All published articles of this journal are available on ScienceDirect.
Transgenic Pro-Vitamin A Biofortified Crops for Improving Vitamin A Deficiency and Their Challenges
Abstract
Vitamin A Deficiency (VAD) has been a public health problem among children in developing countries. To alleviate VAD, Vitamin A Supplementation (VAS), food fortification, biofortification and nutrition education have been implemented in various degrees of success with their own merits and limits. While VAS is the most widely utilized intervention in developing countries to ease the burden of VAD, some have raised questions on VAS’ effectiveness. Biofortification, often touted as an effective alternative to VAS, has received significant attention. Among the available biofortification methods, adopting transgenic technology has not only facilitated rapid progress in science for enhanced pro-Vitamin A (pVA) levels in target crops, but drawn considerable skepticism in politics for safety issues. Additionally, VAD-afflicted target regions of transgenic pVA crops widely vary in their national stance on Genetically Modified (GM) products, which further complicates crop development and release. This paper briefly reviews VAS and its controversy which partly demanded shifts to food-based VAD interventions, and updates the current status of transgenic pVA crops. Also, this paper presents a framework to provide potential influencers for transgenic pVA crop development under politically challenging climates with GM products. The framework could be applicable to other transgenic micronutrient biofortification.
1. INTRODUCTION
The 2016 World Food Prize (WFP), the prestigious prize for agricultural research, celebrated four recipients on their achievement with the pro-vitamin A (pVA) biofortified sweet potato to combat Vitamin A Deficiency (VAD) [1]. According to WFP, nearly two million households across ten African countries are currently planting or purchasing this sweet potato. In comparison, Golden Rice (GR), the most well-known pVA biofortified crop, still grapples with regulatory approvals and public skepticism for its release. The GR project, initiated in the 1990's with humanitarian concerns, aims at achieving the same goal as the sweet potato. A critical difference, however, in the two crops’ divergent fate is how they are created, i.e., conventional breeding for the sweet potato and transgenic technologies for GR.
Vitamin A deficiency is a major public health issue especially among preschool-aged children in developing countries. It affects about 190 million of those children, and estimated 6.9 million die annually due to VAD by the age of five [2]. With serum retinol lower than 0.7 μML-1 which defines VAD, the estimated prevalence of VAD was 29% in 2013 among children aged 6 to 59 months, with the highest rates in Sub-Saharan African countries ranging from 25% to 75%, and South Asian 13% to 79% [3]. Vitamin A is required for normal functioning of the visual system, maintenance of cell function for growth, epithelial integrity, immunity, and reproduction. Vitamin A deficiency, thus, increases vulnerability to a range of illnesses including diarrhea, measles, and respiratory infections [4] consequently leading to higher mortality [5]. Accordingly, international and national entities have implemented policy and behavior interventions to combat VAD. Policy interventions include Vitamin A Supplementation (VAS) and food fortification while behavior interventions focus on individual knowledge and practices for VA rich diets [6].
The 1992 International Conference on Nutrition, which called for VAD elimination, facilitated urgent VAS implantation. Many countries have since integrated periodic VAS to children in their national health programs [2]. Currently, the United Nations Children's Fund (UNICEF), the VAS flagship organization, supports national-level VAS programs in its 82 priority countries as a child survival intervention. In 2014, 69% of the target children in the priority countries received VAS compared to 30% in 2000 [7]. However, VAS programs may depend on compliant behaviors, consistent funding from the government or international donors, and accessible national healthcare distribution channels to target populations [8].
Other interventions to alleviate VAD are more food-based, including promotion of dietary diversification, food fortification, and biofortification. A diversified diet including animal-origin foods rich in VA might satisfy VA daily requirements. However, in many developing countries, diets are mainly based on cereals and legumes that are lacking or low in VA. Moreover, some readily available vegetables have low VA bioavailability [9]; for instance, raw green leafy vegetables rank the lowest in the VA bioavailability pyramid [10]. Dietary diversification depends little on external funding or healthcare systems, yet often it is difficult to change established dietary behaviors. Therefore, rapid impacts from dietary diversification on VAD are less likely achievable in a short period [8]. Food fortification, which is fortifying processed foods such as table sugar, cooking oil or cereal flour with VA, is another VAD intervention strategy [11]. A successful example is VA-fortified sugar which decreased VAD across target populations in Guatemala [12, 13]. For effective food fortification, good vehicle foods for target nutrients should be available and widely consumed. Also, the nutrient in the vehicle must be stable under normal conditions of storage and usage [13]. For instance, VA fortified rice was estimated less effective in Cambodia where rice provides 70% of the daily energy intake because of up to 93% loss of fortified VA, depending on the fortification methods and storage conditions [8]. Centralized processing facilities are also needed with regulatory frameworks in place to minimize trading conflicts and monitoring issues [12]. However, many developing countries do not have the food industry sufficiently developed for centralized fortification, and national fortification programs are often subject to political priorities [10]. Biofortification differs from food fortification by aiming to increase crop nutrient levels during crop growth, not through crop processing. Biofortification can be achieved by applying nutrient-rich fertilizers to facilitate micronutrient accumulation in edible parts, conventional breeding by creating elite hybrids with enhanced target nutrients, and transgenic methods to obtain specific nutritional traits from donor organisms [2]. In recent decades, biofortification has targeted three globally important micronutrients: iron, zinc and pVA. While transgenic methods are touted advantageous over conventional breeding in that they could transfer specific traits to target crops with precision in shorter time periods [14], safety issues on human and the environment hinder their wide acceptance.
Other than VA delivery mechanisms of those interventions, the form of VA also differs. VAS uses Retinyl Palmitate (RP), retinyl acetate or retinol, and RP is the most commercially available VA form [15]. These chemicals are typically diluted in vegetable oil with vitamin E [16], and this oil-based VA solution is orally delivered in soft gelatin capsules [17]. The oil matrix protects VA during storage, improves stability, and facilitates absorption in the gastrointestinal tract [15]. Likewise, food fortification widely utilizes RP although RP’s efficacy in fortified foods might vary depending on food vehicles and storage conditions [18, 19]. While the VAs are well absorbed in the body, adverse effects on health were indicated if consumed in large amounts [20]. The VA form of biofortification is pVA carotenoids which plants produce naturally for multiple functions [21]. Of the carotenoids, β-carotene has the highest pVA activity due to its two unsubstituted β-rings. Plant β-carotene is considered safe because its intestinal conversion to retinal for absorption decreases with increased β-carotene [20]. Yet, its bioconversion rate varies with food matrix, or chemical relationships between the nutrient and non-nutrient components of food [22], food preparation techniques and amount of fat [20]. Recently, Bechoff and Dhuique-Mayer (2016) indicated human factors such as nutritional status, genetic factors, and diseases that need to be investigated for their influence on biofortified pVA, as well as dietary factors [23].
This paper covers VAD issues from three angles. The first part briefly discusses VAS and arguments around it, which partly triggered a paradigm shift towards food-based approaches to better combat VAD. The second part updates the status of transgenic pVA crops as a food-based intervention. Few VAD interventions perhaps have invited more debates than transgenic biofortification due to controversies on Genetically Modified Organisms (GMOs). The third part discusses political climates for adopting transgenic pVA crops, and presents a framework for transgenic pVA crop development.
2. VITAMIN A SUPPLEMENTATION AND CONTRADICTING VIEWPOINTS ON ITS EFFECTIVENESS
According to the World Health Organization (WHO), oral provision of high-dose VA every 6 months until the age of five is grounded on that a single, large dose of VA is well absorbed and stored in the liver, and mobilized as needed over an extended period of time [17]. This scheme is considered effective, swift and inexpensive for developing countries as it does not require a sterile injectable preparation [24]. WHO recommends a dose of 100,000 International Units (IU) in infants aged 6-11 months and 200,000 IU in children aged 12-59 months for adequate protection up to 6 months [17]. On a per-child basis, VAS can be a low-cost intervention; each VA gelatin capsule costs approximately US$ 0.02 with an estimated annual delivery cost of US$ 1-2 per child [17].
The evidential ground for the WHO recommendations largely came from the studies conducted during the 1980's, which found a direct relationship between VAS and reduced child morbidity and mortality [17]. One of the landmark studies, conducted in Indonesia, associated VAD with excess mortality in children suffering mild xerophthalmia [25]. The findings indicated mild xerophthalmia might justify community-wide interventions to prevent blindness and reduce child mortality [25]. Following this association, 200,000 IU VA capsules were distributed to preschool-aged children twice a year in 450 Indonesian villages, and the authors found VAS could decrease child mortality by 34%, compared to the control [26]. When the 34% reduction in child mortality was interpreted for formulating intervention policy, VAS became part of a child survival measure [27]. For child morbidity, a meta-analysis showed VAS effects varied depending on diseases in children aged 6-59 months; VAS significantly reduced incidence of diarrhea and measles while not significant on respiratory diseases [28]. This study mentioned reductions in diarrhea and measles could be a potential pathway to reducing all-cause child mortality. However, the authors cautioned levels of evidence and quality of the analyzed studies ranged from moderate to very low, indicating difficulties in separately studying child morbidity from VAD [28].
Some studies, however, questioned VAS’ effectiveness in reducing child mortality. For instance, Awasthi et al. (2013) evaluated the periodic 200,000 IU VA capsule delivery on child mortality over five years in India [29]. Their mortality ratio between the VAS and control group was 0.96 with 95% confidence interval (CI) 0.89-1.03 and p-value 0.22. Their results did not support the expected 20-30% reduction in child mortality with VAS [29]. Substantial interest in VAS during the neonatal period also exists. Neonatal VAS (within the first 28 days after birth) was proposed to improve infant survival by increased VA body storage. Presently, WHO does not recommend neonatal VAS due to conflicting evidence on neonatal mortality [30], which prompted large-scale trials for more conclusive evidence and better decision-making [16]. With WHO funding, three large-scale randomized trials were conducted in Ghana, Tanzania and India to assess whether an oral dose of 50,000 IU VA to newborn infants reduced post-VAS mortality by at least 15% in the first six months of life [31]. The three studies concluded that their findings did not support inclusion of newborn VAS as a child survival strategy. In Ghana, the Relative Risk Ratio (RRR) between the VAS and control group was 1.12 with 95% CI 0.95-1.33 and p-value 0.18 [31]. In Tanzania, RRR was 1.10 with 95% CI 0.95-1.26 and p-value 0.19 [16]. In India, RRR was 0.90 with 95% CI 0.81-1.0 and p-value 0.06 [32].
Despite extensive distribution of periodic VAS over the past 20 years, decrease in VAD has been stagnant; Mason et al. (2014) estimated an annual global improvement rate of VAD as 0.3 percentage points since 1990, and at this rate the authors argued it would take another 100 years to eliminate VAD [33]. While criticizing VAS, Greiner (2013) endorsed food-based approaches for their reachability and safety to all community members, and promotion of self-sufficiency and food security [34]. He argued little emphasis on food-based approaches comes largely from political reasons as food-based programs are complex to implement and evaluate [34]. As a food-based approach, conventionally-bred pVA biofortified crops such as the sweet potato, cassava and maize have been released [35]. However, no transgenic pVA biofortified crops are currently available despite significant scientific progress in various crops such as wheat, potato, maize, banana, tomato, canola as well as rice. Some of the cited reasons for transgenic crop disapproval are their potential threats to human health through toxins or allergens, erosion of biodiversity via vertical or horizontal gene flows, new reliance of small-holder farmers on transgenic seed supply, and oversimplification of hunger and poverty issues in developing countries [36]. The next section updates the status of transgenic pVA crops as of November 2016.
3. SCIENTIFIC PROGRESS OF PRO-VITAMIN A ENHANCED TRANSGENIC CROPS
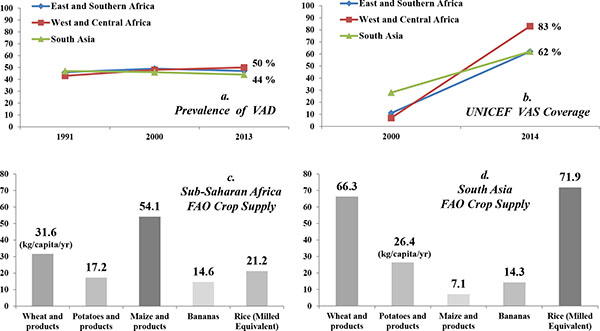
Four global crops, wheat, maize, potato and banana were selected for the updates. Fig. (1) shows changes in VAD prevalence and VAS coverage rates over time in Sub-Saharan Africa and South Asia, and annual per-capita crop supply to indicate crops’ relevance to each region. For instance, maize would be more important in Sub-Saharan Africa whereas rice and wheat in South Asia at a whole population level, not specifically for children due to data limitation.

For transgenic pVA enhancement in crops, identification of the carotenogenesis pathway and its regulating genes in plants and bacteria enabled ectopic expression of specific carotenoids in edible parts of crops [38]. Briefly, carotenogenesis begins with the formation of geranylgeranyl-diphosphate (GGPP) followed by condensation of two GGPPs (Fig. 2). This produces a colorless carotene, phytoene with phytoene synthase (PSY). A series of desaturation reactions with phytoene desaturase and ζ-carotene desaturase leads to a colored lycopene, followed by subsequent cyclization reactions to yield carotenoids. Of the carotenoids, only β-carotene has a high pVA activity [39].
Three strategies can summarize transgenic production of pVA carotenoids. First, with the push strategy, genes encoding rate-limiting steps in the pVA biosynthetic pathway are inserted to produce carotenoids. Golden Rice is an example of this strategy with the multiple-genes inserted to produce β-carotene in rice endosperm [40]. Second, the blocked strategy silences a biosynthetic step immediately downstream of the compound whose level is to be increased. Higher lycopene accumulation in tomato is a result of silenced lycopene β-cyclases [41]. The third strategy, termed sink engineering, regulates carotenoid storage structures to stably store carotenoids [42]. Table 1 summarizes outcomes on transgenic pVA production in the select crops.
3.1. Development of Transgenic Pro-Vitamin A Enhanced Wheat
Wheat (Triticum spp.) is a major cereal crop in many parts of the world [37] supplying the greatest amount of calories [21]. Durum wheat (T. turgidum) for pasta and couscous was selected for increased yellow pigments [44] of which the main components are carotenoids [37]. However, the major carotenoid in durum wheat is lutein, non-pVA carotenoid. Moreover, 94% of wheat currently grown is bread wheat (T. aestivum) which was selected for white flour with little pVA carotenoid [44].
Cong et al. (2009) showed bread wheat grains could produce high levels of carotenoids by introducing the maize PSY1 and bacterial carotene desaturases (CrtI) genes [37]. Their transgenic wheat endosperm increased carotenoid contents 10.8 folds compared with the non-transgenic counterpart [37]. To further increase carotenoids and investigate transgene effects, Cong et al. (2014) introduced the bacterial phytoene synthase gene (CrtB) instead of the maize PSY1 gene into bread wheat, Bobwhite [21]. Expression of either CrtB or CrtI alone slightly increased carotenoid contents while co-expression of both genes in transgenic grain significantly enhanced total carotenoids up to 8 folds, pVA contents as a sum of α-, β-carotenes, and β-cryptoxanthin up to 76 folds, and β-carotene alone up to 65 folds [21]. Zeng et al. (2015) altered wheat β-carotene with lycopene β-cyclase gene (LCYb) [43]. Lycopene cyclization is the first branch point for the two competing carotenes, α- and β-carotene (Fig. 2). And LCYb is a key enzyme catalyzing β-carotene biosynthesis while lycopene ε-cyclase (LCYe) for α-carotene. A wheat LCYb (TaLCYb) was cloned from bread wheat, Chinese Spring, and silencing the gene decreased β-carotene [43]. Also, carotenoid cleavage dioxygenases (CCDs) modified β-carotene contents by converting β-carotene into smaller molecules called apocarotenoids. Qin et al. (2016) cloned two wheat CCDs genes and characterized them with β-carotene synthesis; CCD1 homoeologs contributed to the degradation of β-carotene by cleaving it [44].
| Crop | Gene | Cultivar or Line | Outcomes | Publication | |
|---|---|---|---|---|---|
| pVA production increase or narrative indication |
Compound level (Transgenic vs. control) # |
||||
| Wheat | Maize PSY * and CrtI * | Variety EM12 | - ~ * 10.8 folds increase in total carotenoids with expression of yellow grain color in transgenic lines | - 4.96 μg g-1 DW * vs. 0.46 | [37] |
| CrtB *, CrtI | Cultivar Bobwhite | - ~ 8 folds increase in total carotenoids with expression of red/yellow grain color in transgenic lines | - 4.06 ± 0.49 μg g-1 DW vs. 0.58 ± 0.02 | [21] | |
| - ~ 76 folds increase in pVA content § | - 3.21± 0.37 μg g-1 DW vs. 0.05±0.01 | ||||
| Wheat LCYb * |
Chinese Spring | - β-carotene decrease by silenced LCYb gene | - 0.09 μg g-1 DW vs. 0.22 | [43] | |
| Wheat CCDs * |
Tetraploid Kronos and hexaploid line UC1041 | - β-carotene decrease by CCD1 cleaving and degrading β-carotene; indicated from increased ratio of β,ε- and β,β-branch carotenoids in endosperm | - No numerical data provided | [44] | |
| Maize | Maize PSY and CrtI | White kernel Hi-II |
- ~ 34 folds increase in total carotenoids with preferential accumulation of pVA | - 33.6 mg g-1 DW vs. 1.01 | [45] |
| - ~ 25 folds increase in pVA | - 9.8 mg g-1 DW vs. 0.39 | ||||
|
Zmpsy1 *, PacrtI *, Gllycb * , Glbch * , ParacrtW * |
South African elite white variety M37W | - ~ 142 folds increases in total carotenoids with both Zmpsy1and PacrtI expression | - 156.14 mg g-1 DW vs. 1.01 | [46] | |
| - Zmpsy1 and PacrtI genes needed for changes in carotenoids and color from white to yellow, orange, red | - No numerical data provided | ||||
| Maize PSY, CrtI | South African elite white variety M37W | - ~ 169 folds increase in β-carotene with orange color endosperm | - 59.32 mg g-1 DW vs. 0.35 | [47] | |
| Potato | CrtI | Cultivar Desiree | - ~ 6.3 folds increase in tuber total carotenoids | - 35 lg g-1 DW vs. 5.6 | [48] |
| - β-carotene increase from undetectable levels | - 10.30 lg g-1 DW vs. negligible level | ||||
| LCYe * | - ~ 14 folds increase in tuber β-carotene by silenced LCYe | - 43.56 ng g-1 DW vs. 3.17 | [41] | ||
| CHY1, CHY2 | - ~ 4.5 folds increase in tuber total carotenoids by silencing CHYs | - 21758.57 ng g-1 DW vs. 4887.95 | [49] | ||
| - ~ 35 folds increase in tuber β-carotene by silencing CHYs | - 85.3 ng g-1 DW vs. 2.25 | ||||
| CrtB, CrtI, CrtY * | - ~20 folds increase in total carotenoids | - 114.4 mcg g-1 DW vs. 5.8 | [50] | ||
| - 3600 folds increase in β-carotene | - 47 mcg g-1 DW vs. 0.013 | ||||
| CrtB, CrtI, CrtY | - ~ 20 folds increase in total carotenoids - 50% of total carotenoids as pVA carotenoids resulting from their favorable shift |
- Shown in graphical charts | [42] | ||
| Cauliflower Or (Orange) gene | - ~ 10 folds increase in total carotenoids during storage vs. starting point in fresh tubers - ~ 20 folds increase in β-carotene during storage vs. starting point in fresh tubers |
- Shown in graphical charts | [51] | ||
| * ~: up to, DW: dry weight PSY: phytoene synthases, CrtI: bacterial carotene desaturases, CrtB: bacterial phytoene synthase, LCYb: Lycopene β-cyclase gene, CCDs: Carotenoid cleavage dioxygenases, DW: seed dry weight, Zmpsy1: maize phytoene synthase 1, PacrtI: Pantoea ananatis phytoene desaturase, Gllycb: Gentiana lutea lycopene b-cyclase, Glbch: G. lutea β-carotene hydroxylase, ParacrtW: Paracoccus β-carotene ketolase, LCYe: lycopene ε-cyclase, CHY: non-heme β -carotene hydroxylases, CrtY: lycopene β-cyclase. § As sum of α-carotene, β-carotene, and β-cryptoxanthin. # Highest levels among transgenic lines when given specific numerical data; otherwise without numerical data, shown in bar charts or graphs for graphical comparisons. |
|||||
3.2. Development of Transgenic Pro-vitamin A Enhanced Maize
Maize (Zea mays) is a dominant subsistence crop in many parts of Sub-Saharan Africa [45]. While β-carotene is low in maize kernels [52], maize displays a genetic variation in carotenoid contents [53]. In yellow kernels, β-carotene ranges from 0.01 to 4.7 mg g-1 [54] although most yellow varieties consumed contain only 0.5 to 1.5 mg g-1 [53]. Additionally, utilization of yellow maize for livestock feed hinders human consumption in parts of Southern Africa where white maize, lacking pVA, is preferred [55].
Aluru et al. (2008) employed bacterial CrtB and CrtI genes for carotenoid production in white maize, Hi-II which contains undetectable amounts of carotenoids [45]. The transgenic lines expressing the two genes increased β-carotene 7-13 folds compared to the control [45]. Zhu et al. (2008) correlated specific transgenes’ expressions with carotenoid profiles in white maize, M37W which lacks endosperm carotenoids [46]. They introduced five carotenogenic genes in M37W, and identified transgenic lines carrying combinations of the five transgenes. Of the combinations, ones carrying the maize PSY1 and Pantoea ananatis CrtI produced orange-red phenotypes with high pVA carotenoids [46]. Naqvi et al. (2009) created transgenic maize with three multiple vitamins, pVA, ascorbate and folate, by simultaneously modifying three separate metabolic pathways [47]. This transgenic maize is expected to combat multiple micronutrient deficiencies. Expression of the maize PSY1 and CrtI increased β-carotene 169 folds in transgenic maize, and this level of β-carotene could provide its full recommended daily intake with a typical portion of 100-200 g of maize grain [47]. In 2014, the European Commission granted consent for experimental release of the transgenic maize (Carolight TM) in Spain to measure its agronomic and other characteristics [56]. Recently, Zanga et al. (2016) reported Carolight TM was indistinguishable from its near isogenic line for agronomic performance including yield [57]. Rice has made similar progress with iron and zinc; Trijatmiko et al. (2016) succeeded in attaining 30% Estimated Average Requirement of iron and zinc in transgenic indica rice [58]. Rice thus can combine enhanced iron, zinc and pVA to complement multi-micronutrients interventions [58].
3.3. Development of Transgenic Pro-vitamin A Enhanced Potato
Potato (Solanum tuberosum) comes after wheat, rice and maize as a significant energy source [41], but cultivated potatoes are low in carotenoids ranging between 0.5 and 2.5 mg g-1 Fresh Weight (FW) [50]. The main carotenoids are xanthophylls, lutein and violaxanthin, which lack pVA activities, and β-carotene ranges from undetectable to 0.03 mg g-1 FW. Some wild potato species reach high levels of carotenoids, but low in β-carotene [50].
Ducreux et al. (2005) enhanced carotenoids with the CrtB in a potato cultivar, Desiree [48]. The transgenic lines increased total tuber carotenoids up to 6.3 folds, and reached 11 lg g-1 DW in β-carotene compared to the negligible amount in the control [48]. Diretto et al. (2006) increased β-carotene up to 14 folds in transgenic potato by silencing the LCYe gene, which favorably altered a proportion of α- to β-carotene [41]. Diretto et al. (2007 a) adopted a different strategy to further increase β-carotene [49]. They silenced genes encoding a non-heme β-carotene hydroxylase (CHY) to prevent β-carotene from converting into zeaxanthin, the immediate product of CHY. Their CHY-silenced tubers increased β-carotene up to 38 folds and total carotenoids up to 4.5 folds with zeaxanthin decreased [49]. Diretto et al. (2007 b) introduced a bacterial-origin pathway with CrtB, CrtI and CrtY (lycopene beta-cyclase) genes in potato to direct β-carotene synthesis from GGPP [50]. Expression of all three genes increased β-carotene up to 3600 folds in a deep yellow phenotype, which has been the highest increase reported in all pVA biofortified crops. With an assumed β-carotene to retinol conversion ratio 6:1 and 250g of potato consumption, they can provide 50% of VA Recommended Dietary Allowance [50].
3.4. Development of Pro-vitamin A Enhanced Banana: Blurring the Line Between Conventional and Transgenic Methods
Bananas (Musa spp.) are perennial monocots that are vegetatively propagated [59]. Natural variations in banana pVA contents contribute to the fruit color variation from white to dark orange. A major banana cultivar, Cavendish (M. acuminata) is low in β-carotene, 0.72 lg g-1 FW while noncommercial Asupina (Fei group Musa cultivar) with orange pulp contains high β-carotene, 14.5 lg g-1 FW. However, the fact that the majority of banana cultivars are sterile triploids challenges their conventional breeding. Therefore, genetic modification within the genetic variation in Musa spp., or cisgenics, can be a viable option [60].
Mlalazi et al. (2012) characterized banana PSY genes and proposed at least two PSY paralogs, PSY1 and PSY2a are present in Cavendish and Asupina [60]. The Asupina PSY enzyme exhibited 2-fold higher enzymatic activity than that of Cavendish, and β-carotene contents in Asupina and Cavendish differed by at least an order of magnitude. Thus, utilization of Asupina PSY gene could increase β-carotene in widely consumed banana cultivars, facilitate GMO regulatory approval, and improve consumer acceptance of pVA bananas [60]. Buah et al. (2016) further identified key differences between the two bananas; expression of CCD4 in Cavendish which decreased β-carotene in Cavendish, and conversion of amyloplasts to chromoplasts in Asupina which increased β-carotene in Auspina [59]. As in wheat CCD enzymes, expression of CCD4 gene cleaved more Cavendish β-carotenes to form apocarotenoids. Chromoplasts play critical roles in β-carotene storage under the Orange gene (Or) control, which a later section discusses. Therefore, both greater CCD4 expression and little presence of chromoplasts in Cavendish attribute to its low β-carotene [59].
3.5. Stability and Storage of Enhanced Pro-Vitamin A in Transgenic Crops
Harvested crops are consumed and stored until the next harvest. Degradation of pVA carotenoids during storage is problematic for VAD-afflicted populations. Moreover, β-carotene is considerably susceptible to oxidation due to its conjugated double-bond system [61]. In high-carotenoid yellow maize lines, up to 56% of carotenoids were lost during a long-term storage and in yellow wheat, up to 48% [51]. It is equally critical to retain enhanced pVA during crop consumption and storage. Li et al. (2001) investigated a mutant cauliflower Or gene and showed the gene is associated with increases in carotenogenic activity and chromoplasts which sequester large amounts of β-carotene in cauliflower [62]. Currently, the Or gene is the only known gene that triggers differentiation of non-colored plastids into chromoplasts. Chromoplasts accumulate massive amounts of carotenoids by generating carotenoid-lipoprotein sequestering substructures. These substructures stimulate continuous carotenoid biosynthesis and stably store the synthesized products. Chromoplast biogenesis, therefore, can be part of a strategy to enhance pVA contents and their stability [51]. Expression of the cauliflower Or gene was investigated for carotenoid accumulation and stability in potato [51, 63]. Transgenic potato tubers enhanced carotenoids and β-carotene, and continued accumulating them during long-term cold storage with induced formation of chromoplasts [63]. Thus, the formation of a proper sink structure may enable target crops to promote and stabilize pVA accumulation during crop growth and post-harvest storage [51].
Transgenic pVA enhancement has been successful in various crops. However, a decisive question is whether those transgenic crops will be released in target regions to ease the VAD burden amidst GMO controversies. The next section discusses relevant political issues, and presents a framework to assist in achieving research goals.
4. CAVEATS OF DEVELOPING TRANSGENIC PRO-VITAMIN A ENHANCED CROPS AND A FRAMEWORK FOR BETTER ALIGNED AGRICULTURE-NUTRITION LINKAGE
Domestic regulations determine commercialization of GM crops, and regulatory approval for their cultivation, marketing and usage is country-specific [64]. Thus, governmental stance on GMOs predicts availability of GM crops at market. Genetic modification has been touted as precise, flexible and rapid compared to its alternatives under limited time and resources [65], yet there are persistent concerns about safety and trade aspects of GM products, which necessitate regulations [66]. In formulating national policies of GM products, countries weigh in opportunities and potential risks in social, political and economic contexts. If VAD-afflicted countries prohibit transgenic pVA crops on the ground of their national GMO regulations, investment in multi-stage development, release and marketing of such crops is not viable.
Regarding transgenic crop adoption, countries in Sub-Saharan Africa and South Asia are a political mosaic of national stance, from permissive, pre-cautionary to prohibitive [66, 67]. Likewise, developmental stages of legislative infrastructure on GM crops vary from established, partially built to virtually non-existent [67]. In South Asia, for instance, Bhutan adopted a restrictive GMO-free strategy banning any introduction, release, or research of GMOs while India established federal and state agencies to regulate already commercialized GM crops [67]. In sub-Saharan Africa, South Africa among few others is a frontrunner of GM crop adoption and enacted GMO laws in 2000 while Angola prohibits GMOs with no enacted GMO laws [66, 68]. Mabaya et al. (2015) identified some key factors affecting GM crop policies in Africa [68], which might be applicable to South Asia. First, positions of line ministries are important; the ministry of agriculture tends to favor GM crops whereas the ministry of environment tends to view them as potential threats. Also, the more ministries are involved, the less likely or more slowly GM crops would be adopted due to their conflicting interests. Second, peer country influence is significant because GM crop adoption by one country can trigger repercussions across porous borders. The repercussions include spreading agricultural technology, sharing knowledge-experience, trading unapproved GM products, and unintended environmental contaminations [68]. Herrington (2008) indicated that unauthorized transgenic crop planting might be considerable [5, 65]. This partly results from ‘asynchronous approval’ of new GM products; approval of a new GM product does not occur simultaneously across countries [64]. In Sub-Saharan Africa, this concern contributed to creating COMESA or Common Market for Eastern and Southern Africa to guide safe and responsible management of trans-boundary movements of GMOs [69]. Third, developmental stages of seed sectors affect GM crop adoption; more matured seed sectors are correlated with more positive attitudes to GM crops. Fourth, advocacy by key political figures and public influence are identified important; the media, often criticized for sensationalism, sway public opinions that in turn influence political decisions. Fifth, food security crisis plays a role in GM crop adoption; with production surplus, there is more reluctance to adopt GM crops whereas under food insecurity they are considered a workable alternative [68].
In addition to those domestic-oriented factors, external factors influence GM policy differences, one of which is the mixed signal that America and Europe send to market. The American policy stance on GMOs emphasizes product similarities between conventional and GM breeding. This approach is based on the principle of ‘substantial equivalence’, in which GMOs are viewed as comparable to the products of conventional breeding. As such, creating new regulations to oversee GMOs is not necessary [70]. On the other hand, European skepticism about GMOs arises from the precautionary principle, in which GMOs are viewed as fundamentally different from conventional breeding. Thus, GMOs are subject to strict measures for approval, monitoring and liability, influencing policies both in Europe and abroad [70]. Furthermore, the divergent stances on GMOs complicate food aid policies. Responding to the severe drought in 2001 and 2002 in southern Africa, only Swaziland accepted the GM yellow maize as food aid from the World Food Program while Zimbabwe, Mozambique, Lesotho, and Malawi limited or refused it [69].
Given those complicated circumstances, utilization of transgenic pVA crops hinges on political dynamics in target countries since the current GMO policies do not discriminate trait characteristics whether they are disease resistance, or micronutrient biofortification. Transgenic biofortified crops should be more carefully viewed in that they serve different purposes and benefit different populations. For this reason, the reductionist’s viewpoint on GMOs, either for or against, may not be the best way to see transgenic biofortification.
Overall, the segmented GMO policies across the target regions require cautious considerations prior to, during and post development of transgenic pVA crops. The framework thus intends to provide potential influencers for transgenic pVA crop development (Table 2). Although other influencers can enter and interplay in specific contexts, the framework may offer important factors to consider, probably applicable to other transgenic micronutrient biofortification.
| Stage | Consideration * |
Influencer Positive/yes as facilitator and negative/no as barrier § |
|---|---|---|
| Feasibility for launching pVA biofortification program | Current VAD status, VAD interventions in target country and population | - Availability of accurate data on VAD status in target population. - Effectiveness and acceptance of existing VAD program and on-going activity. - Prioritization of VAD reduction in target country. |
| Target crop, its cultivation and local consumption in target country and population | - Availability of accurate data on candidate local variety of target crop, its cultivation, utilization and consumption by target population. | |
| Domestic GMO regulation and relevant political climate | - Stance of line ministry on GMO. - Availability of financial and political assistance during target crop development. |
|
| International or regional GMO regulation ¶ | - Potential conflict to international and regional GMO standard and regulation. | |
| pVA crop development, test and distribution | Pre safety estimate and evaluation # | - Safety to local ecological system, human health, and potential contamination i.e. gene flow from target crop. |
| Information communication | - Existence of transparent channel for safety test result on health and environment, potential benefit and risk, IPRs arrangement ʃ to stakeholders (free-of-charge or fee-based with potential conflict of interest). | |
| Adoption assessment | - Public perception of transgenic target crop. - Awareness of VAD and its health impact. - Economics of target crop for cultivation, distribution, utilization and consumption. |
|
| Verification assessment | - Feasibility of large-scale test for yield, safety and bioavaiability of pVA. - Significant anti-GMO activity against large-scale test. |
|
| Distribution channel | - Existence of mature seed sector and infrastructure. - Potential for private-public distribution partnership. |
|
| Monitoring and evaluation | Post pVA quality monitoring and evaluation in VAD change | - Existence of effective monitoring procedure, indicator, trained staff and budget. |
| Monitoring legal/illegal trading of target crop | - Harmonized GMO regulation with neighbor country. - Potential scale-up of target crop to neighbor country. |
|
| Abbreviations: VAD; vitamin A deficiency, pVA; pro-vitamin A, GMO; genetically modified organism. * Not in the order of importance, but flexible depending on target crop development program. § If influencer is positive/yes, it can facilitate target crop development; otherwise act as barrier. ¶ Such as Codex Alimentarius, Cartagena Protocol on Biosafety, COMESA guidelines, FAO GM guidelines etc. # Probably via literature-review on target crop, laboratory-generated data, small-scale experiment. ʃ Intellectual property rights probably not applicable to vegetative propagation, depending on IPRs arrangement. |
||
In sum, political climates and public attitudes towards GMOs in target countries should be realistically evaluated. Their different political stance and attitude would enable or disable and support or hinder transgenic pVA crop development and adoption to combat VAD. As such, consultation and partnership with line ministries should guide initial research decisions. Notably, patterns of target crop production, consumption and cultural practices should be assessed. Wheat, maize, potato and banana are global commodities but this does not guarantee that specific target populations readily adopt them due to different food-related cultures, social norms or economies. Developers may consider whether a target population grows a target crop for subsistence or income-generation, the population rejects the transgenic crop from any changes in its attribute, or growing the crop involves additional costs compared to the conventional counterpart, which is related to the Intellectual Property Rights (IPRs) to utilize the pVA trait. The IPR arrangement affects affordability of a pVA crop, allowing it to be free-of-charge under a humanitarian aid or fee-based under a market principle. As Zanga et al. (2016) recommended, developers should allocate resources for IPRs investigations and partnership-building with patent licensors to minimize future conflicts [71]. Although often overlooked, efficacy of existing VAD interventions in target regions must be factored in; when the current interventions work well within the healthcare system with low costs, approval of transgenic pVA crops is not a political priority. Presence of transparent communication channels is required to disseminate scientifically-sound information, and discriminate against misleading information, either positive or negative, on transgenic pVA crops. These channels promote or impede informing stakeholders of benefits and risks for fair judgment, and proactive, not reactive, GMO policy modifications. Finally, when target populations utilize transgenic pVA crops, quality monitoring and health evaluation should be hand-in-hand to ensure a claimed level of pVA and its efficacy for health effects, and to scale up pVA crop programs.
CONCLUSION
Humanitarian efforts to release pVA crops should not be hindered for political reasons when a safe means is available with proven benefits. Yet, without careful proactive strategies to factor those major influencers in, however effective to combat VAD, transgenic pVA crops would unlikely reach to market shelves from research facilities under political challenges.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The author thank Steven E. Ullrich and Nuan Wen at Department of Crop and Soil Sciences, Washington State University, Pullman, WA, USA, and Sheldon Krimsky at Department of Urban and Environmental Policy and Planning, Tufts University, Medford, MA, USA for their valuable comments on the manuscript.


