All published articles of this journal are available on ScienceDirect.
Physical and Chemical Properties of Boiled Oil: A Traditional Method of Extracting Oil from Boiled Olive Fruits
Abstract
Background
In some villages in Northern Jordan, farmers boil part of their olive fruit harvest before oil extraction to increase the quantity of oil, get a desirable acquired taste, as they claim, and get additional health benefits. Locals call this oil Boiled oil (BO), and its price is about 20% higher than virgin olive oil (VOO) produced by the same farmer.
Objective
The hypothesis was that boiling olive fruits may affect the quality of the produced oil. Therefore, our study aimed to determine the chemical and physical characteristics of boiled oil in comparison with VOO and the effect of storage of both types of oils for one year.
Methods
Total phenolic compounds, ferric-reducing antioxidant power assay (FRAP), and radical scavenging activity (RSA) were evaluated. Moreover, the quality indices of BO and VOO were also evaluated.
Results
Total phenolic compounds decreased significantly (p <0.05) from 8.7 mg GAE/100g in VOO to 2.47 mg GAE/100g in BO. The antioxidant activity measured using FRAP assay also decreased significantly (p <0.05) from 962 to 379 micromole/kg.
Half-maximum inhibitory concentration (IC50) of the RSA was significantly higher (p <0.05) in BO samples (414 mg/ml) in comparison with VOO samples (38.9 mg/ml). Moreover, there was a significant increase (p <0.05) in acid value in BO samples (0.943%) in comparison with VOO samples (0.518%). However, the increase in acid value after one year of storage was higher in VOO than in BO. The peroxide value also increased significantly in boiled oil (500 meq/kg) in comparison with VOO (19 meq/kg). Additionally, a significant increase in ultraviolet absorption was observed in BO at k232 and k270 (3.5), which is considered unsuitable for human consumption compared with VOO (2.43).
Conclusion
In conclusion, these results showed that boiling olive fruits before oil extraction deteriorates oil quality as expected, and consumers should be educated that this type of oil is hazardous to human health and is a waste of effort and money.
1. INTRODUCTION
The Olive tree is the oldest cultivated tree in the Mediterranean region [1], and it is the most cultivated fruit tree in Jordan [2]. Olive oil produced in Jordan is known for its distinctive taste and aroma [2]. Fatty acid composition and phenolic compounds found in olive oil play a major role in disease prevention [3]. These phenolic compounds affect the quality and the distinctive taste of virgin olive oil [4]. The quality of olive oil is affected by many factors, such as growth conditions, harvest date, cultivar, degree of ripeness of the olive fruit, storage conditions, method of extraction, and temperature [3]. The effect of heating during cooking is usually studied in the literature. Kmiecik et al., 2023 studied the effect of frying on different cultivars using 170 and 200 degrees Celsius. They reported that the higher the temperature, the higher the degradation of olive oil, depending on the cultivar [3]. Since boiling olive fruits in water is found in very small localities, this type of information is not found in the literature. In some villages in Northern Jordan, the farmers boil olive fruits for about ten minutes, then sun-dry them for two weeks before oil extraction. The dried boiled olive fruits are first crushed using an ancient traditional stone mill. The paste is then manually spread by pressing mats 2-3 cm thick layers, and the automatic hydrostatic force is applied to separate the oil droplets from the paste at room temperature. Finally, the oil is collected in 20-litre containers and stored in a dry, dark place. The inhabitants of Tubneh village claim that the taste, colour, and health benefits of olive oil produced by this method are enhanced [5-8].
This type of oil has never been studied earlier, so the present study aimed to investigate the effect of boiling olive fruits and sun-drying them on the quality of the produced oil compared to virgin olive oil produced from the same olive trees. This was accomplished by evaluating quality indices of the oil, such as acid value, peroxide value, and ultraviolet absorption, in addition to antioxidant activity and total phenolic compounds.
2. MATERIALS AND METHODS
2.1. Experimental Design
Freshly pressed and one-year-old boiled oil and virgin olive oil samples were purchased from the same farmer. Parameters of olive oil quality, such as acid value peroxide value, radical scavenging assay against DPPH, and ultraviolet absorption at 232 nm and 279 nm, were measured. Moreover, total phenolic compounds and antioxidant activity using FRAP assay were assessed using oil sample extracts. All measurements were done in triplicates.
2.2. Olive Oil Samples
Virgin olive oil and boiled oil samples were purchased from a farmer from Tubneh village in the province of Irbid/Northern Jordan. Samples of boiled oil and virgin olive oil were purchased for two consecutive years to evaluate the effect of storage on boiled oil and virgin olive oil. The freshly pressed samples were purchased immediately after pressing, the oil was stored in a dark, dry room in a 20-litre canister, and these were placed on wooden shipping crates and not directly on the ground. The idea of recapitulating the mode of production in the laboratory was deliberated but dismissed because pressing a few kilogrammes of boiled olive fruits using a modern mill is practically impossible because all mills open during olive pressing season only, and people stay in queues for several days to press their harvest in some instances. So, the benefit of controlled conditions that would have been accomplished by controlling the temperature would disappear. Moreover, traditional mills are very rare in Jordan, and they are found in remote locations. However, since the objective was to evaluate the quality of the actual product, then using boiled oil produced by the farmers would serve the purpose.
2.3. Chemicals
Folin-Ciocalteu phenol reagent and Gallic acid and diethyl ether were purchased from Alpha Chemika, 2, 4, 6- tripyridyl- s- triazine (TPTZ) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from TCI (USA), Ferrous sulphate was purchased from VWR chemicals, sodium thiosulphate. Cyclohexane, ethyl acetate, methanol, and n-hexane were HPLC grade.
2.4. Methods
2.4.1. Preparation of Olive Oil Extracts
Olive oil extracts were prepared according to Favati et al., 1995 [9]. Fifty grams of olive oil samples were diluted with hexane. Then, the diluent was washed three times with a 30 mL methanol/water mixture (60% methanol). The complex mixture was agitated for 2 min before allowing the two phases to separate in a separatory funnel. Finally, the methanolic extracts were then washed with 50 mL of hexane filled up to 100 mL in a volumetric flask and kept at -18°C until use.
2.4.2. Total Phenolic Compounds
Three replicates of boiled oil extract (two hundred microliters) were taken. Each replicate was mixed with 1.5 ml of 10% Folin-Ciocalteu reagent and incubated for five minutes. Then 1.5 ml of a 6% sodium bicarbonate solution (NaHCO3) was added. The sample was left in the test tube to complete the reaction within 90 minutes in a dark place at room temperature, and the absorption was measured at 725 nm using a spectrophotometer (GENESYS 10S UV-Vis) against a blank, which contained 200μL of 60% methanol. The gallic acid calibration curve was used, and the results were expressed as mg gallic acid equivalent (mg GAE /100g fresh weight) (y = 8.5227x-.0052; R2 = 0.9997) [10].
2.4.3. Antioxidant Activities
2.4.3.1. Ferric Reducing Antioxidant Power Assay (FRAP)
The antioxidant activity of olive oil was determined spectrophotometrically using a ferric-reducing antioxidant power assay (FRAP). Ferric reducing/antioxidant power reagent containing 5 mL of a (10 mmol L-1) TPTZ (2, 4, 6- tripyridyl- s- triazine) solution in 40 mmol L-1 HCl plus 5 mL of (20 mmol L-1) FeCl3 and 50 mL of (0.3 mmol L-1) acetate buffer (pH 3.6) was prepared freshly and warmed at 37°C for 10 min before use. Aliquots of 100 μL extracts were mixed with 3 mL FRAP reagent, and the absorbance of the reaction mixture at 593 nm was measured using a spectrophotometer (GENESYS 10S UV-Vis) against a blank after incubation at 37°C for 10 min. For the construction of the calibration curve, ferrous ions of ferrous sulfate were used with concentrations ranging from 100 to 1000 micromole/L, and the results were expressed as micromoles of ferrous ions per kg. Another calibration curve was used, where the concentrations ranged between 50 to 1000 micromole/L, and the FRAP value was expressed in micromole/L of ascorbic acid [11].
2.4.3.2. Radical Scavenging Assay (RSA)
The radical scavenging assay was performed according to Kalantzakis et al., 2006 method [12], and the antioxidant activity of boiled oil was measured by measuring their RSA against DPPH0. Oil samples were dissolved in different concentrations of ethyl acetate (10%, 30%, 50% wt./vol) until IC50 was reached. One ml of oil extract was added to 4 ml of DPPH solution 104 M in freshly prepared ethyl acetate. The reaction mixture was shaken vigorously for 10 seconds using a vortex, and the tubes were then kept in complete darkness for 30 minutes. The reaction mixture was then measured at 515 nm against a blank (which did not contain a radical), and the control sample contained a DPPH solution without oil. The constituents of the oil samples were expressed as RSA toward DPPH0 as a % reduction in DPPH0 concentration:
% [DPPH.] reduction = 100% (1–[DPPH.]30/[DPPH.]0)
Where [DPPH.]0 was the concentration of [DPPH.] in the control sample (t = 0) and [DPPH.] 30 in the test mixture after the 30-minute reaction and inhibitory percent IC50.
2.4.4. Quality Control Parameters
2.4.4.1. Acid Value
Acid value was determined according to International Olive Council regulation Method COI/T.20/DOC. 34/Rev. 1 – 2017 [13], the acid value was expressed as a percent of oleic acid. It was determined by titration of an oil sample dissolved in a methanol/diethyl ether mixture (1:1, v/v).
2.4.4.2. Peroxide Value
Peroxide value was determined as follows: a mixture of oil and chloroform/acetic acid 3:2 (v/v) was left to react in darkness with saturated potassium iodide solution, and then the free iodine was titrated with 0.01 M sodium thiosulfate solution in the presence of starch indicator. Peroxide value was carried out according to the International Olive Council Regulation Method COI/T.20/DOC. 35/Rev. 1 – 2017 [14].
2.4.4.3. Ultraviolet Absorption
Ultraviolet absorption was carried out according to the International Olive Council Regulation Method COI/T.20/ DOC. 19/rev. 5/2019 [15]. The presence of dienes and triene that resulted from oxidation was measured by dissolving the oil samples in 1% cyclohexane. The samples were then transferred to a quartz cuvette (10 mm path length) and read at 232nm and 270nm).
2.5. Statistical Analysis
A statistical package for social science (SPSS, version 23.0) was used. Three replicates of each sample were used. Data were subjected to analysis of variance; the one-way analysis of variance (ANOVA) assessment was done to test the difference between different types of oil, and means were compared by least significant difference (LSD). P-values were calculated using the student t-test, and means with different superscripts (a, b, c) differed significantly at (P<0.05). Pearson correlation was used to investigate the association between phenolic content and FRAP value.
3. RESULTS AND DISCUSSION
3.1. Total Phenolic Compounds
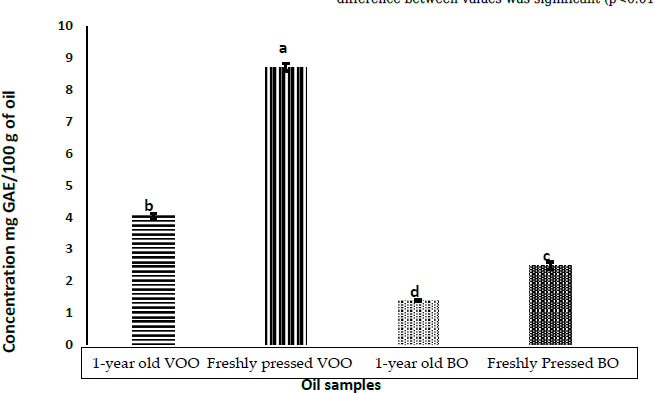
Fig. (1) shows the effect of boiling olive fruits before oil extraction and the effect of storage for one year on total phenolic compounds in comparison with virgin olive oil from the corresponding year. Total phenolic compounds decreased significantly (P< 0.05) in freshly pressed boiled oil samples (2.47 ± 0.12 mg GAE/100g oil) in comparison with freshly pressed virgin olive oil samples (8.71 ± 0.12 mg GAE/100g oil). Similarly, total phenolic compounds decreased significantly (P<0.05) in stored boiled oil samples (1.38 ± 0.03 mg GAE/100g oil) in comparison with stored olive oil samples (4.05± 0.08 mg GAE/100g oil). This decrease in total phenolic compounds was foreseen, such as boiling olive fruits and sun-drying them for 2 weeks was expected to have this effect, as it is well documented that total phenolic compounds concentration in olive oil is affected by many factors, such as the cultivar, extraction method, and processing conditions. In this study, we used the same cultivar (Rumi), the same method of extraction, and the same storage conditions. The only variable was boiling olive fruits and sun-drying them before extraction [16-18].
3.2. Antioxidant Activity
3.2.1. Ferric-reducing Antioxidant Power Assay (FRAP)
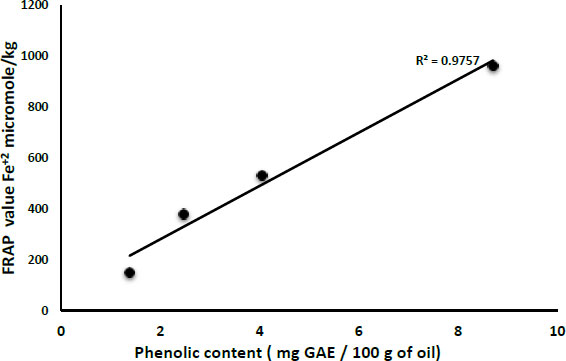
Table 1 shows the effect of boiling olive fruits and sun drying on FRAP values of boiled oil in comparison with virgin olive oil and the effect of storage of both types of oils for one year. The values of FRAP were obtained by using a Ferrous ion (Fe+2) calibration curve. Both freshly pressed and one-year-old boiled oil samples showed lower FRAP values than the corresponding virgin olive oil samples. Moreover, freshly pressed boiled oil samples showed significantly higher FRAP values in comparison with the one-year-old BO samples (Approximately 60%). Although the difference between freshly pressed virgin olive oil and one-year-old virgin olive oil was significant, it was not as extreme as that of boiled oil (approximately 45%), which indicates that antioxidants in boiled oil are more susceptible to degradation than that of freshly pressed boiled oil. These results might be attributed to higher total phenolic compounds in virgin olive oil than in boiled oil, as it is known that phenolic compounds can inhibit oxidation basically by hydrogen atom transfer, metal-chelating attributes, and radical scavenging activity [19]. Fig. (2) demonstrates the correlation between total phenolic compounds and FRAP values for all samples. In addition to the high Pearson correlation seen between the FRAP value and phenolic content (Pearson r = 0.992), the difference between values was significant (p<0.01**).
| Type of Oil |
FRAP value Fe+2 Micromole/kg Mean ± SE |
FRAP value Ascorbic Acid Micromole/kg Mean ± SE |
|---|---|---|
| Freshly pressed BO | 379.808± 4.19 c | 276± 8.08 c |
| Freshly pressed VOO | 962.66± 4.04 a | 847.43± 6.35 a |
| One-year-old BO | 150.4± 5.77 d | 250± 9.81 d |
| One-year-old VOO | 532.2± 4.62 b | 704± 4.04 b |
| - | - |
3.2.2. Radical Scavenging Assay
Table 2 depicts the reaction of compounds of boiled oil with DPPH measured in polar and non-polar solvents. In the polar fraction of boiled oil, the radical scavenging activity did not reach half the maximal inhibitory concentration IC50, which makes the extraction of phenolic compounds from the polar fraction a necessity. The extracted phenolic compounds were dissolved in an appropriate solvent, and then the percent reactive scavenging activity was found. Boiled oil had minimal phenolic content, corresponding to refined vegetable oils where almost no polyphenols were present; ethyl acetate could be the solvent of choice [20]. The reaction of compounds of the total fraction of boiled oil with DPPH in ethyl acetate shows an increase in freshly pressed BO up to 414 mg/ml compared to freshly pressed VOO (38.9 mg/ml), which means that we need ten times more active component from boiled oil to reach IC50 in comparison with virgin olive oil. This may be explained by the loss of total phenolic compounds and tocopherols, which results in the loss of antioxidant activity. In previous studies, Valavanidis (2004) used the same DPPH protocol on olive oil exposed to heating for two hours at 190°C, where IC50 for olive oil total fraction increased twofold [21], which means that the boiled oil preparation process is more deteriorating in quality and antioxidant activity than heating olive oil.
3.2.3. Acid Value
The acid values of freshly pressed boiled oil samples and one-year-old samples were 0.78% and 0.94%, respectively, as shown in Table 3. Boiled oil did not exceed 1% acidity, which indicates that the heat used to boil olive fruits inhibited free fatty acid formation due to the denaturation of lipase found in ordinary olive oil [22]. The free fatty acid content in olive oil is one of the most used parameters to determine olive oil quality [23], resulting from the hydrolysis of oil, in addition to other factors leading to an increase in the acid value, such as insect or fungal infections, bad handling, and storage of olive oil [24]. Lipase activity in olive fruits is the main cause of free fatty acid formation, starting while the fruits are on the tree during ripening, through milling and malaxation, with an optimum temperature of 35°C. Therefore, boiling olive fruits might have denatured the enzyme, leading to less acid value in the boiled oil [22].
| Type of Oil |
IC50 in a Concentration of Oil in Ethyl Acetate mg Oil Mean± SE |
|---|---|
| Freshly pressed boiled oil | 414± 7.5 C |
| Freshly pressed virgin olive oil | 38.9± 3.23 d |
| One-year-old boiled oil | 598± 3.17 a |
| One-year-old virgin olive oil | 204± 15.1 b |
| Type of Oil | Acidity % | Class |
|---|---|---|
| Freshly pressed boiled oil | 0.943± 0.04 b | VOO |
| Freshly pressed olive oil | 0.518± 0.09 c | EVOO |
| One-year-old boiled oil | 0.784±0.15 c | EVOO |
| One-year-old olive oil | 1.554±0.063 a | VOO |
3.2.4. Peroxide Value
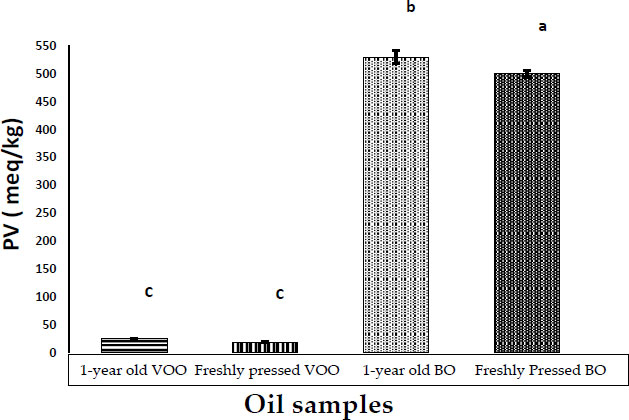
Fig. (3) depicts the peroxide value of freshly pressed boiled oil and stored boiled oil in comparison with freshly pressed VOO and stored VOO. The results showed a significant (P<0.05) increase in peroxide value in the freshly pressed boiled oil samples (500 ± 5.77 meq/kg) in comparison with the freshly pressed VOO l samples (19 ± 1.15 meq/kg). Moreover, the stored boiled oil samples showed a significant (P<0.05) increase in peroxide value (530 ± 11.45 meq/kg) in comparison with stored olive oil samples (25 ± 0.58 meq/kg). The deterioration of olive oil quality begins in the fruit [25], so chances that boiled oil will deteriorate more intensely might be due to high temperature and infusion of water into olive fruits during boiling [26] and exposure to sunlight for 2 weeks.
3.2.5. Ultraviolet Absorption
Ultraviolet absorption is considered an oxidative stability test: an indication of the oxidation degree of the oil, and it is an expression of the extent of extinction coefficient at wavelength 232 and 270 nm and abbreviated as K232 and K270. The lower the values, the better the oil quality is. The result of K232 demonstrated in Table 4 depicts a significant (P<0.05) increase in the freshly pressed boiled oil samples (3.50 ± 0.115) compared with the freshly pressed VOO samples (2.43 ± 0.002). Moreover, in K270, the results depict a significant (P<0.05) increase in the freshly pressed boiled oil samples (0.57 ± 0.002) compared with the freshly pressed samples (0.13 ± 0.002). According to previous studies, boiled oil is not classified as EVOO and VOO [27, 28]; it may be classified as lampante olive oil because the value is below 3.7 and above 0.25. The observed increase in ultraviolet absorption for boiled oil samples compared with olive oil samples may be explained by the low total polyphenolic compounds values, where polyphenols degrade at high temperatures and leach to water, and high peroxide values [18]. Therefore, the boiled oil can be classified as lampante olive oil.
| Type of oil |
K232 Mean ± SE |
K270 Mean ± SE |
ΔK Mean ± SE |
|---|---|---|---|
| Freshly pressed boiled oil | 3.50 ± 0.115 a | 0.57± 0.002 a | 0.023± 0.002 a |
| Freshly pressed olive oil | 2.43 ± 0.002b | 0.13 ± 0 .002 bc | 0.010 ± 0.001 bc |
| One-year-old boiled oil | 3.54 ± 0.002 a | 0.29 ± 0.002 c | 0.007 ± 0.001 c |
| One-year-old olive oil | 3.55 ± 0.00260 a | 0.35 ± 0.0289 b | 0.0117 ± 0.002 b |
Ultraviolet absorption of K232 showed a non-significant increase (P> 0.05) in one-year-old boiled oil samples (3.54 ± .002) compared to freshly pressed BO samples (3.50 ± 0.115). Whereas, ultraviolet absorption of freshly pressed boiled oil at K270 showed a significant (P<0.05) (0.57 ± 0.002) compared with one-year-old BO samples (0.29 ± 0.002). Ultraviolet absorption at K270 depends on the secondary oxidation products formed from the initial compounds detected at 232 nm, indicating that peroxides were transformed into other products like aldehydes and ketones.
The (ΔK) index is a criterion of discrimination between bad quality olive oil and refined olive oil. It is a method by which we can distinguish the presence of pomace oil. In this study, ΔK in freshly pressed boiled oil samples was significantly (p<0.05) higher than (0.023 ± 0.002) than that of freshly pressed EVOO samples (0.010 ± 0.002). According to EU regulation, the limit according to EU regulation of ΔK value for virgin olive oil should not exceed 0.01 [16].
CONCLUSION
An inverse relationship between the quality of olive oil and temperature has already been established in the literature. In this study, the traditional method of boiling olive fruits and sun drying them for two weeks revealed a significant loss of total phenolic compounds, leading to a loss of antioxidant activity. Moreover, a higher peroxide value was observed in both freshly pressed and one-year-old boiled oil. On the other hand, the difference in acid values between freshly pressed boiled oil and one-year-old boiled oil was less than that between freshly pressed olive oil and one-year-old olive oil, which indicates that the lipases might have been deactivated by boiling, leading to less free fatty acid liberation during storage. However, this does not justify the use of boiling because the produced oil is considered hazardous to human health.
LIST OF ABBREVIATIONS
| FRAP | = Ferric-reducing antioxidant power assay |
| RSA | = Radical-scavenging activity |
| IC50 | = Half-maximum inhibitory concentration |
| VOO | = Virgin olive oil |
| BO | = Boiled oil |
CONSENT FOR PUBLICATION
Both authors consented for publication.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available from the corresponding author [S.J] upon reasonable request.
FUNDING
This project was funded by the Deanship of Research at Jordan University of Science and Technology (JUST) grant number 20220241.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We would like to thank the Deanship of Research at Jordan University of Science and Technology (JUST) for providing financial support for this project, grant number 20220241.


