RESEARCH ARTICLE
Effects of Alternate Irrigation with Saline and Non-Saline Water on Sorghum Crop Manured with Elaeagnus angustifolia Leaves Using 15N
Farid Al-Ain, Mohamad Al-Chamma'a, Fawaz Kurdali*
Article Information
Identifiers and Pagination:
Year: 2017Volume: 11
First Page: 24
Last Page: 34
Publisher ID: TOASJ-11-24
DOI: 10.2174/1874331501711010024
Article History:
Received Date: 14/11/2016Revision Received Date: 20/02/2017
Acceptance Date: 20/02/2017
Electronic publication date: 28/04/2017
Collection year: 2017

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
A pot experiment was conducted to determine the effects of alternate irrigation with saline (S) and fresh (F) water on growth, nitrogen uptake and nitrogen use efficiency in Sorghum crop (Sorghum bicolor L.) manured with Elaeagnus angustifolia leaves (GM) using 15N.
Method:
Five types of irrigation systems abbreviated as (F, 2F:1S, 1F:1S, 1F:2S and S) and one rate of Green Manure (GM) were employed.
Result:
Results showed reductions in both Dry Matter yield (DM) and nitrogen uptake (NY) in sorghum grown under different types of irrigation systems as compared with the control (F). The reduction rates of these two parameters increased with increasing number of irrigations with saline water. However, E. angustifolia leaves applied as green manure mitigated the harmful effect of salinity on plant growth parameters. The percent increments as a result of GM application were 9, 19, 43, 43 and 65% for DM, and 33, 30, 42, 36 and 60% for NY in F, 2F:1S, 1F:1S, 1F:2S and S, respectively. Nitrogen use efficiency of added green manure ranged between 25 and 47% in the different types of irrigation systems. Our results indicated that increment of NY in green manured sorghum plants was mainly attributed to its nitrogen availability. According to the availability of S and F water resources, and the economic returns aimed by farmers in the semi-arid regions, it is recommended to use (2F:1S, 1F:1S and 1F:2S) in combination with E. angustifolia leaf GM. Such a procedure can be considered as a promising agricultural practice to improve yield with a proper water resource investment.
1. INTRODUCTION
The increasing demand for water resources in the world, especially in the arid and semi-arid regions, has forced farmers to use low quality water such as agricultural drainage water and marginal quality ground water for irrigation. Irrigation with this low quality water during the whole growing season of the crops, even the tolerant ones, does not always produce acceptable yield. Therefore, mixing agricultural drainage water as well as low quality ground water with good quality water in ratios to keep the salinity of the irrigation water below the threshold of the target crop is an acceptable practice and is used by many scientists [1-3].
Alternating good quality water with saline water is an another management practice. Its application is easier because it does not need reservoirs for mixing two sources of irrigation water. Moreover, some scientists used a good quality water during the sensitive stages of plant growth and a poor quality water during the non-sensitive stages [4, 5]. Alternate irrigation system maintains low salt accumulation in the wetting zone and produce a higher yield of tomato (24%), as compared with the mixing water managment practice [6]. Sharma et al. [7] mentioned that cyclic irrigations with canal and drainage water at different sequences resulted in wheat yields of 88% to 94% of the potential, at the same time it did not cause any significant reduction in pearl-millet and sorghum yields. Also Sharma et al. [8] reported a sunflower yield of 82% of the potential using the same manner of irrigation technique. Investigating the impact of irrigation by saline water at different growth stages of wheat, Mojid and Zahid Hossain [9] reported that irrigation by saline water (7 ds m-1) at grain filling stage resulted in a lower grain yield (by 30% as compared to the control), this revealing that the grain filling stage of wheat is the most sensitive to irrigation water salinity. Mao et al. [10] observed that irrigation by brackish water was economically attractive to farmers for a short term time, however, ecological hazards may occur in the long term use of the water, like soil salinization which cause low water and nutrients availability and lead to physiological crop diseases [11].
On the other hand, bioreclamation of salt-affected soils through green manuring of the growing crops is considered one of the most important ways of improving their fertility. This soil-ameliorating practice is increasing in recent years because of the high cost of chemical fertilizers, and the increased risk of environmental pollution [12, 13].
Benefits of using legume-green manure as a biofertilizer may also arise from improving soil properties, nutrient availability, increasing water storage and providing some essential nutrients other than N [14]. Moreover, it has been reported that the mineralization rate of incorporated plant materials in soil depends on a number of factors including quality and quantity of organic materials, soil type, temperature, moisture, and time of application [15]. Kurdali and Al-Shamma'a [16] found that the addition of 120 kg N ha-1 of Leucaena leucocephala leaves as a Green Manure (GM) at the sowing time of sorghum resulted in obtaining higher amounts of N derived from GM, a lower soil N uptake, and greater dry matter and N yield in sorghum leaves.
Elaeagnus angustifolia (Russian olive) is an actinorhizal plant which is able to grow in symbiosis with the N2-fixing bacteria Frankia. These plant species are highly tolerant against a variety of abiotic stresses (water, temperature, salt, and other enviromental factors) and can be grown under a wide range of climatic conditions [17]. Elaeagnus angustifolia is a widespread species in Syria and has effective nodules on its roots under arid conditions [18]. Khamzina et al. [19] reported that Elaeagnus angustifolia, grown on highly salinized, nutrient-depleted croplands, fixed N2 in the range of 24-514 kg N ha-1 year-1, depending on plantation age. The high N2 fixation and N concentration in E. angustifolia leaves with a lower C/N ratio [18, 20] would allow this actinorhizal plant to make significant contributions to the N economy of the ecosystem. Therefore, it can be effectively used for land recultivation, soil rehabilitation and bioamelioration in the degraded irrigated croplands [19, 21, 22].
To evaluate the benefit of GM for crop production, it is necessary to quantify N derived from this material. The 15N-isotopic dilution technique is widely used to estimate N2 fixation in legumes and can be utilized to determine the nitrogen fraction derived from green manure [13, 23].
Several reports highlighted the effect of legume green manures in improving the plant growth in salt-affected soils [13, 24]. However, information is lacking on the use of actinorhizal plant residues for green manuring of other crops either irrigated by saline or fresh water. Moreover, there is a need to increase the area and productivity of agricultural land through green manuring of the growing crops and the proper use of available water resources.
Therefore, the objective of this study was to determine the effect of green manuring with Elaeagnus angustifolia leaves on a dry matter yield, nitrogen uptake, amount and percent of nitrogen derived from the different resources and nitrogen use efficiency of green manure in sorghum plants alternatively irrigated with saline and/or fresh water using 15N isotopic dilution technique.
2. MATERIALS AND METHODS
2.1. Soil Properties
The experiment was conducted in pots, each containing 10 kg of throughly mixed soil collected from Deir AL-Hajar research station, located south east of Damascus, Syria (36° 28' E, 33° 21' N) at 617 m above sea level. Soil was sandy clay loam in texture with pH 7.5; Electrical Conductivity (EC) 0.42 dS m-1; organic matter 0.91%; total nitrogen 0.07%; available phosphorus 6.1 µgg-1; ionic content (meq L-1) chloride (Cl-) 0.74, bicarbonate (HCO3-) 0.97, sulfate (SO4--) 1.27, calcium (Ca++) 1.1, potassium (K+) 0.14, sodium (Na+) 1.27, and magnesium (Mg++) 0.47; Cation Exchange Capacity (CEC) 29.08 meq 100 g-1; nitrate (NO3-) 32 µgg-1 and ammonium (NH4+) 16.1 µgg-1.
2.2. Green Manure Preparation
Young leaves of Elaeagnus angustifolia used as green manure were collected from man- made plantation trees [18], oven dried at 70 oC for 72 hours, milled to pass through 1 mm sieve for total N and C determinations. Mean values of total N and C in leaves were 3.75% and 49%, respectively (e.g., C/N ratio was 13).
2.3. Experiment Details
Seeds of sorghum plants (Sorghum bicolor L.), were sown in pots, and set outdoors under natural climatic conditions. After germination, seedlings were thinned to two plants per pot. The design was a split plot, with manure treatments being the main treats and the irrigation systems are the sub-main. The green manure was incorporated into the soil at planting in two rates: 0 (-GM) and 64 mg N kg-1 soil (+GM). Five types of irrigation systems abbreviated as (F, 2F:1S, 1F:1S, 1F:2S and S) were applied within these two manure treatments, where:
- F: control, irrigation only with fresh water (ECiw ≈ 1 dS m-1).
- 2F:1S alternate irrigation with fresh (F) and saline (S) water (ECiw ≈ 7 dS m-1), laboratory prepared (NaCl), at a sequence (2:1).
- 1F:1S alternate irrigation with fresh (F) and saline (S) water, at a sequence (1:1).
- 1F:2S alternate irrigation with fresh (F) and saline (S) water, at a sequence (1:2).
- S: irrigation only with a saline water (ECiw ≈ 7 dS m-1).
Total number of pots was 40 (i.e. 2 manure × 5 irrigation systems × 4 replicates).
At the time of each irrigation, all pots were weighed to keep soil water content around 75% of field capacity throughout the experimental period. Fresh water was used from planting up to the complete germination, thereafter; plants were subjected to the above-mentioned five different irrigation systems. The crop required a total number of irrigations equal to 18, starting from complete germination to the harvest time at a rate of 0.81 L pot-1 per irrigation (Table 1).
An equivalent fertilizer rate of 20 kg Nha-1 (8.5 mg N kg-1 soil) in the form of urea enriched with 5% 15N atom excess was applied to the soils. The N fertilizer was applied to all treatments in four equally split applications at 10 days intervals after planting. This procedure was followed to stabilize the 15N enrichment of the N pool and to minimize N immobilization.
After harvest, composite soil samples representing each treatment were taken to monitor mineral nitrogen, organic matter and electrical conductivity under the different treatments.
| Irrigation Treatments | No. of Irrigations | Quantity of Water (L) | ||
|---|---|---|---|---|
| Fresh Water | Saline Water | Fresh Water | Saline Water | |
| F | 18 | - | 14.6 | - |
| 2F: 1S | 12 | 6 | 9.73 | 4.87 |
| 1F: 1S | 9 | 9 | 7.30 | 7.30 |
| 1F: 2S | 6 | 12 | 4.87 | 9.73 |
| S | - | 18 | - | 14.6 |
| Note: F: irrigation only with a fresh water, S: irrigation only with a saline water, 2F:1S, 1F:1S and 1F:2S: alternate irrigation with fresh (F) and saline (S) water at a sequence (2:1, 1:1 and 1:2, respectively). | ||||
2.4. Plant Harvest and Analysis
Sorghum plants were harvested at physiological maturity and separated into shoots, roots, and panicles. Samples were dried to a constant weight at 70 oC and grounded to a fine powder. Kjeldahle procedure was used to determine total nitrogen in the samples, and the 15N/14N isotopic ratio was determined by the emission spectrometry (Jasco-150, Japan). The nitrogen fractions derived from the available sources were calculated using the indirect 15N isotopic dilution method [23, 25]. This method is based on the fact that plant received unlabelled organic residues (e.g. green manure) with 15N labeled fertilizer to the soil resulted in a lower atom %15N excess in plant tissues compared to the control receiving no residues. This indicates that a dilution effect is taken place, and that a portion of sorghum N is derived from green manure. Accordingly, the percent N derived from green manure (Ndfgm) using the indirect method was calculated using the following equation:
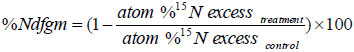 |
Where, Treatment: plants grown with green manure. Control: plants grown without any green manure application.
The amount of N derived from green manure (mg N pot-1) was calculated as follows:
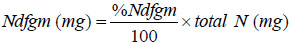 |
The percent of N recovery from the green manure was calculated by the following equation:
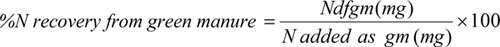 |
Percent (%) and amount (mg N pot-1) of N derived from fertilizer (Ndff), and N recovery from the added fertilizer were calculated using the following equations:
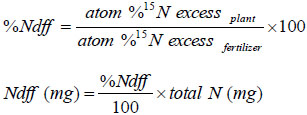 |
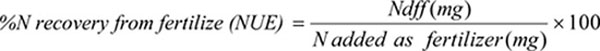 |
The percent N derived from soil (%Ndfs) was calculated as follows:
%Ndfs = 100 - (%Ndfgm + %Ndff)
Data were subjected to analysis of variance (ANOVA) test by using the statistical program Statview, and means were compared using the Least Significant Difference (LSD) test at a probability level of P <0.05.
3. RESULTS AND DISCUSSION
3.1. Soil After Harvest
Table 2 shows that the Electrical Conductivity (EC) of soils increased under the different irrigation systems, relative to the control (F). Such increases were slightly higher in the unmanured treatments than those in the manured ones within the same irrigation system. In spite of the increased salinity, soils under the different treatments were classified as non-saline soils according to [26], indicating the safe employment of the different irrigation systems used in this study. On the other hand, soils mineral nitrogen content increased with increasing the number of irrigations with saline water, (i.e., increased NaCl concentration in those soils). This might be explained by the decreased nutrients assimilation with increasing soil solution salinity due to the competition between ions, particularly nitrate (NO3-) and chloride (Cl-) [27, 28].
| Parameters | Irrigation Treatments | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | 2F: 1S | 1F: 1S | 1F: 2S | S | ||||||
| -GM | +GM | -GM | +GM | -GM | +GM | -GM | +GM | -GM | +GM | |
| EC1:5 (dS m-1) | 0.49 | 0.47 | 1.32 | 1.22 | 1.69 | 1.54 | 1.80 | 1.74 | 2.33 | 2.33 |
| OM (%) | 0.96 | 1.03 | 0.95 | 1.04 | 0.95 | 1.05 | 0.94 | 1.06 | 0.93 | 1.13 |
| Mineral N (ppm) | 53.2 | 63.0 | 65.8 | 68.2 | 67.2 | 73.5 | 71.4 | 96.6 | 81.2 | 108.0 |
| Note: F: irrigation only with a fresh water, S: irrigation only with a saline water, 2F:1S, 1F:1S and 1F:2S: alternate irrigation with fresh (F) and saline (S) water at a sequence (2:1, 1:1 and 1:2, respectively). -GM: without green manure, +GM: with green manure . | ||||||||||
3.2. Dry Matter Production (DM) and Nitrogen Yield (NY)
Total dry matter yields were 59.9, 37.7, 26.4, 22.4 and 13.6 g pot-1, in the F, 2F:1S, 1F:1S, 1F:2S and S treatments, respectively (Table 3). It was evident from the data that increasing the number of irrigations with saline water had negative effect on plant growth. However, GM addition increased dry matter productions by 9, 19, 43, 43 and 65% in F, 2F:1S, 1F:1S, 1F:2S and S, respectively. Moreover, there were similarity in (DM) production between (2F:1S -GM) and (1F:1S +GM); (1F:2S -GM) and (S +GM). These results reflected the positive role of GM in decreasing the harmful effect of salinity on dry matter production. Our results were in agreement with those of Rao and Pathak [24], Tejada et al. [29] and Kurdali and Al-Shamma'a [16] who mentioned the importance of GM in alleviating the negative impact of salinity on (DM) due its positive role in improving physical, chemical and biological soil properties. However, the reduction in (DM) yield caused by alternate irrigation could be attributed to a lower water availability in the rhizosphere, leading to a reduction ability of roots to absorb the water and essential nutrients required to plant growth [30]. Salinity can also cause nutrients deficiency or toxicity due to imbalance in nutrients uptake, and disturb many biological processes that affect plant growth [31-33].
Total Nitrogen Uptake (NY) by (Sorghum bicolor L.) responded to saline conditions in a manner similar to dry matter yield. The observed values were 711, 540, 403, 343 and 199 mg N pot-1, in F, 2F:1S, 1F:1S, 1F:2S and S, respectively. The reduction in (NY) as the result of increasing the number of irrigations with saline water could be most probably due to increasing sodium chloride content in soil solution, leading to an increase in its osmotic potential and a reduction in NO3- and other nutrients availability [28, 33-35].
| Green Manure | Irrigation Treatments | |||||
|---|---|---|---|---|---|---|
| F | 2F: 1S | 1F: 1S | 1F: 2S | S | LSD 0.05 | |
| Dry Matter | ||||||
| -GM | 59.91 B,a | 37.68 B,b | 26.40 B,c | 22.35 B,d | 13.63 B,e | 1.68 |
| +GM | 65.25 A,a | 44.75 A,b | 37.75 A,c | 32.05 A,d | 22.50 A,e | 2.34 |
| LSD 0.05 | 3.75 | 1.95 | 1.01 | 1.41 | 2.53 | |
| N-Uptak | ||||||
| -GM | 711 B,a | 540 B,b | 403 B,c | 343 B,d | 199 B,e | 28.3 |
| +GM | 945 A,a | 702 A,b | 574 A,c | 466 A,d | 318 A,e | 37.0 |
| LSD 0.05 | 54 | 49 | 22 | 23 | 30 | |
| Note: Means within a column (capital letter) and within a row (small letter) followed by the same letter are not significantly different (P<0.05). F: irrigation only with a fresh water, S: irrigation only with a saline water, 2F:1S, 1F:1S and 1F:2S: alternate irrigation with fresh (F) and saline (S) water at a sequence (2:1, 1:1 and 1:2, respectively). -GM: without green manure, +GM: with green manure . | ||||||
The amounts of N uptake in sorghum manured with E. angustifolia leaves were considerably higher than those of the unmanured treatments under the different irrigation systems. The percent increments of sorghum (NY) due to the addition of (GM), were 33, 30, 42, 36 and 60%, in F, 2F:1S, 1F:1S, 1F:2S and S, respectively. It could also be noticed that (GM) addition induced similarity in the amounts of (NY) in either (2F:1S +GM) and (F -GM) or (1F:2S -GM) and (S +GM). These results indicated a positive impact for the use of (GM) to enhance plant growth through providing a portion of its nitrogen demands, alleviating the adverse effect of salinity on (NY) [25, 36, 37], and increasing the available soil mineral nitrogen content [22, 38].
3.3. Nitrogen Uptake from Various Nitrogen Sources
Percentages of nitrogen derived from fertilizer (%Ndff) in sorghum plants ranged between 9.30 and 11.23%; whereas, percentages of nitrogen derived from soil (%Ndfs) ranged between 88.77 and 90.7% under the different irrigation systems. E. angustifolia leaves, applied as green manure, resulted in significant decreases in both (%Ndff) and (%Ndfs) within all irrigation systems (Table 4). Values of %N derived from green manure (%Ndfgm) were: 30.91, 29.58, 27.92, 34.11 and 49.67% in F, 2F:1S, 1F:1S, 1F:2S and S, respectively. In this regards, it can be noted that %Ndff values under manured treatments were (6-8%), approximately in the same range of %Ndff values (4-6%) reported for sorghum plants manured with Sesbania aculeata [25]. The amounts of (Ndfgm) were reduced as the result of increasing the number of irrigations with saline water. The percent reductions in the amount of Ndfgm were 29, 45, 46 and 46% as compared to the control (F +GM) in 2F:1S, 1F:1S, 1F:2S and S, respectively. These results showed no significant differences in the amounts of Ndfgm in 1F:1S, 1F:2S and S, respectively. All treatments, receiving green manure, resulted in lower amounts of Ndff and Ndfs in sorghum under all irrigation systems as compared to the unmanured treatments. This indicated that (GM) provided a substantial portion of N requirements in sorghum. Similar results were reported by Kurdali [25] and Malik et al. [37] using different kinds of green manure. The reduction in the amounts of Ndff and Ndfs as the result of added E. angustifolia leaves (GM) is useful for maintaining soil fertility and substituting considerable amounts of the costly and environmentally pollutant inorganic N-fertilizer, particularly at early growth stages of sorghum [22, 38]. However, several reports [13, 39, 40] showed an increase in the amounts of Ndfs in plants receiving (GM), such increments could be explained as the mineralized N in soil originated from (GM), which is taken by plants and referred to as Ndfgm, has already been a part of soil N pool. Therefore, the absorbed N from resources other than N-fertilizer, is the sum of Ndfgm and Ndfs, and were: 872, 647, 527, 432 and 300 mg N pot-1 in F, 2F:1S, 1F:1S, 1F:2S and S, respectively. In comparing the aforementioned values with the amounts of Ndfs in unmanured treatments (632, 480, 358, 305 and 181 mg N pot-1 in F, 2F:1S, 1F:1S, 1F:2S and S, respectively), the percent increments in the amounts of Ndfs were 38, 35, 47, 42 and 66%, respectively. Such results indicate a positive effect of (GM) in the enhancement of soil N pool (Ndfgm and Ndfs) to meet sorghum N demands.
| Green Manure | Irrigation Treatments | |||||
|---|---|---|---|---|---|---|
| F | 2F: 1S | 1F: 1S | 1F: 2S | S | LSD 0.05 | |
| %Ndff | ||||||
| -GM | 11.23 A,a | 11.21 A,a | 11.20 A,a | 11.03 A,a | 9.30 A,b | 0.35 |
| +GM | 7.76 B,b | 7.91 B,ab | 8.10 B,a | 7.40 B,c | 5.65 B,d | 0.23 |
| LSD 0.05 | 0.45 | 0.23 | 0.25 | 0.40 | 0.30 | |
| Ndff (mg N pot-1) | ||||||
| -GM | 79.82 A,a | 60.58 A,b | 45.18 A,c | 37.84 A,d | 18.54 A,e | 2.2 |
| +GM | 73.37 B,a | 55.53 B,b | 46.46 A,c | 34.49 B,d | 17.96 A,e | 3.5 |
| LSD 0.05 | 4.45 | 4.72 | n.s. | 1.75 | n.s. | |
| %Ndfs | ||||||
| -GM | 88.77 A,b | 88.79 A,b | 88.80 A,b | 88.97 A,b | 90.70 A,a | 0.35 |
| +GM | 61.33 B,b | 62.51 B,ab | 63.99 B,a | 58.49 B,c | 44.68 B,d | 1.79 |
| LSD 0.05 | 1.54 | 1.26 | 0.97 | 1.55 | 1.91 | |
| Ndfs (mg N pot-1) | ||||||
| -GM | 631.5 A,a | 479.6 A,b | 358.2 A,c | 305.4 A,d | 180.6 A,e | 26.5 |
| +GM | 579.8 B,a | 438.8 B,b | 367.1 A,c | 272.6 B,d | 141.9 B,e | 28.0 |
| LSD 0.05 | 50.3 | 34.6 | n.s. | 20.0 | 19.5 | |
| %Ndfgm | ||||||
| +GM | 30.91 c | 29.58 cd | 27.92 d | 34.11 b | 49.67 a | 2.0 |
| Ndfgm (mg N pot-1) | ||||||
| +GM | 292.0 a | 207.5 b | 160.1 c | 159.0 c | 157.7 c | 14.5 |
| Note: Means within a column (capital letter) and within a row (small letter) followed by the same letter are not significantly different (P<0.05). F: irrigation only with a fresh water, S: irrigation only with a saline water, 2F:1S, 1F:1S and 1F:2S: alternate irrigation with fresh (F) and saline (S) water at a sequence (2:1, 1:1 and 1:2, respectively). -GM: without green manure, +GM: with green manure . | ||||||
Jenkinson [41] has introduced the term “Added Nitrogen Interaction” (ANI) or priming effect. ANI may result from an increase in the volume of soil exploited by the roots thereby increasing the uptake of soil N by the green manured–plants. Furthermore, Rees et al. [40] reported that such effects are likely to be a result of either increased root growth leading to a greater uptake of N, or increased N mineralization. The study of Azam [39] using an organic N source demonstrated positive added N interactions to be associated with increased root growth. In this study, a positive effect on sorghum root dry matter yield was observed following the addition of green manure (data not shown) where the percent increments ranged between (25 and 40%) as compared to the control. Moreover, there is a reason to believe that a priming effect may occur in soils because soil microorganisms react to the addition of energy-rich materials, and the increased microbial activity will involve mineralization of the soil organic matter [39]. Therefore, it can be suggested that the beneficial effect of GM on total N uptake by sorghum may be attributed to root growth stimulation (i.e. DM) and N-mineralization of added GM (i.e., Ndfgm) [39-41].
3.4. Nitrogen Fertilizer Use Efficiency (%NUE)
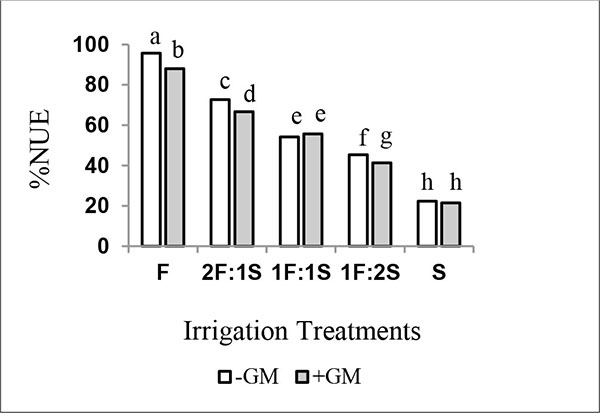
Several researchers reported improvement in the percentage of nitrogen fertilizer use efficiency (%NUE) following the application of non organic N-fertilizer in the presence of organic manure. Such improvement was mainly attributed to greater water availability and greater biological activity resulting from the presence of green manure [13, 14, 16]. In this study, values of NUE ranged between 22 and 96% under the different treatments (Fig. 1). The increasing number of irrigations with saline water resulted in decreases in NUE values. Moreover, the application of (GM) did not lead to an improvement in NUE, probably due to the small added amount of N (e.g., 20 kg ha-1 as 15N) which was mainly used to estimate %Ndfgm. Regardless of GM amendments, the efficient use of N-fertilizer was considerably higher in plants irrigated with non-saline than those irrigated with saline water.
Maas and Grattan [42] found reverse relationship between soil solution salinity and plant response to fertilization. This response depends on the degree of salt stress in the root zone. Similar trends were also reported by Albassam [43] and Irshad et al. [44] who mentioned that salinity could lead to a decrease in NUE. Moreover, Esmaili et al. [33] noted a negative effect of soil salinity on mineralization process. Therefore, the decreases in the %NUE of urea fertilizer as the result of increasing the number of irrigations with saline water most probably attributed to the application of green manure of high N-content with low 14N/15N ratio and to the decreasing in the microbial activity involved in N-mineralization process as salinity increased.
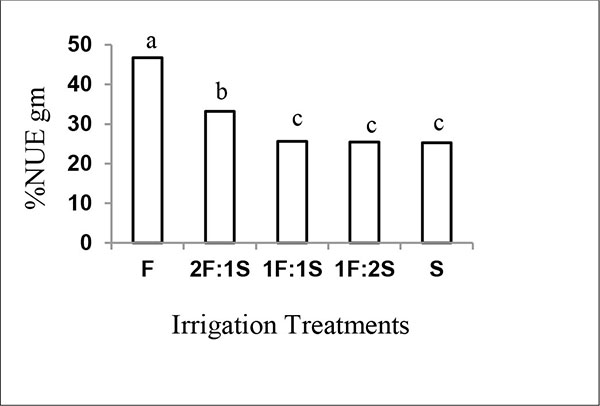
3.5. Nitrogen Recovery of Added Green Manure (%NUEgm)
The efficient use of green manure decreased with increasing the number of irrigations with saline water (Fig. 2) with values being 47, 33, 26, 25 and 25% in F, 2F:1S, 1F:1S, 1F:2S and S, respectively. Approximately, the same range of %NUEgm values (20-52%) has been reported for sorghum manured with sesbania leaves grown in saline soil [13, 25]. The reduction in %NUEgm could be attributed to the increase in soil salinity (Table 2), as salinity has a negative effect on green manure use efficiency. Several workers: Rees et al. [40], Biederbeck et al. [45], Pathak and Rao [46] and Morrissey et al. [47] reported that the benefit from the green manure to be dependent on the type of green manure and soil salinity. Azam [39] showed that %NUEgm was 19.3% in rice manured with Sesbania aculeata. For other Sesbania species, Diekmann et al. [48] reported that rice utilized 46% of the N applied in the form of Sesbania rostrata.
The mineralization rate of plant material depends on a number of factors including quality and quantity of the organic matter, soil type, temperature, and moisture [15, 16]. Giller and Wilson [49] reported that the mineralization rate of plant material with a C/N ratio lower than 30 is high. The application of green manure with low C/N ratio, such as legume plants, would lead to a high mineralization rate, if the application was made even at planting [13]. On the other hand, Rietz and Haynes [50] reported that agriculture-induced salinity greatly affects soil microbial and biochemical properties. Therefore, it can be concluded that the high %NUEgm in treatments irrigated with non-saline water may be mainly attributed to a high mineralization rate of Elaeagnus angustifolia leaves which attributed to have a low C/N ratio (13) [18], as well as to greater soil microbial activity under fresh conditions.
The indirect isotopic dilution method used herein is feasible and simple for measuring N release from organic residues (e.g. green manure). Hood et al. [51] reported that estimates of the percent N derived from alfalfa residues in the ryegrass were similar for the direct and indirect methods. Similary, Kurdali [25] showed that both methods gave similar results in estimating Ndfgm as a result of adding Sesbania leaf residues (C18: N1) prior to sorghum planting.
3.6. Water Study
From Table 1 it is clear that percentages of saving fresh water were 33, 50, and 67% in 2F:1S, 1F:1S, and 1F:2S, respectively. It is noteworthy that the reduction in dry matter production of manured sorghum in 2F:1S, 1F:1S, and 1F:2S Table 3 did not exceed 50% compared with the control (F -GM) which is considered producible yield [2]. This suggests the possibility of employment the aforementioned alternate irrigation systems, according to the availability of saline and non-saline water resources, along with the economic returns aimed to farmers. However, our results revealed unsuitable use of 1F:1S or 1F:2S treatments in the unmanured sorghum plants, since the reduction in (DM) yield exceeded 50% compared with the control. This reflects the necessity of applying (GM) to enhance dry matter production when using the abovementioned alternate irrigation treatments.
The use of saline water in irrigation could substitute a significant amount of fresh water, which in turn, enables us to irrigate additional agricultural lands and increase farmer's income. However, under the experiment conditions, irrigation of sorghum only with saline water (S) cannot be followed as the loss in dry matter production exceeded 50% as compared with the control (F).
Overall, the use of salt tolerant N2- fixing plants could be suggested as a promising approach to revegetate salt affected lands. Elaeagnus angustifolia can be used as part of a larger set of strategies to exploit salt-affected irrigated croplands. The desirable characteristics of this plant species (e.g. high foliage N content, ability to fix atmospheric and high salt and drought tolerent) can contribute to an effective use of its leaves and pruning residues as green manures for growth of economic crops.
CONCLUSION
The undertaken study is the first report on the use of Elaeagnus angustifolia L. to be used as a green manure for growth of sorghum crop grown under saline and non-saline conditions.
Results showed that:
- Alternate irrigation with saline and fresh water (i.e. 2F:1S, 1F:1S, and 1F:2S) in combination with green manuring of sorghum plants with of Elaeagnus angustifolia leaves can be considered as a promising approach for enhancing plant growth performance (i.e. dry matter production and N yield) and for a proper use of water resources by substituting a significant amount of fresh water for irrigation.
- The beneficial effect of Elaeagnus angustifolia leaves in total N yield was mainly attributed to the additional N availability to the plants.
- Under prevailing experimental conditions, the 1F:1S and 1F:2S seemed to be not suitable treatments for irrigation the unmanured sorghum plants, suggesting the importance of testing other salt tolerant varities.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ACKNOWLEDGEMENTS
The authors would like to thank the Director General of the Atomic Energy Commission of Syria, for his support, encouragement and providing necessary facilities during the course of the experiment.