RESEARCH ARTICLE
The Effect of Rhizobia Isolates Against Black Root Rot Disease of Faba Bean (Vicia faba L) Caused by Fusarium solani
Gedyon Tamiru1, *, Diriba Muleta2
Article Information
Identifiers and Pagination:
Year: 2018Volume: 12
First Page: 131
Last Page: 147
Publisher ID: TOASJ-12-131
DOI: 10.2174/1874331501812010131
Article History:
Received Date: 04/4/2018Revision Received Date: 01/6/2018
Acceptance Date: 03/6/2018
Electronic publication date: 31/7/2018
Collection year: 2018

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective:
Evaluate for potential biocontrol agent by assessing isolates for in vitro inhibitory efficacy, probable mechanisms to inhibit fungal pathogen and effect on growth of Faba bean infected with F. solani.
Methods:
The effect of Rhizobium isolates on the development of radial mycelium of F. solani in PDA medium were tested in vitro. The experiments were carried out using the dual culture technique. Isolates that showed inhibitory effect against F. solani in vitro were tested to assess hydrolytic enzymes and growth promoting traits. Subsequently, the three Rhizobium isolates that showed the greatest inhibitions and their combinations were tested in the greenhouse against F. solani root rot on seedlings by applying cell suspensions at three different times of exposure to the pathogen.
Results:
In dual culture, 27 rhizobium isolates inhibited the radial growth of F. solani mycelium more than 25%. Isolates JU26(1), JU15(2) and Ho-1WG, inhibited fungal radial growth by 70.5 %, 64.7% and 63.7%, respectively. Among the 27 Rhizobium isolates tested for hydrolytic enzymes 26.1%, 44.4%, 14.8% were positive for chitinase, protease and lipase production, respectively. Chitinase, protease and lipase positive isolates showed significant fungal mycelia inhibition. Eight (29.6%) were positive for hydrogen cyanide production. Also, 24(88.8%) were positive for IAA production and over 50% formed visible dissolution haloes on PA. Concurrent production of protease, lipases, chitinase, IAA and phosphate solubilization coupled with anti-fungal activity suggests potential plant growth promotion and broad-spectrum bio control of these isolates. Furthermore, combination and Ho-1WG consistently reduced disease incidence and severity; and increased growth parameters on seedling in greenhouse at all times of application compared to diseased (control). Maximum disease severity (73.3%) reduction was observed with application of combination before the pathogen. The combination formulation provided the highest (48 cm/plant) shoot height when applied before the pathogen.
Conclusion:
Beneficial traits strongly assist the efficiency of candidate antagonists for desired biocontrol, emphasizing the value of concerted mechanisms of action. The result indicated the possible use of Rhizobial isolates as an alternative means of BRR management but further study is needed to verify actual use in agricultural production.
1. INTRODUCTION
In Ethiopia, grain crops are grown annually on approximately 12.5 million hectares of land, of these, 1.5 million hectares is covered by pulses out of which 443,074.68 hectares is dedicated to Faba bean with annual production of about 8,389,438.97 quintals [1]. Faba bean makes a significant contribution to soil fertility restoration as a suitable rotation crop that fixes atmospheric nitrogen and reduces the dependence on external fertilizer inputs and also provides an important source of income for farmers and generates foreign currency for the country [2].
In spite of their huge importance, the productivity of Faba bean in Ethiopia remains far below the crop’s potential greater than 3 ton/ha. Production of Faba bean has been constrained by several biotic and abiotic factors [2]. Surveys on diseases of Faba bean in Ethiopia showed that 17 pathogens infect Faba bean in different parts of the country [3]. Some diseases that are economically most important in the major Faba bean growing regions include Black Root Rot (BRR), chocolate spot and rust caused by Fusarium solani (Mart.) Appel and Wr., Botrytis fabae Sard., and Uromyces viciae-fabae (Pers.) Schr. important, respectively [3].
Root rot is among the major production constraints limiting the yield of Faba bean in many countries of the world especially where poor nutrient supply and overly wet soil conditions prevail [4]. The fungus Fusarium solani has been encountered on a large number of hosts in Ethiopia including Faba bean [5]. Complete crop losses could occur in severe infection conditions and when favorable conditions prevail for the pathogen. In farmers’ fields, a loss of about 45% was estimated due to this disease [5].
Numerous efforts have been made to manage BRR in Ethiopia as such using host plant resistance, cultural practice and synthetic chemicals [6]. Nevertheless, increasing use of chemical inputs causes several negative effects, i.e., development of pathogen resistance to the applied agents and their nontarget environmental impacts [7]. Furthermore, the growing cost of pesticides, particularly in less-affluent regions of the world, and consumer demand for pesticide-free food has led to a search for substitutes for these products [8]. Thus, there is a need for supplementary plant disease management options that provide effective management of the disease under question while minimizing cost and negative consequences to human health and the environment [9].
Many species of Rhizobium are reported to inhibit significantly the growth of pathogenic fungi [10]. The rhizobia have several mechanisms of action to control pathogens that include competition for iron by production of siderophores [11], synthesis of Rhizobiotoxin [12], phosphate solubilization and promotion of plant growth in terms of better shoot height, root length, dry weight and root nodulation [13]. Antoun et al [14] showed that rhizobia are able to produce IAA.
In spite of the existence of rhizobia in faba bean rhizosphere, there is little information on rhizobia antagonists against black root rot. Therefore, the major aims of the current study were to determine in vitro inhibitory effect of Rhizobium isolates against Fusarium solani; and to evaluate the probable mechanisms used by rhizobial isolates to inhibit fungal pathogen by studying their ability to produce: Protease, Chitinase, Lipase, Hydrogen Cyanide (HCN), Indole Acetic Acid (IAA); and to mobilize phosphate. In addition to evaluate effect of Rhizobium isolates on in vivo growth of Faba bean (Vicia faba L) infected with Fusarium solani.
2. MATERIALS AND METHODS
2.1. Description of the Study Area
The study was conducted at Jimma University College of Agriculture and Veterinary Medicine (JUCAVM), Jimma, Ethiopia. Laboratory activities were conducted in Plant Pathology Laboratory. Pot experiments were conducted in JUCAVM which is located at 7042 N Latitude 36057 E Longitude and at altitude 1710 m. a.s.l. The maximum and minimum temperature of the area is 26.8 and 11.8 0c, respectively, with relative humidity of 91% and the mean rainfall of 1500 mm per annum [15].
2.1.1. Origin of Rhizobium Isolates and Culture Conditions
Fourteen (14) rhizobium isolates were obtained from the culture collection of Holeta Agricultural Research Centre, Microbial Biotechnology Laboratory. The other twenty seven (27) isolates were obtained from Jimma University, Department of Biology, Applied Microbiology Laboratory (Table 1). Jimma University isolates have been morphologically, and biochemically tested in Applied Microbiology Laboratory at Department of Biology [16].
| Isolates Code | Host | Geographic Origin | Source | Isolates Code | Host | Geographic Origin | Source |
|---|---|---|---|---|---|---|---|
| JU2(4) | Faba bean | Mena | Jimma | JU3(1) | Faba bean | Mena | Jimma |
| JU-E(1) | Haricot bean | Mena | Jimma | Ho-4-1SG | Faba bean | S. Gonder | Holeta |
| JU1(2) | Faba bean | Mena | Jimma | JU47(2) | Faba bean | Mena | Jimma |
| JU1(3) | Faba bean | Mena | Jimma | JU2(2) | Faba bean | Mena | Jimma |
| JU8(1) | Pea | Mena | Jimma | JU2(3) | Faba bean | Mena | Jimma |
| JU1(5) | Faba bean | Mena | Jimma | JU-Y(1) | Haricot b | Yebu | Jimma |
| Ho-1-1NG | Faba bean | N. Gonder | Holeta | JU3(2) | Faba bean | Mena | Jimma |
| Ho-1018 | Faba bean | N. Gonder | Holeta | JU8(2) | Faba bean | Mena | Jimma |
| Ho-3WG | Faba bean | W. Gonder | Holeta | JU8(3) | Faba bean | Mena | Jimma |
| JU26(1) | Faba bean | Mena | Jimma | JU-D(1) | Haricot b | Dedo | Jimma |
| JU3(1) | Faba bean | Mena | Jimma | Ho-3-1SG | Faba bean | S. Gonder | Holeta |
| JU15(2) | Faba bean | Mena | Jimma | JU5(2) | Faba bean | Mena | Jimma |
| JU-S(1) | Haricot b | Seribo | Jimma | JU21(4) | Faba bean | Mena | Jimma |
| JU11(1) | Faba bean | Mena | Jimma | JU-Sr(1) | Haricot b | Seribo | Jimma |
| JU-G(2) | Haricot b | Ginibbe | Jimma | JU26(2) | Faba bean | Mena | Jimma |
| Ho-2ST | Faba bean | S. Tigria | Holeta | Ho-7EG | Faba bean | E. Gonder | Holeta |
| Ho-1WG | Faba bean | W. Gonder | Holeta | Ho-1035 | Faba bean | N. Gonder | Holeta |
| JU3(3) | Faba bean | Mena | Jimma | Ho-2-1ST | Faba bean | S. Tigria | Holeta |
| Ho-5EG | Faba bean | E. Gonder | Holeta | Ho-2-1WG | Faba bean | W. Gonder | Holeta |
| Ho-2EG | Faba bean | E. Gonder | Holeta | ||||
| Ho-1EG | Faba bean | E. Gonder | Holeta | ||||
| JU1(4) | Faba bean | Mena | Jimma |
2.2. In vitro Screening for Antagonism
The inhibitory effect of the Rhizobium isolates against the radial mycelial growth of the F. solani (JUFS1) was evaluated using the dual culture technique [18]. In order to quantify the percent reduction, a small fungal agar block (5 mm in diameter) was cut from a plat of potato dextrose agar after seven days of fungal growth at 25°C. The plug was centrally placed on pre-solidified PDA medium. One loopful of 24 h old culture (108cfu/ml) bacterial cultures at the exponential growth phase were streaked on PDA as a broad band (making a straight short bar) approx. 2 cm away from the fungal agar block at one opposite edge of triplicate Petri dishes (90 mm diameter). In all antagonistic studies, a plate inoculated with only sterile liquid bacterial medium and the test fungus place at the center served as a control. Both experimental and control Petri plates were arranged in a completely randomized design with three replicates per treatment. Petri plates were incubated at 28 ± 2 °C for 7 days. The percentage fungal radial growth inhibition was calculated by using the following formula of [19].
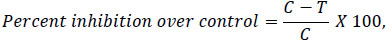 |
Where, C is the radial growth of fungus in control,and T is the radial growth of fungus in dual culture after 7 days of incubation.
2.3. In vitro Phytobeneficial Trait Tests
The rhizobial isolates showing antagonistic activities against mycelium growth were further studied with different biochemical tests to determine the probable mechanisms of antimicrobial activities of the isolates.
2.3.1. Chitinolytic Test
To test for chitinolytic activity of isolates were spot inoculated individually on medium containing fine powdered chitin [20] (g/L): chitin powder, 5.0; yeast extract, 0.5; K2HPO4, 0.5; MgSO4· 7H2O, 0.2; NaCl, 0.1; agar, 20.0. The pH of the medium was adjusted to 7.0 and the medium was sterilized by autoclaving at 121oC for 15 min. The uninoculated plates were used as control in the experiment. The plates were incubated at 28oC for 72 to 96 h until zones of chitin clearing could be seen around the colonies.
2.3.2. Proteolytic Test
Proteolytic activity was assessed on skimmed milk agar [21]. Uninoculated plates were used as control. The plates were incubated at 28oC for 2 to 3 days until zones of clearing could be seen around the colonies.
2.3.3. Lipolytic Test
Lipolytic activity was assessed on Yeast Extract Mannitol Agar medium containing 1% Tween 80R. Single bacterial isolate was spot inoculated onto solidified plates of medium. The uninoculated plates served as control. The presence of lipase activity was indicated by insoluble olic acid forming halos around the bacterial colonies [22].
2.3.4. Hydrogen Cyanide Test
Hydrogen cyanide production was detected as described by [23]. Single bacterial isolates were streaked onto Trypticase soy agar (TSA, Difco, Franklin Lakes, NJ USA) supplemented with 4.4 g glycine per liter to screen for cyanide production. A piece of filter paper impregnated with 0.5% picric acid (yellow) and 2.0% sodium carbonate was placed in the lid of each Petri dish. The Petri dishes were sealed with parafilm and held at 28oC for 3 to 5 days. Discoloration of the filter paper to orange brown after incubation indicates microbial production of cyanide.
2.4. Plant Growth Promoting Mechanisms Tests
2.4.1. Production of Indole Acetic Acid (IAA)
Indole Acetic Acid (IAA) production was detected as described by Gordon and Weber [24]. Rhizobium isolates were grown separately in YEM broth medium supplemented with L-tryptophan (100μg/ml). The flasks were incubated at room temperature on rotatory shaker at 150 rpm for 72 h. After incubation, the medium was centrifuged at 6000 rpm for 15 min and the cell-free supernatant was used for IAA test. The supernatant (2 ml) was mixed with two drops of orthophosphoric acid and 4 ml of the Salkowski reagent (50 ml, 35% of perchloric acid, 1 ml 0.5 M FeCl3 solution). Uninoculated liquid media were used as control. Development of pink colour indicates IAA production.
2.4.2. Phosphate Solubilization
Isolates were tested for phosphate solubilizing ability on Pikovskaya medium [25]. The medium was distributed in 9 cm diameter Petri plates and inoculated with a loopful (108cfu/ml) of single isolate at the center. Uninoculated plates were used as control. Both inoculated and uninoculated plates were incubated at 28+2 oC for 7 days and clear zone (halo) diameter surrounding the bacterial colony was measured. The ability of the rhizobium isolates to solubilise insoluble phosphate was primarily described by Edi-Premono et al [26].
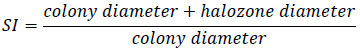 |
2.5. Preparation of Rhizobium and Fungal Inoculum for In Vivo Seedling Test
One of our Fusarium isolate (JUFS1) was obtained from the purified culture slant in test tube and maintained on PDA medium to produce conidia. The culture of fungal pathogen was multiplied by cultivating on Potato Dextrose Agar (PDA) medium in Petri plates (9 cm) at 25±2 oC for 15 days in an incubator. The colonies were harvested by scraping the surface with spatula and homogenized with 200 ml of sterile distilled water and filtered through a cheese cloth to make a fungal suspension for inoculation. The spores 1 x 106 / mL were counted by haemocytometr [10]. The isolates of Rhizobium (JU15(2), JU26(1) and Ho-1WG) which showed high potential of in vitro inhibition were used for experiment. For single isolate inoculum production, a loopful of isolate (JU26(1), JU15(2) and Ho-1WG) was separately inoculated into the 250 ml Erlenmeyer flasks contain sterilized yeast extract mannitol broth [17]. The medium had the following composition (g/l): mannitol 10, KH2PO4 0.5, MgSO4.7H20 0.2, NaCI 0.1, yeast extract 0.5. The pH of the medium was adjusted to 6.8 before autoclaving at 121oC for 15 minutes. The flasks were incubated on a rotary shaker at 120 rpm for 72 h at room temperature (28 ± 2 oC). After 48 h of incubation, bacterial concentration was adjusted to 108CFU/ml (OD620 0.8-0.9) using spectrophotometer [27]. Bacterial concentrations were confirmed by dilution plating. For preparation of combination of inoculums, the bacterial isolates JU26(1), JU15(2) and Ho-1WG were grown separately and mixed with equal volume (1:1:1 v/v ratio) [28].
2.6. In Vivo Seedling Test
Antagonistic effect of Rhizobium isolates against Fusarium isolate was studied under greenhouse conditions (25-28oC) by pot culture method [29]. The pots were surface sterilized for 3 min with 5% sodium hypochlorite and washed five times with sterile water and left to dry before use. Surface sterilized healthy looking seeds of Faba bean (Variety ‘Kasa’ susceptible to Root rot) were sown (4 seeds/pot) in 18-cm diameter plastic pots containing sterilized clay soil. After 10 days of sowing, 20 ml of pathogen (1 × 106 condia/ml) and 4 ml (108 CFU/ ml) of Rhizobium were added per seedling to the base of stem at 2-cm depth of soil. The antagonists (JU26(1), JU15(2), Ho-1WG and Combination) were inoculated to seedling in pot before or at the same time or after the pathogen inoculation with an interval of 7 days. The following treatments were carried out in triplicate: T1 = health (uninoculated); T2 = Diseased (control, only F. solani isolate [JUFS1]); T3 = JU26(1) + JUFS1; T4 = JU15(2) + JUFS1; T5 = Ho-1WG + JUFS1; T6 = JU26(1) + JU15(2) + Ho-1WG) + JUFS1 (Combination). Pots were arranged in a randomized complete block design.
All plants were harvested from each pot at full bloom flowering stage (45 days). Excess soil was removed from the roots by placing the root mass on a sieve and washing with running tap water. Cleaned plants were separated into nodules, root (everything below the first node except nodules) and shoots and the following parameter were assessed. Disease incidence was evaluated after 45 days of planting. The number of plants exhibiting root rot symptoms and percent disease incidence were estimated according the equation by Reddy et al [30].
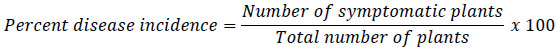 |
To determine disease severity, individual plant root tissues assessed according to a 1-9 scale proposed by Abawi and Pastor-Corrales [31]. Where 1 = No visible symptoms, 3 = Light discoloration either without necrotic lesions or with approximately 10% of the hypocotyl and root tissues covered with lesions, 5 = Approximately 25% of the hypocotyls and root tissues covered with lesions but tissues remain firm, with some deterioration of the root system, 7 = Approximately 50% of the hypocotyls and root tissues covered with lesions combined with considerable softening, rotting, and reduction of root system, 9 = Approximately 75% or more of the hypocotyl and root tissues affected with advanced stages of rotting combined with severe reduction in the root system. With these data, for each replicates a Disease Severity Index (DSI) was calculated as follows
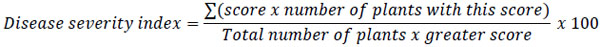 |
The percentage of disease reduction (DR %) was calculated according to Edginton et al [32]:
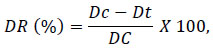 |
where Dc is disease on the control plants that treated with only pathogen and Dt is disease on the treated with antagonist and pathogen.
Shoot, Root, root nodulation and also flower and leaf number were recorded. In order to measure dry weight of nodules, roots and shoots from each plant was placed in paper bags, dried at 70oC for 72 h in a hot oven, and weighed [33].
2.7. Data Analysis
The data were subjected to analysis of variance (ANOVA) using the General Linear Modeling (GLM) procedure of SAS-9.2 software [34]. The disease incidence and severity data were arcsine transformed before subjected to ANOVA to normalize the data [35]. Tukey’s test was performed at 5% level of significance to denote significant difference between the treatments.
3. RESULTS AND DISCUSSION
3.1. In Vitro Screening for Antagonism
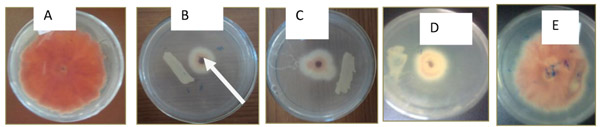
A total of 41 Rhizobium isolates were screened for their potential to inhibit the Fusarium solani under in vitro assays (Table 2). The antagonistic isolates tested showed varying levels of effects against the Fusarium solani. Most of the Rhizobium isolates showed potent antifungal activity by restricting mycelial expansion on dual culture medium (Figs. 1B, C and D). Some of the Rhizobium isolates did not show any antagonistic effects (Fig. 1E).
| Isolates Code | Host | Percent Inhibition | Isolates Code | Host | Percent Inhibition |
|---|---|---|---|---|---|
| JU2(4) | Faba bean | 50.0f | JU1(4) | Faba bean | 36.2ji |
| JU-E(1) | Haricot bean | 29.4lmn | JU13(1) | Faba bean | 24.5op |
| JU1(2) | Faba bean | 56.9e | Ho-4-1SG | Faba bean | 35.2jk |
| JU1(3) | Faba bean | 44.1g | JU47(2) | Faba bean | 26.4opn |
| JU8(1) | Pea | 25.4op | JU2(2) | Faba bean | 23.5qp |
| JU1(5) | Faba bean | 14.7r | JU2(3) | Faba bean | 30.3lm |
| Ho-1-1NG | Faba bean | 0.0s | JU-Y(1) | Haricot bean | 61.7cbd |
| Ho-1018 | Faba bean | 27.4omn | JU3(2) | Faba bean | 32.3lk |
| Ho-3WG | Faba bean | 62.7cbd | JU8(2) | Faba bean | 24.5op |
| JU15(2) | Faba bean | 64.7b | JU8(3) | Faba bean | 59.8ed |
| JU3(1) | Faba bean | 41.1hg | JU-D(1) | Haricot bean | 60.7cd |
| JU26(1) | Faba bean | 70.5a | Ho-3-1SG | Faba bean | 41.1hg |
| JU S(1) | Haricot bean | 29.4lmn | JU5(2) | Faba bean | 29.4lmn |
| JU11(1) | Faba bean | 39.2hi | JU21(4) | Faba bean | 39.2hi |
| JU-G(2) | Haricot bean | 59.8ed | JU-Sr(1) | Haricot bean | 20.5q |
| Ho-2ST | Faba bean | 0.0s | JU26(2) | Faba bean | 20.5q |
| Ho-1WG | Faba bean | 63.7cb | Ho-7EG | Faba bean | 35.2jk |
| JU3(3) | Faba bean | 24.5op | Ho-1035 | Faba bean | 20.5q |
| Ho-5EG | Faba bean | 11.7r | Ho-2-1ST | Faba bean | 11.7r |
| Ho-2EG | Faba bean | 20.5q | Ho-2-1WG | Faba bean | 26.4opn |
| Ho-1EG | Faba bean | 32.3lk | Control | - | 0.0s |
| CV (%) | 2.98 | ||||
There were significant differences (p < 0.05) between rhizobial antagonists in inhibiting the mycelial expansion of F. solani, ranging from 0.0-70.5% radial growth inhibition, respectively (Table 2). Over 65.8% of the rhizobial antagonists tested (n = 27) exhibited remarkable fungal radial growth inhibition, against F. solani as compared to the control. The isolates Ho-1WG, JU15(2) and JU26(1), exerted their maximum inhibitory effect 63.7%, 64.7% and 70.5% against F. solani, respectively (Table 2). However, the isolates Ho-5EG, Ho-2-1ST and JU1(5) showed the least inhibition percent 11.7%, 11.7% and 14.7% against F.solani, respectively. It was pointed out by Chao [36] that the Rhizobium leguminosarum biovar phaseoli had an effect on the inhibition of the Pythium, Fusarium and Rhizoctonia species. Also in a study Omar and Abd-Alla [37] determined that rhizobia significantly inhibited the mycelial growth of Fusarium solani and Rhizoctonia solani. In general, Lalande and Bissonette [38] showed that the inhibitory effect of the Rhizobium isolates vary from one isolates to another.
3.2. In Vitro Assay of Possible Biocontrol Mechanisms of Rhizobium Isolates
The seven (25.9%) isolates that showed remarkable inhibitory effect against Fusarium solani tested positive for chitinase (Table 3). Variation was observed among the isolates for the chitinase production. Mazen et al. [33] reported that chitinase enzyme was produced by rhizobial isolates but with different degrees. The Rhizobium isolates that were positive for chitinase activity in this study had significant mycelia radial growth inhibition (26.4-70.5%) against Fusarium solani (Table 2). In recent study, the rhizobacterial isolates that were positive for chitinase activity had significant radial growth inhibition (> 40%) against F. oxysporum and F. stilboides and caused large inhibition zones (> 3 cm) against F. xylarioides [9].
In this investigation, twelve (44.4%) out of those that showed inhibitory effect against Fusarium solani tested positive for protease (Table 3). Variation was observed among the isolates for the protease activity. The variation in enzymatic activities of rhizobial isolates was reported by various workers. Kumari et al. [22] reported that out of 5 strains of rhizobia studied for protease production, 4 were positive.
| Isolates code | Hydrolytic enzymes | HCN | IAA |
Growth promoting traits Phosphate solubilisation |
|||
|---|---|---|---|---|---|---|---|
| Chitinase | Protease | Lipase | |||||
| (halo zone diameter in cm) | SI | ||||||
| JU-D(1) | + | + | - | - | + | 0.1 | 1.08h |
| Ho-1 WG | + | + | - | - | + | 1.3 | 1.90a |
| JU-Y(1) | - | + | + | - | - | 0.2 | 1.12fhg |
| JU47(2) | + | + | - | - | + | 0.2 | 1.11hg |
| JU8(3) | - | + | - | - | + | - | - |
| JU1(2) | - | + | - | - | + | 0.3 | 1.08h |
| JU-E(1) | - | - | - | - | - | - | - |
| JU-S(1) | + | - | - | - | + | - | - |
| Ho-2-1WG | - | - | - | - | + | 0.5 | 1.37c |
| JU11(1) | - | - | + | - | + | 0.3 | 1.18feg |
| JU4-1SG | - | - | - | + | + | - | - |
| JU-G(2) | - | + | - | - | + | 0.2 | 1.20fe |
| JU1(3) | - | - | - | + | + | - | - |
| JU3(2) | + | + | - | - | + | - | - |
| JU3(1) | - | - | - | - | + | 0.4 | 1.67b |
| Ho-3-1SG | - | - | - | + | + | 0.2 | 1.09h |
| JU21(4) | - | + | - | - | - | 0.7 | 1.23de |
| Ho-3WG | - | - | + | - | + | - | - |
| JU5(2) | - | - | - | + | + | - | - |
| JU26(1) | + | + | - | - | + | 0.3 | 1.32dc |
| JU1(4) | - | - | - | + | + | - | - |
| JU2(3) | - | - | - | + | + | 0.5 | 1.38c |
| JU8(1) | - | + | - | - | + | 0.7 | 1.88a |
| Ho-1EG | - | - | - | + | + | - | - |
| JU2(4) | - | - | + | - | + | - | - |
| JU15(2) | + | + | - | - | + | 0.2 | 1.17fheg |
| Ho-1018 | - | - | - | + | + | - | - |
| Control | - | - | - | - | - | 0.0 | 0.00i |
The Rhizobium isolates that were positive for protease activity in this study showed significant mycelial growth inhibition (25.4-70.5%) against Fusarium solani (Table 3). Kishore et al. [39] demonstrated that Pseudomonas aeruginosa which produced protease had significant inhibition (> 32%) against Sclerotium rolfsii. This is an indication that the enzyme protease has responsible effect on the phytopathogens. Earlier, Dunne et al. [40] confirmed that biocontrol of Pythium ultimum in the rhizosphere of sugar beet with Stenotrophomonas maltophila (Hugh) Palleroni and Bradbury W81 was due to the production of extracellular protease. Although, chitinolytic activity appears less essential for plant growth promoting bacteria such as S. plymutica IC14 when used to suppress S. sclerotiorum and Botrytis cinerea, synthesis of proteases are involved [41]. Our result seems to confirm the previous study on the responsibility of protease on biocontrol activity.
In this study, 4(14.8%) of those that showed inhibitory effect against Fusarium solnai were positive for lipase (Table 3). Variation was observed among the isolates for the lipase activity as reported elsewhere [22]. The Rhizobium isolates that were positive for lipase activity in this study showed significant (p< 0.05) mycelial growth inhibition (39.2-62.7%) against Fusarium solani (Table 2). Muleta et al. [9] indicated that all rhizobacterial isolates which were able to produce lipase had significant radial growth inhibition (> 37%) against Fusarium stilboides. This indicates that lipase enzyme is responsible for antifungal activity exhibited by the rhizobial isolates. Srividya et al. [42] verified that Streptomyces sp. 9p which produced lipase enzyme showed a significant inhibitory effect against fungal soilborne and foliar phytopathogens such as Alternaria brassisicola (OCA1), Alternaria brassiceae (OCA3) Alternaria alternata (OTA36) and Rhizoctonia solani (MTCC 4633).
Color of the picrate/ Na2CO3-impregnated paper strips changed from yellow (control) to light brown, brown or reddish brown as an indication of the strength of the cyanide produced (Fig. 2). In the current study, eight (29.6%) of the antagonists produced HCN (Table 3). This proportion is by far higher than that reported by Beauchamp et al. [43]. These authors further remarked that among the rhizobial isolates tested, 12.5% of them were found to be HCN producers.
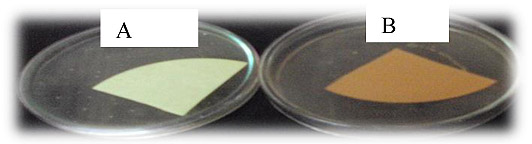 |
Fig. (2). HCN production by Rhizobium isolates: (A) Control and (B) HCN production positive (JU-G(2). |
A growing body of evidence showed that the inhibitory effect of Bradyrhizobium (Arachis) on Macrophomina phaseolina was due to HCN production [12]. Moreover, some research explained the antagonistic properties of Rhizobium leguminosarum against Fusarium oxysporum f.sp. lentis was due to excretion of antibiotics that have fungicidal action on condia of F. oxysporum [44]. Rhizobium was reported to produce toxic metabolites which have inhibitory effect against soilborne plant pathogens [45]. Defago et al. [46] have also demonstrated by mutational analysis and complementation that production of HCN by Pseudomonas fluorescens strain, CHAO accounted for about 60% of the biocontrol activity.
Most of the Rhizobium isolates tested in the present work were able to produce IAA in the presence of tryptophan (Table 3). Among the 27 rhizobial isolates tested for IAA, 24(88.8%) of them produced IAA. Wang et al. [47] observed nodulating and non-nodulating strains of Rhizobium leguminosarum were able to produce indole-3-acetic acid. Indole-3-acetic acid which was produced by rhizobacteria increased root hair density, length and enhanced rice seed germination [48].
Over 50% of the total Rhizobium isolates showed clearly visible haloes (>0.15 cm) around their colonies (Fig. 3B) on PA after 7 days of incubation. The phosphate solubilizing frequency of isolates of this study is comparable to other studies [49]. Among the 27 isolates tested for phosphate solubilisation, isolate JU21(4) produced the largest halo zone (1.3 cm), on Piskovskaya’s Agar plates (Table 3). Solubilisation Indices (SI) in PA are presented in Table 3.
The SI of the potential P solubilising rhizobial isolates differed significantly (p< 0.05) and ranged from 1.0 to 1.9. The bacterial strain Ho-1WG showed the largest SI (1.9 cm) of solubilization, followed by JU8(1) and JU3(1). Phosphate solubilizing rhizobacteria convert insoluble phosphates into soluble monobasic form for plant uptake [50]. In a field study, Chabot et al. [51] have observed that phosphate solubilization by strains of R. leguminosarum bv. phaseoli was the most important mechanism of maize and lettuce growth promotion, in moderately fertile and very fertile soils. A recent extensive review Muleta [52] from both greenhouse and field trials has demonstrated a remarkable improvement in growth responses of various crops to phosphate solubilizing microorganisms’ inoculations.
3.3. Effects of the Rhizobium Isolates on Seedlings
3.3.1. Disease
The results showed that there was significant (p< 0.05) interaction effect among types of treatment used and time of application of the isolates in reduction of disease incidence (Table 4). Under greenhouse conditions, all of the treatment with the Rhizobium isolates applied at time of pathogen inoculation and 7 days before and after have significantly reduced incidence of disease compared to the diseased (control) (Table 4).
| Treatments | Time | Incidence | Incidence Reduction % | Severity | Severity Reduction % |
|---|---|---|---|---|---|
| JU26(1) | Before | (50)45.0b±0.0 | 50.0 | (55.5)48.1cd±0.0 | 37.5 |
| At time | (50)45.0b±0.0 | 50.0 | (61.1)51.4bc±0.0 | 27.7 | |
| After | (50)45.0b±0.0 | 50.0 | (64.8)53.6b±1.90 | 27.0 | |
| JU15(2) | Before | (25)30.0c±0.0 | 75.0 | (38.8)38.5e±0.00 | 56.3 |
| At time | (25)30.0c±0.0 | 75.0 | (51.8)46.0d ±1.8 | 41.6 | |
| After | (41)40.0b±8.6 | 59.0 | (55.5)48.1cd±0.0 | 37.5 | |
| Ho-1WG | Before | (25)30.0c±0.0 | 75.0 | (31.4)34.0f±2.00 | 64.6 |
| At time | (25)30.0c±0.0 | 75.0 | (38.5)38.5e ±0.0 | 56.6 | |
| After | (50)45.0b±0.0 | 50.0 | (57.3)49.2cd±1.8 | 35.4 | |
| Combination | Before | (0)1.4d ±0.0 | 100.0 | (23.7)29.1g±1.8 | 73.3 |
| At time | (0)1.4d ±0.0 | 100.0 | (31.2)33.9f ±2.3 | 64.8 | |
| After | (25)30.0c±0.0 | 75.0 | (35.1)36.3ef±1.9 | 60.4 | |
| Control (diseased) | Before | (100)88.6a±0.0 | 0 | (88.8)70.4a±0.00 | 0 |
| Mean | (35.85)35.5±0.6 | 64.1 | 48.73(44.4)±1.04 | 44.8 | |
| CV (%) | 5.3 | 2.8 | |||
Application of combination of Rhizobium isolates to seedling before the time of pathogen inoculation gave the highest (88.6%) reduction in disease incidence of root rot on Faba bean plants. The lowest (50.0%) percentage of disease incidence reduction was obtained by application of JU26(1) to seedling. There was no significant (p>0.05) difference obtained in the disease incidence when the rhizobial treatments were added 7 days before pathogen inoculation and at the same time of pathogen inoculation (Table 4). Among different treatments applied to seedlings, disease (black root rot) count was maximum (100%) in diseased (control).
All of the Rhizobium treatments applied at the same time with pathogen and 7 days before and after significantly reduced disease severity compared to the control (Table 4). However, the highest reduction in black root rot (BRR) severity (73.3%) was observed with application of combination of isolates before the pathogen inoculation followed by at the same time with pathogen inoculation with mean value of 64.8%. The lowest (27.0%) percentage of disease severity reduction was obtained by application of JU26(1) to seedling after 7 days of pathogen inoculation followed by at the same time of pathogen inoculation with mean value of 27.7%.
The present study showed that Rhizobium isolates proved to be effective in controlling F. solani the causative agent of Faba bean root rot disease. Combination of the isolates proved to be more effective in controlling the disease than all the tested individual isolates. This indicates Rhizobium isolates were beneficial for suppressing root rot. Rhizobia have been reported as best control of root infecting fungi on both leguminous and non-leguminous plants [53]. The potentiality of Rhizobium as biocontrol agents of phytopathogenic fungi in soybean is well known especially to Macrophomina phaseolina, causative agent of charcoal rot disease [45].
Combined application of plant growth promoting rhizobacteria has significantly lowered Fusarium wilt disease of Capsicum annum L. caused by Fusarium solani compared to individual isolate [54]. Advantages of strain mixtures include, broad spectrum of action, enhanced efficacy, reliability and also allow combination of various traits without genetic engineering [55].
The reduction of root rot incidence and severity obtained in Rhizobium isolates inoculated plants in this study could be related to antifungal activity of Rhizobium isolates. There are many mechanisms suggested to clarify the role of antagonistic organisms in suppression of pathogens growth and thus to control diseases. Rhizobium spp. isolated from Algerian soil, were found to produce bacteriocins with antimicrobial activities against Pseudomonas savastanoi, the agent responsible for olive knot disease [56]. The Rhizobia present in the rhizosphere of plants presumably prevent the contact of pathogenic fungi on roots by covering the hyphal tip of Rhizoctonia solani and by parasitizing it [57]. The isolates used for inoculation in the present study exhibited the capacity to produce protease, lipase, chitinase and hydrogen cyanide (Table 5) and therefore might have significantly contributed to the reduction of disease incidence and severity of root rot.
| Treatment | Time of Application | Nodulation | |
|---|---|---|---|
| Number | Dry Weight | ||
| JU26(1) | Before | 0.97g ± 0.0 | 0.04g ± 0.0 |
| At time | 0.0g ± 0.0 | 0.0g ± 0.0 | |
| After | 0.0g ± 0.0 | 0.0g ± 0.0 | |
| JU15(2) | Before | 4.43f ± 0.0 | 0.02ef ± 0.0 |
| At time | 1.0g ± 0.0 | 0.01fg ± 0.0 | |
| After | 0.0g ± 0.0 | 0.0g ± 0.0 | |
| Ho-1WG | Before | 26.42a ± 0.9 | 0.15b ±0.0 |
| At time | 24.3b ± 0.2 | 0.02de± 0.0 | |
| After | 16.3d± 0.1 | 0.01fg ± 0.0 | |
| Combination | Before | 25.33b ± 0.5 | 0.17a ± 0.0 |
| At time | 22.3c ± 0.5 | 0.1c ± 0.0 | |
| After | 14.4e ± 0.3 | 0.03d ± 0.0 | |
| Health (un-inoculated) | 0.0g± 0.0 | 0.0g± 0.0 | |
| Control (diseased) | 0.0g± 0.0 | 0.0g± 0.0 | |
Timing of Rhizobium inoculation was also a factor in determining the level of root rot reduction. The incidence and severity of root rot incited by the Fusarium solani were highly reduced when Faba bean plants were inoculated 7 days before the pathogen whereas, the damage was of intermediate order in simultaneous application. These findings are in agreement with [58]. It seems that earlier establishment of Rhizobium protected the plant. It might have due to induced systemic resistance.
3.3.2. Growth Parameters
The roots of uninoculated and those infected with Fusarium solani only (control) were devoid of any nodules (Table 5). Root nodule number of control plant infected by Fusarium solani was not significantly (p>0.05) different from that of uninoculated (Table 5). Under greenhouse conditions, all treatments applied before pathogen inoculation significantly increased root nodule number compared to the control except JU26(1) inoculation. Application of Ho-1WG isolates to seedling before pathogen inoculation gave the highest (26.4) root nodule number followed by at the same time of pathogen inoculation and after inoculation. The lowest (0.0) root nodule number was obtained by application of JU26(1) after and at the same time of pathogen inoculation and followed by before inoculation.
Before and at time of pathogen inoculation of Ho-1WG, combination and JU15(2) were significantly (p<0.05) increased nodule dry weight compared to the control (diseased) and health (uninoculated). The of suspension isolate JU26(1) was not significantly increased nodule dry weight in either of time of application. Application of isolate combination resulted in the highest (0.17 g/plant) nodule dry weight when applied before followed by at the same time of pathogen inoculation.
Results reported herein indicated that Rhizobium isolates treatments not only suppressed both disease incidence and severity but also enhanced nodule number, nodule fresh weight and nodule dry weight of Faba bean plants compared to infected control. The findings suggest that incorporation of Rhizobium is important for plant growth promotion by increasing mineral uptake of plants and producing IAA. Similar studies Akhtar and Siddiqui [59] have shown that the addition of specific Rhizobium and Bacillus isolates to the rhizosphere can result in increased nodulation of chickpea and soybean plants.
IAA synthesizing rhizobia have been found to nodulate more intensely than IAA negative mutants [60]. Sheng [61] reported that besides fixation of atmospheric nitrogen, the nodulation effect of rhizobial isolates is due to the production of plant growth regulators such as Auxins and cytokinins like substance. The isolates used for inoculation in the present study exhibited the capacity to solubilize phosphorus and produced IAA (Table 5) and therefore might have contributed to the enhanced nodulation.
Over all, significant results have been shown in before application followed by at time of pathogen inoculation. The result of this study corresponds with work done by Yaqub et al. [29] who found that increased nodulation in treatments with prior application of Bradyrhizobium and decreased nodule formation in simultaneous or delayed applications on Meloidogyne incognita inoculated black gram, Vigna mungo plants.
Shoot height of control plant (infected with Fusarium solani) was significantly lower than that of uninfected (health) (Table 6). Generally, all treatments applied at time of pathogen inoculation and 7 days before and after pathogen inoculation significantly increased shoot height compared to the control (diseased).The combination of isolates produced the maximum (48.0 cm/plant) shoots height in response to before inoculation followed by at the same time of pathogen inoculation. Isolate JU26(1) produced the lowest (34.3 cm/plant) shoot height when applied after pathogen inoculation followed by before inoculation and at the same time of pathogen inoculation. There was no significant (p>0.05) difference between isolates JU15(2) and JU26(1) with regard to shoot height in three time of applications. The significant shoot height variation was observed between treatments.
Shoot dry weight of control plant infected with Fusarium solani was significantly lower than that of uninfected (health). Combination of isolates produced the highest (3.6 g/plant) shoot dry weight per plant in case of before inoculation, which was not significantly different than at time of inoculation. After inoculation treatment in case of JU26(1) produced lowest (1.0 g/plant) shoot dry weight followed by at time inoculation, which not significantly different with diseased control.
Means with the same letter are not significantly different at p = 0.05 according to Tukey‘s honest significant difference (HSD). There were 3 replicates in each treatment (mean ± SD)
Root length was significantly lower in plants infected with Fusarium solani than in uninfected plants, or those inoculated with either treatment (Table 6). The pathogenic influence on root length in Fusarium solani -inoculated plants was significantly less when applied at the same time of pathogen inoculation and 7 days before and after inoculated with each biocontrol agent compared to the infected control (diseased control). The combination of isolates produced the maximum (35.6 cm/plant) root length in response to before inoculation followed by at the same time of pathogen inoculation. After inoculation treatment with isolate JU15(2) produced the lowest (21.0 cm/plant) root length followed by other time of applications.
| Treatment | Time of Application | Growth Parameter | |||||
|---|---|---|---|---|---|---|---|
| Shoot | Root | Flower | Leaf | ||||
| Height | Dry wt. | Length | Dry wt. | Number | Number | ||
| JU26(1) | Before | 38.2cd ± 0.6 | 1.8ef± 0.0 | 28.6bcd±1.1 | 1.0e ± 0.0 | 14.6cd ±0.5 | 30.6cd ± 0.2 |
| At time | 35.8de ± 1.5 | 1.4gh± 0.0 | 25.3e±0.5 | 0.8f ± 0.0 | 9.6e ± 0.5 | 28.9def± 0.7 | |
| After | 34.3de± 1.8 | 1.0h ± 0.0 | 22.3fg±0.5 | 0.6gh ± 0.0 | 6.6f ±0.5 | 27.3efg± 0.2 | |
| JU15(2) | Before | 37.0cde± 0.6 | 2.0e± 0.0 | 29.6bc±0.5 | 1.0e ± 0.0 | 17.0b ± 1.0 | 29.4de± 0.1 |
| At time | 35.8de ± 1.5 | 1.3gh± 0.0 | 29.6bc±0.5 | 1.0e ± 0.0 | 13.6d ± 0.5 | 28.0ef ± 0.6 | |
| After | 35.0de ± 1.3 | 1.1gh± 0.0 | 21.0g±1.0 | 0.7g ± 0.0 | 13.0d ±1.0 | 25.2gh ± 0.4 | |
| Ho-1WG | Before | 40.6bc ± 1.8 | 2.9cd ± 0.0 | 31.0b±1.0 | 1.5c ± 0.0 | 20.3a ±1.1 | 33.9ab± 0.9 |
| At time | 36.4de±0.3 | 2.6d± 0.0 | 29.3bc±1.1 | 1.2d ± 0.0 | 14.6cd ±0.5 | 32.3bc ± 1.1 | |
| After | 36.8cde± 2.5 | 1.5fg ± 0.5 | 26.0de±1.0 | 0.9ef ± 0.0 | 14.3cd ± 0.5 | 29.0def± 0.1 | |
| Combination | Before | 48.0a± 0.7 | 3.6a± 0.1 | 35.6a±1.1 | 2.7a ± 0.0 | 20.6a ±0.5 | 36.0a ± 0.7 |
| At time | 42.8b± 1.0 | 3.3ab ± 0.0 | 27.3cde±1.1 | 2.5b ± 0.0 | 15.6bc ±1.1 | 32.0bc ± 1.0 | |
| After | 37.5cde±1.0 | 3.1bc ± 0.0 | 24.6ef±0.5 | 0.7gh ±0.0 | 14.3cd ±0.5 | 27.0fg ± 0.9 | |
| Health (un-inoculated) | 34.0e ± 0.0 | 1.5fg ± 0.0 | 27.0cde±1.0 | 1.2d ± 0.0 | 7.3f ±0.2 | 24.6h ± 0.7 | |
| Control (diseased) | 25.2f±1.2 | 1.0h± 0.0 | 17.3h± 0.5 | 0.5h ± 0.0 | 4.3g ±0.2 | 20.6i ± 0.7 | |
Root dry weight was significantly lower in plants infected with Fusarium solani than in uninfected plants (Table 6). Root dry weight was significantly (p<0.05) less when applied at the same time of pathogen inoculation and 7 days before inoculated with each of treatments compared to the infected control. The highest (2.7 g/plant) root dry weight was measured in response to before inoculation followed by at the same time of pathogen inoculation with combination of isolates. After inoculation treatment with JU26(1) produced the lowest (0.6 g/plant) root dry weight followed by at the same time and before inoculations. The highest (20.6/plant) flower number was recorded in response to before inoculation followed by after inoculation.
No significant (p>0.05) difference was observed between combination and Ho-1WG application on flower number of Faba bean plants. The combination of isolates produced the highest (36.0/plant) leaf number in response to before inoculation followed by at time of pathogen inoculation. After inoculation of JU15(2) produced the lowest (25.2/plant) leaf number followed by at the same time.
The Faba bean plants remained stunted and showed poor growth response in the absence of Rhizobium isolates. However, the biocontrol agents posed increased shoot height, shoot weight, root length, and lesser damage of flower number to Fusarium solani inoculated plants. Multiple lines of evidence Antoun et al. [14] demonstrate that rhizobial isolates enhance plant health and growth through multiple mechanisms. Increase in shoot and root length of several crop plants due to inoculation of phosphate solublizing Rhizobium isolates have been reported [10]. Increased cell elongation and multiplication due to enhanced nutrient uptake by plants following inoculation of Phosphate solubilizing microorganisms may have caused the increased plant height [62]. In nonlegumes, IAA produced by rhizobia may stimulate plant shoot and root systems [63]. The isolates used for inoculation in the present study exhibited the capacity to solubilize phosphorus and produce IAA which could contribute to improved growth parameters.
The findings suggest that incorporation of Rhizobium is important for plant growth promotion by increasing mineral uptake of plants and producing IAA. Similar studies Noel et al. [64] observed that several strains of R. leguminosarum bv. viciae promoted the early seedling root growth of canola and lettuce. The observed growth stimulation was associated with the production of the plant growth regulators Indole- 3-Acetic Acid (IAA) and cytokinin. The role of inorganic P-solubilization as a mechanism in maize growth promotion was analyzed by using two Lux+ mutants of R. leguminosarum bv. phaseoli R1 with reduced solubilization activity [65].
Inoculation of combinations of Rhizobium isolates resulted in greater plant height, shoot dry weight, root length, root dry weight, flower and leaf number as compared to just single isolates. This indicates that Rhizobium isolates have synergetic interactions. The combination of biocontrol agents may better adapt to the environmental changes, protect against a broader range of pathogens, increase the genetic diversity of biocontrol systems that persist longer in the rhizosphere, utilize a wider array of biocontrol mechanisms [66], enhance the efficacy and reliability of control [67], and allow the combination of various mechanisms of biocontrol without the need for genetic engineering [68].
The average disease reduction for mixtures was 45.1% compared to 29.2% for individual strains. In addition to disease reduction strain mixtures increased biomatter production and yield compared to individual strains [28]. Under gnotobiotic conditions, in dual inoculation trials of lettuce, a very significant interaction was observed between Sinorhizobium meliloti and the AM fungus Glomus mosseae. This translated into a 476% increase in shoot dry matter yield of 40-day old plants [69], and the growth promoting effect reported was not accompanied by an increase in root colonization by the AM fungus. This observation suggests that the PGPR rhizobial strain did not act as a mycorrhizal helper bacterium [70], but that rather that the fungus stimulated the PGPR activity.
It was suggested that these multiples of beneficial traits strongly assist the efficiency of candidate antagonists for desired biocontrol methods, emphasizing the great value of concerted mechanisms of action. It has been strongly suggested that the main success of biocontrol agents is largely attributable to their multifunctional characteristics [71] and synergistic interactions [72].
CONCLUSION
The result of study clearly demonstrated that rhizobial isolates exhibited inhibition of the radial growth of F. solani under in vitro conditions. Rhizobial isolates tested showed varying levels of effects against the F. solani. The fungal radial mycelia reduction could be occurred partly due to several modes of actions (Chitinase, Lipase, Protease and HCN production). In pot experiment, the isolates showed significant diseases reduction relative to the positive control. In general, rhizobial antagonists were found to reduce black root rot disease incidence and severity, and enhanced faba bean growth relative to control indicating that rhizobial biological control has considerable promise in suppression of F. solani population. Combination of the isolates applied 7 days before pathogen inoculation was found to be the most efficient in reducing black root rot incidence and severity compared to other rhizobial treatment. Therefore, further field evaluation will be important to ascertain the antagonistic efficacy of those promising rhizobial isolates.
APPENDIX
| Properties | Units | Amount |
|---|---|---|
| Sand | % | 8.00 |
| Silt | % | 18.00 |
| Clay | % | 74.00 |
| Texture class | - | Clay |
| pH (1:2.5 soil: water) | - | 5.29 |
| E. C. (1:2.5 soil: water) | ds/m | 0.07 |
| Organic matter | % | 3.83 |
| Organic carbon | % | 2.22 |
| Total nitrogen | % | 0.19 |
| Available phosphorus | ppm | 3.41 |
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The research is financed by Jimma University and Humbo Woreda Administration. Thanks for Microbial Biotechnology Unit, Holeta Agricultural Research Center.