resistant MRSA (OR-MRSA) and oxacillin-susceptible MRSA (OS-MRSA), respectively. OR-MRSA showed the highest susceptibility to florfenicol (100.00%), followed by doxycycline (95.83%), cefepime (91.67%), tetracycline (75.00%), and trimethoprim/sulphamethoxazole (70.83%). OS-MRSA showed the highest susceptibility to florfenicol (100.00%) and trimethoprim/sulphamethoxazole (100.00%), followed by cefoxitin (96.43%), ciprofloxacin (92.86%), enrofloxacin (92.86%), lincomycin + spectinomycin (89.29%), cefuroxime (89.29%), and cefonicid (89.29%).
Conclusion:
This study may facilitate treatment and prevention of MRSA infections and further benefit not only horses, but also people related with horse farms, horse riding clubs and animal hospitals.
1. INTRODUCTION
The isolation of Methicillin-Resistant Staphylococcus Aureus (MRSA) from animals was first reported by Devriese and Hommez [1], who isolated the organism from the milk of mastitic cows. Subsequently, MRSA has been isolated from a variety of other domestic species, including dogs [2], cats [3], horses [4], sheep [5], pigs [6], and chickens [7]. MRSA has become one of the most prevalent pathogens in animals. MRSA can induce various diseases, from severe skin infections to fatal bacterial septicemia, in animals and humans. In particular, the incidence of MRSA infection in horses has dramatically increased in the past 10 years, and transmission among horses or between horses and humans has been reported as well.
Equine MRSA infections have also been recorded in Ireland [8], Japan [4], Austria [9], the United Kingdom [10], and North America [11, 12]. Wound and postoperative infections tend to be the most common manifestations. Transmission of infection by the hands of veterinary personnel is considered to be the principal route of transmission within a hospital setting. However, a Canadian study found widespread contamination of the veterinary hospital environment, suggesting that this may be an important source of MRSA infection [12]. Cuny et al. [9] reported an MRSA infection rate of approximately 4.8 cases per 1000 equine cases presenting at a veterinary teaching hospital in Austria. MRSA isolation rates were reported as 4.7% for non-targeted surveillance and, 12% for targeted surveillance, respectively [13]. MRSA has very high potential for transmission not only among horses but also between horses and humans, as a zoonotic pathogen. Further, human-horse interactions frequently involve direct contact, including petting of the face and nose, creating opportunities for interspecies transmission [13].
Staphylococcus (S.) aureus isolated from patients can be classified in two ways: by the origin of isolation and by the level of ß-lactamase resistance. The former category can be divided into two further categories: Hospital-Acquired (HA) and Community-Acquired (CA) S. aureus, for strains isolated from hospital patients and those isolated from outpatients at clinics, respectively [14]. The latter category of S. aureus is divided into two subgroups: MRSA with a Minimum Inhibitory Concentration (MIC) of oxacillin higher than 4 µg/ml (OR-MRSA) and methicillin-susceptible S. aureus, with a MIC of oxacillin lower than 2 µg/ml (OS-MRSA). In addition to its methicillin susceptibility, MRSA can be defined by the presence of the mecA gene, which encodes Penicillin-Binding Protein 2ʹ (PBP 2ʹ). The Clinical and Laboratory Standards Institute (CLSI: formerly NCCLS) has presented guidelines for identification of MRSA: that MRSA has an MIC of oxacillin higher than 4 µg/ml or possesses the mecA gene. MRSA showing an MIC of oxacillin from 1–16 µg/ml is customarily designated as pre-MRSA [15], borderline MRSA [16-18], or low-level and dormant MRSA [19-21].
Most MRSA infections occur in hospitals where antibiotics are commonly used, and S. aureus in the local environment is generally methicillin susceptible. Thus, MRSA has a wide range of antibiotic resistance. Since methicillin first began to be used in England in 1961, MRSA has become an increasingly important hospital pathogen not only in Europe in the 1960s but also in the U.S. in the late 1970s. In Korea, following the introduction of the third-generation antibiotic cephalosporin in the 1980s, MRSA has been considered the main factor in hospital-acquired infections [22].
According to studies published by Moon et al. [22], antibiotic resistance in S. aureus has been particularly serious in Korea. The rate of methicillin resistance is as high as 50%, and reached 70–80% for S. aureus isolated in general hospitals, according to recent research [22]. Nevertheless, these studies focused only on preventing transmission between humans in hospitals. Studies on MRSA in animals have been limited to mastitis, and have not included companion animals such as dogs or cats. In countries outside Korea, as the importance of MRSA infection increases, special organizations have been established to develop new ways of reducing public risk and improving clinical treatment.
This work investigated the isolation frequency and antimicrobial susceptibility of MRSA isolated from horses in Korea.
2. MATERIALS AND METHODS
2.1. Sample Collection and Isolation
A total of 596 samples (376 nasal discharge, 148 vaginal discharge, and 72 skin wound lesions) were collected from different horses with diseases using a cotton-tipped culture swab (BBL, USA) from 2011 to 2015 in five provinces (Gangwon, Geonggi, Jeju, Jeonbuk, and Kyungbuk) in Korea. Swabs were transported to the laboratory in Liquid Stuart Medium on ice packs and refrigerated until used.
2.2. Identification of S. aureus and MRSA
Isolation of S. aureus from samples was performed according to previously described techniques [23], and the isolates were identified by conventional methods and PCR. Screening of MRSA was performed by multiple PCR of isolates, and MRSA was identified by detection of S. aureus species-specific genes and mecA. The primers used for mecA were as follows: SA1 (AATCTTTGTCGGTACACG ATATTCTTCACG) and SA2 (CGTAATGAGATTTCAGTAGATAATACAACA) to detect a 108-bp segment of S. aureus, and MRSA1 (TAGAAATGACTGAACGTCCG) and MRSA2 (TTGCGATCAATGTTACCGTAG) to detect a 154-bp segment (mecA gene). PCR was performed in a total volume of 50 µl of the following mixture: 25 µl of 2× PCR buffer [0.2 mM deoxynucleoside triphosphate, 1.5 U of Taq DNA polymerase, 1.5 mM MgCl2, 75 mM Tris-HCl (pH 8.8 at 25°C), 20 mM (NH4)2SO4, 0.01% (v/v) Tween 20], 1 µM of SA1 and SA2 primers, 1.5 µM of MRSA1 and MRSA2 primers, and 5 µl template DNA. PCR amplification was performed as follows: denaturation for 5 min at 92°C, followed by 35 cycles at 92°C for 1 min, 56°C for 1 min and 72°C for 1 min, followed by a final extension step at 72°C for 5 min [24]. Multiplex PCR amplification was performed in a 9700 thermal cycler (Applied Biosystems, Foster City, CA, U.S.A.). Amplified products were analyzed by 2.5% agarose gel electrophoresis, stained with ethidium bromide, and visualized on a UV transilluminator [24].
2.3. Identification of OR-MRSA and OS-MRSA
The oxacillin (Sigma, MO, U.S.A) MIC for identification of OR-MRSA and OS-MRSA was determined by a broth microdilution test method in accordance with the CLSI guidelines. OR-MRSA and OS-MRSA were determined by the presence of mecA and a MIC of oxacillin of more than 4 µg/ml and less than 2 µg/ml, respectively.
The susceptibility of the isolates to oxacillin was determined in triplicate using a microbroth dilution method in cation-supplemented Mueller–Hinton broth plus 2% NaCl at final inoculation of 5 × 105 CFU/ml from an overnight culture. The MIC was defined as the lowest concentration of an antimicrobial that inhibited the visible growth of the test organism in 24 h of incubation at 35°C. After inoculation, the 96-well microplates were sealed with parafilm and incubated at 37°C, then examined until a transparency change was detected in the drug-free control. The MIC was defined as the lowest concentration of antibiotics that prevented a transparency change at the time when the transparency in the control without antibiotic had changed. MIC50 and MIC90 values represented the MICs of the antibiotic that inhibited at least 50% and 90% of strains. Susceptibility to each antimicrobial was defined using the back point categories of the CLSI recommendations.
2.4. Antimicrobial Susceptibility of MRSA
Disk diffusion susceptibility testing was performed in accordance with the CLSI recommendations. Three to five colonies were inoculated into 4–5 ml of Mueller–Hinton broth and incubated at 37°C for 2–6 h to develop a turbidity equivalent to the 0.5 McFarland turbidity standard (approximately 1–2 × 108 CFU/ml). Plates for susceptibility testing were incubated at 37°C for 24 h and the inhibition zone was measured according to the CLSI guidelines.
3. RESULTS
The frequency of isolation of S. aureus and MRSA from horses is shown in Table 1 and Fig. (1). S. aureus was isolated from 116 (19.46%) of 596 horses and MRSA was identified in 52 (8.72%) isolates. Among the 52 MRSA isolates, 24 (46.15%) and 28 (53.85%) of the strains were OR-MRSA and OS-MRSA, respectively.
| Provine | No. of samples |
No. of S. aureus isolates (%) |
MRSA | Total No. of MRSA isolates (%) | |
|---|---|---|---|---|---|
| No. of OR-MRSA isolates (%) |
No. of OS-MRSA isolates (%) |
||||
| Gangwon | 51 | 10 (19.61) | 0 (0.00) | 1 (1.97) | 1 (1.97) |
| Gyeonggi | 82 | 30 (36.59) | 13 (15.85) | 10 (12.20) | 23 (28.05) |
| Jeju | 330 | 56 (16.97) | 9 (2.73) | 14 (4.24) | 23 (6.97) |
| Jeonbuk | 63 | 8 (12.70) | 0 (0.00) | 1 (1.59) | 1 (1.59) |
| Kyungpook | 70 | 12 (17.14) | 2 (2.86) | 2 (2.86) | 4 (5.71) |
| Total | 596 | 116 (19.46) | 24 (4.03) | 28 (4.70) | 52 (8.72) |
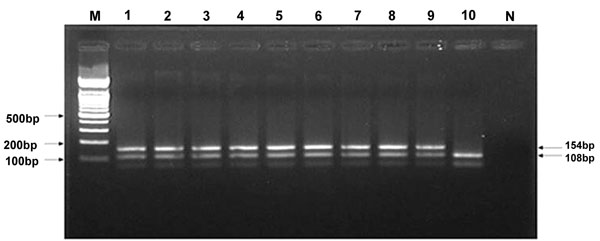
The relationship between isolation frequency and lesion type is presented in Table 2. MRSA strains were isolated most frequently from skin wound lesions (13/72, 18.05%), followed by nasal discharge (33/376, 8.78%), and vaginal discharge (4.05%).
| Sampling site | No. of samples |
MRSA | Total No. of MRSA isolates(%) |
|
|---|---|---|---|---|
| No. of OR-MRSA isolates(%) |
No. of OS-MRSA isolates(%) |
|||
| Skin Wound lesion |
72 | 6 (8.33) | 7 (9.72) | 13 (18.06) |
| Nasal discharge | 376 | 16 (4.26) | 17 (4.52) | 33 (8.78) |
| Vaginal discharge | 148 | 2 (1.35) | 4 (2.70) | 6 (4.05) |
| Total | 596 | 24 (4.03) | 28 (4.70) | 52 (8.72) |
Oxacillin resistance was confirmed by determining the MICs for all 52 MRSA isolates (Table 3). Microbroth dilution testing of the MRSA strains showed that the MIC90 value for oxacillin was 4 µg/ml and the MIC50 value was 2 µg/ml. The oxacillin MIC ranged from 0.125 µg/ml to >512 µg/ml. Of the 52 strains, 36.54% had an oxacillin MIC of 4 µg/ml and 53.84% of strains had an oxacillin MIC <2 µg/ml. Of the 52 MRSA strains, 24 (46.20%) showed 4 µg/ml MIC, above the resistant range indicated by CLSI. In addition, 28 isolates (53.80%) were susceptible to oxacillin.
| Method | MIC (ug/ml) | MRSA | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | <0.125 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4* | 8 | 16 | 32 | 64 | 128 | 256 | 512 | >512 | No. of resistance(%) | No. of susceptible(%) | |||||||||||||||||
| 0 (0.00)* |
1 (1.92) | 5 (9.62) |
8 (15.38) | 6 (11.54) | 8 (15.38) | 19 (36.54) | 1 (1.92) | 0 (0.00) | 1 (1.92) | 1 (1.92) | 0 (0.00) | 0 (0.00) | 1 (1.92) | 1 (1.92) | 24 (46.16) |
28 (53.84) |
||||||||||||||||||
| mecA gene | 0 (0.00) |
1 (1.92) |
5 (9.62) |
8 (15.38) |
6 (11.54) |
8 (15.38) |
19 (36.54) |
1 (1.92) |
0 (0.00) |
1 (1.92) |
1 (1.92) |
0 (0.00) |
0 (0.00) |
1 (1.92) |
1 (1.92) |
52 (100.00) | ||||||||||||||||||
Disk diffusion susceptibility of MRSA is shown in Table 4. OR-MRSA showed the highest susceptibility to florfenicol (100.00%), followed by doxycycline (95.83%), cefepime (91.67%), tetracycline (75.00%), and trimethoprim/sulphamethoxazole (70.83%). OS-MRSA showed highest susceptibility to florfenicol (100.00%) and trimethoprim/sulphamethoxazole (100.00%), followed by cefoxitin (96.43%), ciprofloxacin (92.86%), enrofloxain (92.86%), lincomycin + spectinomycin (89.29%), cefuroxime (89.29%), and cefonicid (89.29%).
| Antimicrobial drugs | 24 OR-MRSA | 28 OS-MRSA | ||||
|---|---|---|---|---|---|---|
| S*(%) | I (%) | R (%) | S (%) | I (%) | R (%) | |
| An | 14(58.33) | 6(25.00) | 4(16.67) | 21(75.00) | 7(25.00) | 0(0.00) |
| Amc | 6(25.00) | 1(4.17) | 17(70.83) | 7(25.00) | 1(3.57) | 20(71.43) |
| Am | 1(4.17) | 0(0.00) | 23(95.83) | 1(3.57) | 0(0.00) | 27(96.43) |
| C | 4(16.67) | 9(37.50) | 11(45.83) | 13(46.43) | 10(35.71) | 5(17.86) |
| Cz | 3(12.50) | 5(20.83) | 16(66.67) | 19(67.86) | 6(21.43) | 3(10.71) |
| Cf | 2(8.33) | 6(25.00) | 16(66.67) | 19(67.86) | 7(25.00) | 2(7.14) |
| Ma | 5(20.83) | 5(20.83) | 15(62.50) | 17(60.71) | 6(21.43) | 5(17.86) |
| Fox | 0(0.00) | 1(4.17) | 23(95.83) | 27(96.43) | 1(3.57) | 0(0.00) |
| Cid | 4(16.67) | 3(12.50) | 17(70.83) | 25(89.29) | 3(10.71) | 0(0.00) |
| Cxm | 0(0.00) | 2(8.33) | 21(87.50) | 25(89.29) | 3(10.71) | 0(0.00) |
| Cpr | 2(8.33) | 2(8.33) | 21(87.50) | 24(85.71) | 2(7.14) | 2(7.14) |
| Ctx | 3(12.50) | 18(75.00) | 3(12.50) | 3(10.71) | 21(75.00) | 4(14.29) |
| Ctt | 2(8.33) | 3(12.50) | 18(75.00) | 22(78.57) | 3(10.71) | 3(10.71) |
| Cff | 4(16.67) | 8(33.33) | 12(50.00) | 15(53.57) | 9(32.14) | 4(14.29) |
| Cfm | 4(16.67) | 8(33.33) | 12(50.00) | 14(50.00) | 10(35.71) | 4(14.29) |
| Cro | 0(0.00) | 8(33.33) | 16(66.67) | 0(0.00) | 9(32.14) | 19(67.86) |
| Fep | 22(91.67) | 2(8.33) | 0(0.00) | 24(85.71) | 3(10.71) | 1(3.57) |
| Cip | 2(8.33) | 6(25.00) | 16(66.67) | 26(92.86) | 0(0.00) | 2(7.14) |
| Cc | 4(16.67) | 0(0.00) | 20(83.33) | 18(64.29) | 7(25.00) | 3(10.71) |
| D | 23(95.83) | 0(0.00) | 1(4.17) | 24(85.71) | 0(0.00) | 4(14.29) |
| Enr | 2(8.33) | 3(12.50) | 19(79.17) | 26(92.86) | 0(0.00) | 2(7.14) |
| E | 2(8.33) | 6(25.00) | 16(66.67) | 3(10.71) | 23(82.14) | 2(7.14) |
| Ffc | 24(100.00) | 0(0.00) | 0 (0.00) | 28(100.00) | 0(0.00) | 0(0.00) |
| Gm | 10(41.67) | 0(0.00) | 14(58.33) | 17(60.71) | 0(0.00) | 11(39.29) |
| LS | 0(0.00) | 2(8.33) | 22(91.67) | 25(89.29) | 3(10.71) | 0(0.00) |
| Neo | 9(37.50) | 11(45.83) | 4(16.67) | 4(14.29) | 13(46.43) | 11(39.29) |
| Nor | 4(16.67) | 0(0.00) | 20(83.33) | 23(82.14) | 1(3.57) | 4(14.29) |
| Ox | 0(0.00) | 2(8.33) | 22(91.67) | 26(92.86) | 2(7.14) | 0(0.00) |
| P | 0(0.00) | 0(0.00) | 24(100.00) | 5(17.86) | 4(14.29) | 19(67.86) |
| Sh | 0(0.00) | 0(0.00) | 24(100.00) | 0(0.00) | 0(0.00) | 28(100.00) |
| Sxt | 17(70.83) | 2(8.33) | 5(20.83) | 28(100.00) | 0(0.00) | 0(0.00) |
| S3 | 14(58.33) | 0(0.00) | 10(41.67) | 16(57.14) | 0(0.00) | 12(42.86) |
| Te | 18(75.00) | 0(0.00) | 6(25.00) | 22(78.57) | 0(0.00) | 6(21.43) |
| Tia | 0 (0.00) | 5(20.83) | 18(75.00) | 0(0.00) | 6(21.43) | 22(78.57) |
| Tyl | 2(8.33) | 13(54.17) | 9(37.50) | 2(7.14) | 15(53.57) | 11(39.29) |
4. DISCUSSION
MRSA appears to be an emerging pathogen in horses. The first report of MRSA in horses was in mares with endometritis in Japan [4]. MRSA infections have been reported in horses in various countries, and transmission of MRSA between horses and humans has been identified, both in veterinary hospitals and in the community [8-10, 12].
S. aureus was isolated and identified by PCR in 116 (19.46%) of 596 clinical specimen samples collected from horses, and MRSA was identified in 52 (8.72%) of those isolates. Among the 52 MRSA isolates, 24 (46.15%) and 28 (53.85%) of the strains were identified as OR-MRSA and OS-MRSA, respectively. The isolation rate (52/596, 8.72%) for MRSA in this study was 4.7% higher than that reported by Cuny et al. [9], and 12% lower than that reported by Weese et al. [12].
Of the 52 MRSA strains, most were found in skin wound (13/72, 18.05%), followed by nasal discharge (33/376, 8.78%), and vaginal discharge (6/148, 4.05%). The number of MRSA isolates may be so high in skin wound lesions because suppurative lesions are a fertile environment for MRSA. These results are concordant with those of other researchers [25], who reported that infections of surgical sites and traumatic wounds are more likely to contain MRSA.
All 24 OR-MRSA strains were mecA positive. These OR-MRSAs showed the least resistance to oxacillin, among the MRSAs tested, and they were within the susceptible range to seven other ß-lactam antibiotics tested. OR-MRSA was noted here to occur with a certain frequency; precautions are called for in the classification of oxacillin-resistant S. aureus and in the treatment of OR-MRSA infections.
Twenty-eight OS-MRSA strains were resistant to spectinomycin (100.00%), ampicillin (96.43%), and tiamulin (78.57%). Of the 24 OR-MRSA isolates, approximately 90% were resistant to penicillin, spectinomycin, ampicillin, tiamulin, and amoxicillin.
In contrast to the multiple resistance of MRSA, Methicillin-Susceptible S. Aureus (MSSA) showed greater susceptibility to all tested antimicrobial agents except for penicillin, ampicillin, and tiamulin. This might be because these agents are currently used heavily in the treatment of general infections. MSSA seemed to be much more susceptible to florfenicol, sulphamethoxazole, and ciprofloxacin, but not penicillin, ampicillin, and co-trimoxozable. We observed that strains isolated from carrier horses showed lower resistance than clinical strains, for all antibiotics tested. In the present study, resistance to penicillin and neomycin was comparatively higher in veterinary hospital isolates than in staphylococcal strains isolated from other farms. This might be due to greater use of these antibiotics for treatment of infections. This study underscores the need for hospital clinicians to be aware of the common bacterial isolates in their unit and their usual antibiotic susceptibility. This is imperative in order to make rational decisions for the prudent use of antibiotics, particularly for empirical therapy. Another important cause of resistance is excessive or inappropriate use of antibiotics in hospitals. The magnitude of the problem of multi-resistance is such that clinicians must be familiar with the causes of antibiotic resistance and the measures for preventing or minimizing the emergence of resistance. In addition, 64 MSSA isolates showed high susceptibility to almost all antimicrobial agents, except erythromycin, spectinomycin, and ceftriaxone.
Study of the characteristics of MRSA in the horse industry could provide significant information for treatment and prevention of infections. It could also facilitate treatment of horse clinicians and people in the horse industry who become infected with MRSA from a horse. Furthermore, datum obtained from this research could be used to protect the spread of this pathogen to other countries. We did not investigate the prevalence of MRSA infection in horse personnel. However, MRSA infection can be transmitted from horses to humans, as it is a zoonotic pathogen. Consequently, the prevalence of MRSA infections in horse personnel should be investigated. Further studies are required to determine the prevalence of MRSA infection and colonization in horses and humans at veterinary hospitals and equine farms, as well as genotyping of the isolates.
This work is the first to investigate clinical patterns and characteristics of MRSA infection in horses. Study of the biochemical, genetic, and pharmacological features of MRSA will further facilitate prevention and treatment. Furthermore, it will benefit not only the horses, but also the people who work on horse farms, horse riding clubs, and animal hospitals.
CONCLUSION
This work is the first to investigate clinical patterns and characteristics of MRSA infection in horses in South Korea. Study of the biochemical, genetic, and pharmacological features of MRSA will further facilitate prevention and treatment. Furthermore, it will benefit not only the horses, but also the people who work on horse farms, horse riding clubs, and animal hospitals.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The reported experiments were in accordance with the standards set forth in the 8th Edition of Guide for the Care and Use of Laboratory Animals (http:// grants.nih.gov/grants/olaw/Guide-for-thecare- and-use-of-laboratory-animals.pdf) published by the National Academy of Sciences, The National Academies Press, Washington DC, United States of America.
HUMAN AND ANIMAL RIGHTS
All antimicrobial test was conducted in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines.
CONSENT FOR PUBLICATION
All authors consented the submission and approved the final manuscript is true.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Seoung-Kyoon Choi designed the study, conducted experiments, Ji-Yong Hwang and Chul-Song Park contributed to data acquisition and interpretation. Gil-Jae Cho executed the study.


