All published articles of this journal are available on ScienceDirect.
Heavy Metal Pollution in Soils near Ilesha Gold Mining Area, Nigeria
Abstract
Background
The discovery of extensive gold deposits has raised concerns about potential heavy metal contamination in waterways and adjacent soils, particularly in developing nations like Nigeria where environmental regulations may not be stringent enough.
Objective
A research study was conducted to assess the levels of heavy metals in surface soils (0 to 25 cm deep) across 30 sites within three selected areas (Epe, Igun, and Ijana) located in the gold mining region of Ilesha, Osun State Nigeria.
Methods
The study employed an atomic absorption spectrophotometer to measure the total concentrations of heavy metals, specifically arsenic (As), cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), nickel (Ni), lead (Pb), and zinc (Zn). Furthermore, the research encompassed an examination of various physicochemical properties of the soil samples, including organic matter content, pH levels, organic carbon content, calcium content, phosphorus levels, cation exchange capacity, and particle size distribution.
Results
Results showed a range mean concentration with standard deviation of As, Cd, Co, Cr, Cu, Ni, Pb and Zn of 1.0 – 7.1 ± 1.77 mg kg-1, 0.9 – 4.2 ± 0.84 mg kg-1, 1.0 – 6.0 ± 1.29 mg kg-1, 1.1 – 6.4 ± 1.57 mg kg-1, 2.90 – 20.9± 4.00 mg kg-1, 1.1 – 6.4 ±1.56 mg kg-1, 0.7 – 6.7 ±1.26 mg kg-1 and 11.7 – 70.7± 17.75 mg kg-1, respectively. The investigation of soils in three specific locations revealed significant contamination by heavy metals. However, it is worth noting that the concentrations of these metals fell below the established intervention levels outlined by environmental protection agencies such as the World Health Organization and the Food and Agriculture Organization. The distribution of heavy metals and their availability exhibited a direct correlation with the distance from the mining sites, spanning from 0 to 900 meters. Two critical factors influencing the concentration of heavy metals in these areas were identified as soil particle size, specifically the clay percentage, and pH levels.
Conclusion
Despite the presence of heavy metal pollution in the soils of Epe, Igun, and Ijana, they managed to maintain metal levels below the intervention thresholds set by environmental protection agencies, with the exception of cadmium (Cd).
1. INTRODUCTION
Soil is a complex ecosystem influenced by a combination of natural forces and human activities, resulting in variations in its mineral and chemical compositions [1]. It has become increasingly evident that the presence of heavy metals in soil poses significant threats to both human health and the environment, primarily due to their non-biodegradable nature. Consequently, assessing soil pollution caused by these toxic elements necessitates a comparison with baseline concentrations in the soil [2].
The improper disposal and accidental release of organic and inorganic compounds into the environment have led to widespread contamination of groundwater and aquatic ecosystems globally [3]. Among the inorganic pollutants, heavy metals are naturally occurring elements present in soil, albeit typically in low concentrations. Their distribution and abundance are determined by the chemical makeup of the underlying rocks [4]. As a result of weathering and evaporation, heavy metal concentrations in the environment can escalate to levels harmful to plants and animals [5].
Certain metals, such as Copper (Cu), Iron (Fe), Manganese (Mn), Nickel (Ni), and Zinc (Zn), are essential micronutrients vital for various life processes in organisms, playing significant roles in regulating physiological functions. Conversely, other metals like Arsenic (As), Cadmium (Cd), Chromium (Cr), and Lead (Pb) lack known physiological functions and can exhibit toxicity even at low concentrations [6, 7].
Human activities, particularly the mining and smelting of metal ores, have significantly contributed to the surge in heavy metal contamination on the Earth's surface. This issue is recognized as a substantial global environmental problem [8]. Soils in the vicinity of mining sites are particularly impacted, and this contamination often extends to other components of the environment, including surface and groundwater sources, the air, and agricultural crops [9-11].
Open-cast mining activities, in particular, have a profound impact on soil and water bodies, resulting in the accumulation of sulfide-rich tailings in substantial quantities. These mine soils exhibit mechanical, physical, chemical, and biological deficiencies [12]. They display instability and limited cohesion, characterized by low nutrient and organic matter content and elevated levels of heavy metals. The oxidation of sulfides in these mining areas leads to acidic drainage, causing leaching of substantial quantities of cations such as Fe2+, Pb2+, Mn2+, Cu2+, and Zn2+ [12]. The contamination of metals and the presence of acidic mine drainage pose significant environmental concerns, particularly in regions where waste materials containing metal-rich sulfides from mining activities have been improperly stored or abandoned [2, 13].
In Osun State, Nigeria, Ilesha and its surrounding areas, including Epe, Igun, and Ijana, are major centers for gold mining due to abundant deposits. However, illegal mining operations have surged, potentially resulting in the contamination of waterways and nearby soils with heavy metals. This situation is particularly concerning in developing countries like Nigeria, which may lack stringent regulations for soil and environmental protection. Studies have indicated that these mining sites are often located near farmlands, where chemicals from the soil may accumulate in arable and cash crops, ultimately leading to severe heavy metal contamination of water sources and posing risks of poisoning to humans and animals if ingested [14, 15].
While several research efforts have recently focused on heavy metals in water in mining areas [16, 17], there remains a notable gap in information regarding heavy metal pollution in agricultural soils within these mining regions. This is especially significant as the majority of people residing near these mining sites are farmers. Thus, there is an urgent need to gather new data on the levels of heavy metals in agricultural soils in the vicinity of the Ilesha gold mining area. This data could serve as a critical resource for assessing the extent of pollution, identifying the specific types of metals involved, and pinpointing areas most susceptible to these pollutants.
The primary objective of this study is to evaluate the concentrations of select heavy metals present in soils across various gold mining sites in the Ilesha area, encompassing Epe, Igun, and Ijana. These findings will be compared to international standard values mandated by regulations. Through this research, we aim to address pressing environmental concerns in gold mining areas, with a particular focus on Nigeria, where metal contamination could have detrimental effects on soil quality, crop production, and human well-being.
2. MATERIALS AND METHODS
A research project was undertaken to investigate the presence of heavy metals within the soil of three distinct locations situated in the Ilesha region of Osun State, located in the southwestern region of Nigeria. The study sites chosen for this investigation were Epe, characterized by a latitude of 7° 13'N and a longitude of 5° 12'E; Igun, situated at a latitude of 7° 35'N and a longitude of 4° 38'E; and Ijana, positioned at a latitude of 7° 33'N and a longitude of 4° 40'E. It is worth noting that these areas rely predominantly on subsistence farming and the cultivation of cocoa (Theobroma cacao) as their primary socio-economic activities [18].
In terms of geological composition, the study area is primarily composed of talc schist and mica schist, with the presence of foliated amphibolites, quartzite, and granite also documented [18].
The soil present at the selected sites was identified as sandy loam, with detailed characteristics provided in Table 1. The region experiences two distinct rainy seasons: one from March to July and the other occurring from mid-August to November. Average temperatures in this region typically range between 26°C to 29°C. The annual average rainfall within the area varies between 1400 and 1500 mm, with relative humidity levels fluctuating between 60% and 80%.
| Site | Sand (%) | Silt (%) | Clay (%) | pH (water) | P (mg kg-1) | Ca (cmol kg-1) | Mg (cmol kg-1) | OM (%) | CEC (cmol kg-1) |
|---|---|---|---|---|---|---|---|---|---|
| Epe | 66.2d | 13.6c | 20.2a | 5.9b | 6.5b | 2.53a | 1.27b | 3.4b | 17b |
| Igun | 72.2b | 16.6a | 11.2c | 4.6c | 9.9a | 2.13ab | 1.07c | 2.39d | 13.1d |
| Ijana | 68.2cd | 14.5b | 17.3b | 5.8b | 3.6d | 1.87c | 0.93d | 3.1c | 14.2c |
| Control | 83.2a | 7.6d | 9.2d | 6.5a | 4.9c | 2.24ab | 1.63a | 8.51a | 19.8a |
2.1. Sample Collection and Treatment
A total of thirty soil samples were meticulously collected from distinct gold mining sites situated in the Ilesha region of Osun state. These sites were specifically pinpointed at three locations: Epe, Igun, and Ijana. The collection process involved ten sampling points, each spaced at 100-meter intervals, extending up to a distance of 900 meters from each of these three locations. In addition to these samples, a control sample was procured from the same geographical area.
The soil extraction process was executed using a soil auger, reaching a depth of 25 cm. To facilitate subsequent laboratory analysis, the soil samples from each location were amalgamated to form composite soil samples. These composite samples were subjected to a series of preparatory steps, which included air-drying, followed by grinding into fine particles using a mortar and pestle. Subsequently, the soil particles were sieved through a 2 mm mesh, effectively eliminating any stones and plant materials and rendering them suitable for further analysis.
The air-dried soil samples were employed for specific analytical procedures. To ascertain the textural composition, the hydrometer method was employed, as referenced [19]. The pH levels were determined within a 1:2 soil-water medium. Organic carbon content was assessed through the Walkley and Black method, utilizing dichromate wet oxidation [20]. The organic matter content was then calculated by multiplying the carbon content by 1.724. For the examination of exchangeable bases, a 1M ammonium acetate solution was utilized for extraction, following the procedure outlined in another study [21]. Instrumental analysis was conducted employing a flame photometer. Available phosphorus (P) was extracted using Bray-1 solution and analyzed colorimetrically utilizing the molybdenum blue procedure [22].
The analysis of heavy metals entailed the digestion of 0.5 grams of each soil sample, employing 0.5 mL of H2SO4, 0.6 mL of concentrated HNO3, and 1.8 mL of concentrated HCl. This digestion process occurred at a temperature of 95°C for a duration of 2 hours. Following cooling, the samples were diluted to a final volume of 10 mL using deionized water. The presence of heavy metals, specifically Arsenic (As), Cadmium (Cd), Cobalt (Co), Chromium (Cr), Copper (Cu), Nickel (Ni), Lead (Pb), and Zinc (Zn), was analyzed utilizing a Perkin-Elmer Atomic Absorption Spectrophotometer, model 303. This instrument functions by measuring the concentration of elements within a liquid sample based on their capacity to absorb light at specific wavelengths. It consists of a light source, a monochromator, a flame or furnace, and a detector. The hollow cathode lamp serves as the light source, emitting light at the precise wavelength for the element under examination. The monochromator selects the desired wavelength of light emitted by the lamp. The flame or furnace vaporizes the sample and atomizes its constituent elements, allowing the atoms to absorb light at the specific wavelength emitted by the lamp. The detector then measures the amount of light absorbed by the sample. To determine the concentration of the element within the sample, this measurement is compared to the amount of light absorbed by a blank sample, which does not contain the element being measured. Three samples were measured for each metal, and the mean concentration was calculated.
2.2. Statistical Analysis
Statistical analysis was conducted on the data collected from each experiment using SPSS 17.0. The analysis involved the application of analysis of variance (ANOVA). To assess the differences between treatment means, the standard error of the difference between means (SEDM) was utilized. Any mentions of statistical significance throughout this study imply a significance threshold of p ≤ 0.05, unless explicitly specified otherwise.
3. RESULTS
Table 1 presents the physicochemical characteristics of soil at various locations: Epe, Igun, Ijana, and the control site at the Ilesha area Gold mine. The control has the highest value of sand particles which was significantly different from those of Epe, Igun and Ijana sites. Epe and Ijana sites have similar values of sand. Igun site has the highest value of silt while the control statistically has the lowest value. The values of clay were in the decreasing order of Epe (20.2) > Ijana (17.3) > Igun (11.2) > control (9.2). The soil's pH ranges from slightly acidic (control = 6.5) to acidic Igun (4.6). Epe, Igun and the control have similar values of Ca which were significantly different from that of the Ijana site. Also, the control has the highest values of Mg, OM and CEC. The Igun site has the lowest values of OM and CEC while the Ijana site has the lowest value of Mg.
| Heavy Metal (mg kg-1) | As | Cd | Co | Cr | Cu | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|
| Mean | 4.97 | 3.10 | 4.45 | 5.10 | 10.86 | 4.86 | 4.23 | 9.80 |
| SE± | 2.11 | 1.10 | 2.58 | 2.10 | 6.01 | 1.64 | 1.82 | 1.161 |
| Min | 0.90 | 0.80 | 1.10 | 0.90 | 2.90 | 1.30 | 0.80 | 10.9 |
| Max | 9.10 | 5.10 | 5.90 | 6.70 | 21.80 | 6.60 | 6.90 | 72.1 |
| WHO/FAO | 20 | 3 | 50 | 100 | 100 | 50 | 100 | 300 |
3.1. Cadmium (Cd)
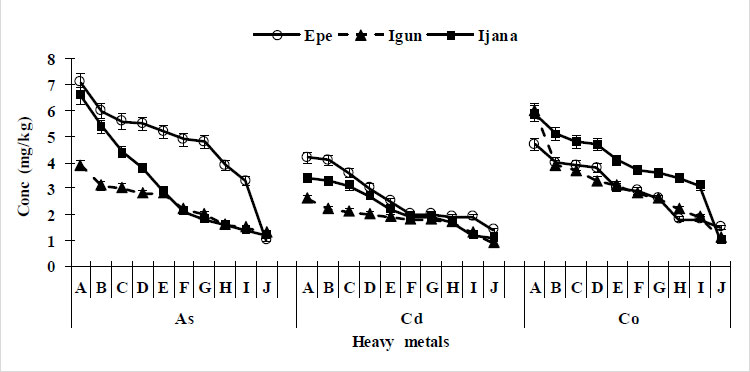
Cadmium levels in the soil exhibited a range of concentrations, spanning from 1.40 to 4.20 mg kg-1 for Epe, 0.8 to 2.6 mg kg-1 for Igun, and 1.10 to 3.40 mg kg-1 for Ijana sites, as depicted in Fig. (1). The respective average values for these three locations were 2.66 mg kg-1, 1.83 mg kg-1, and 2.25 mg kg-1. Notably, the maximum concentrations observed in Epe and Ijana exceeded the permissible soil limit of 3 mg kg-1 recommended by WHO/FAO and the European Union, as documented in Table 2. Specifically, 40% of soil samples from Epe and 30% from Ijana surpassed this threshold.
Across all sites, the concentration of Cadmium in the soil exhibited a decreasing trend as one moved away from the mining sites (0 m) and extended up to 900 m from the source. Additionally, Fig. (1) illustrates that Cadmium concentrations were relatively elevated in the soil of Epe and Ijana when compared to the levels observed at the Igun site.
3.2. Arsenic (As)
Arsenic was found to be present in all sampled locations. The concentrations of arsenic (As) in the soil were relatively low when compared to acceptable limits for agricultural purposes, as outlined in Table 2. Specifically, the As concentrations ranged from 0.90 to 7.10 mg kg-1 in the Epe area, 1.3 to 3.90 mg kg-1 in Igun, and 1.2 to 6.6 mg kg-1 in Ijana. The highest recorded As concentration was observed at the Epe site, as depicted in Fig. (1). Furthermore, it was observed that As levels consistently decreased as the distance from the mining site increased, spanning from 0 meters to 900 meters away.
3.3. Cobalt (Co)
In the case of Cobalt (Co), the concentration range in the Epe region was 1.50 to 4.70 mg kg-1, while both Igun and Ijana exhibited concentrations ranging from 1.10 to 5.9 mg kg-1. The mean Co concentration across all sites was 4.45 mg kg-1, which falls well below the recommended limit of 50 mg kg-1 set by the World Health Organization (WHO), as detailed in Table 2. Notably, the concentrations of Co displayed a gradual decrease as one moved away from the mining site, with the highest concentration observed at the Ijana mining site, as illustrated in Fig. (1).
3.4. Chromium (Cr)
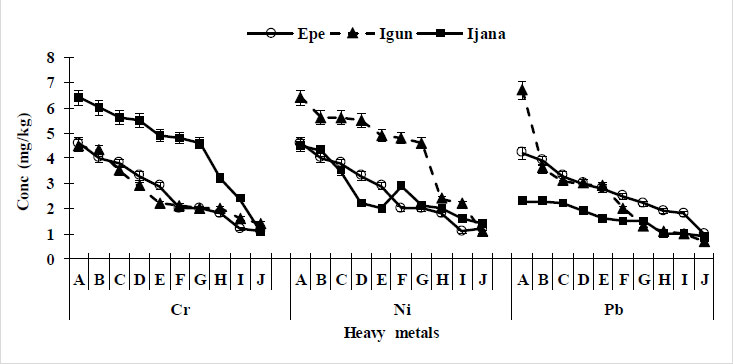
Mean concentrations of Cr in all soil samples were 1.10 – 4.60 mg kg-1 for Epe, 1.40 – 4.50 mg kg-1 for Igun and 0.90 – 6.7 mg kg-1 for Ijana. These values were below the permissible limits of 100 mg kg-1 and 150 mg kg-1 by WHO and European Union, respectively (Table 2). The concentrations decrease gradually along the distance slope across all the regions studied. The highest concentration was found in the Ijana mining site (Fig. 2).
3.5. Nickel (Ni)
Nickel was detected in all sample locations. As distance increases from mining sites the concentration decreases. The mean Ni concentration (4.86 mg kg-1) was within international regulatory agency permissible limits in agricultural and residential soils (Table 2). The values of Ni ranged between 1.30 – 4.60 mg kg-1 for Epe, 1.60 – 4.40 mg kg-1 for Igun and 1.50 – 6.60 mg kg-1 for Ijana. The Ijana site has the highest values of Ni, and the values of Epe and Igun were similar (Fig. 2).
3.6. Lead (Pb)
Lead was detected in all the sample locations. However, the range 1.10 – 4.60 mg kg-1, 0.90 – 2.30 mg kg-1 and 0.8 – 6.9 mg kg-1 for Epe, Igun and Ijana, respectively were below the permissible limit of 100 mg kg-1 by WHO (Table 2) There seems however to be elevated levels of Pb at Epe and Ijana compared with Igun site (Fig. 2), also in all locations, Pb reduces from mine sites (0 m) to 900 m away from the mining site.
3.7. Copper (Cu)
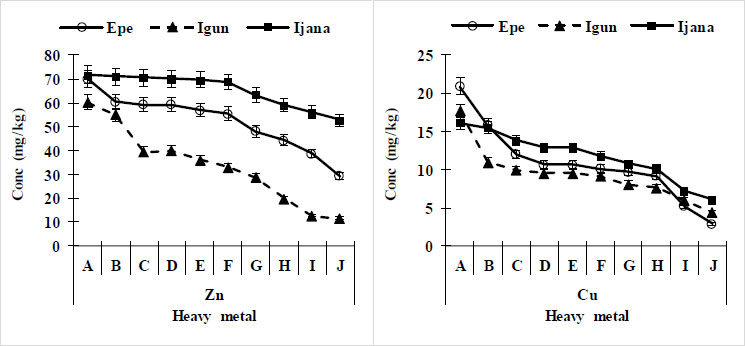
Copper was within the range of 2.9 – 21.8 mg kg-1 with a mean value of 10.72 mg kg-1 for the Epe site. Likewise, 4.4 – 17.7 mg kg-1 was the range of Cu for the Igun site with a mean value of 9.33 while for the Ijana site, Cu has a range of 6.10 – 16.10 mg kg-1 with a mean value of 11.72 mg kg-1. The values of Cu were below the recommended 100 and 140 mg/kg-1 by WHO/FAO and European Union, respectively (Table 2). Cu concentrations in soils for Epe, Igun and Ijana sites reduce from mine sites (0 m) to 900 m away from the mining site. Copper concentrations were higher in the soil at Epe and Ijana compared with the Igun site (Fig. 3).
3.8. Zinc (Zn)
Zinc was detected in all sample locations. The range of Zn for the Epe site was 29.4 – 70.1 mg kg-1. The one for Igun was 10.90 – 60.20 mg kg-1 while Ijana has between 52.80 – 72.10 mg kg-1. The values of Zn in all locations were below the recommended 300 mg kg-1 by WHO (Table 2). Epe (10.72 mg kg-1) and Ijana (11.72 mg kg-1) sites have higher Cu values compared with the Igun site (9.33 mg kg-1) (Fig. 3).
4. DISCUSSION
The soil samples collected from the sites exhibited an acidic pH level, which can be attributed to the leaching of base elements such as Calcium (Ca) and Magnesium (Mg) due to the substantial rainfall in the rainforest environment (approximately 1400 – 1500 mm per annum). The acidic conditions of the soil significantly favor the mobility of trace elements. Under acidic pH, the trace element cations become more soluble and mobile [23]. This phenomenon occurs because protons in acidic solutions efficiently neutralize the negative charges on the soil particles' surfaces, thereby releasing the trace elements from their binding sites. Additionally, in acidic soils, there is often a lower organic matter content. Organic matter can bind to trace elements and immobilize them within the soil. When the organic matter content is low, the trace elements are more likely to remain in the soil solution and be mobile. A similar report of soil acidity was documented around a mining and smelting industry in Spain [24] and Alloway [25].
Cadmium concentrations in the soil from the Epe, Igun, and Ijana sites were measured to be in the ranges of 1.40 – 4.20 mg kg-1, 0.8 – 2.6 mg kg-1, and 1.10 – 3.40 mg kg-1, respectively. It was observed that the maximum values at the Epe and Ijana sites exceeded the permissible limit of 3 mg kg-1 for soil, as recommended by WHO/FAO and the European Union.
The elevated levels of Cadmium in the Epe and Ijana sites were likely due to their proximity to gold smelters, with distances of approximately 300 m for Epe and 200 m for Ijana, from where the samples were taken [2]. Cadmium is a heavy metal commonly found in gold ores and is a byproduct of gold mining. Consequently, it can enter the environment through various pathways, including (1) Tailings: After gold ore has been processed, the remaining waste products, known as tailings, may contain high concentrations of Cadmium. When these tailings are not properly disposed of, they can contaminate soil, water, and air. (2) Acid mine drainage: This form of water pollution arises from the oxidation of sulfide minerals in mine tailings, leading to the release of Cadmium and other harmful substances. (3) Gold recovery methods: Certain gold recovery techniques, like amalgamation, can also release Cadmium into the environment. In a separate study conducted by Ekwue et al [26] in Osun State, Nigeria, high concentrations of Cadmium ranging from 4.4 to 7.6 mg kg-1 were detected in soils from abandoned secondary and primary goldmines. It is crucial to address and monitor Cadmium pollution resulting from gold mining activities to protect the environment and public health, especially in areas with gold smelters and abandoned mining sites. Implementing proper waste management practices and adopting environmentally friendly gold recovery methods can help mitigate the release of Cadmium and other toxic substances into the surroundings.
In this study, the Cu levels were found to be elevated, although still below the permissible limit set by WHO/FAO. These findings align with previous research by Ayantobo [16], who also reported elevated Cu levels in water within a 1 km radius of a gold mining site at Igun. Similarly, Ekwue et al. [26] found elevated Cu levels ranging from 27 to 40 mg kg-1 around gold mine sites.
Across all locations, there were significant positive linear correlations between soil Pb and other heavy metals such as As, Cd, Co, and Ni (R = 0.894, 0.999, 0.914, 0.946, respectively, at P < 0.05). These correlations suggest that soil contamination from gold mining activities contributed to elevated concentrations of Pb in top soils.
The concentration of Zn varied across the study sites, with values ranging from 29.4 to 70.1 mg kg-1 at Epe, 10.70 to 60.20 mg kg-1 at Igun, and 52.80 to 72.70 mg kg-1 at Ijana. These values were similar to those reported by Jinadasa et al. [27] for the Greater Sydney Region, Australia, where Zn levels ranged from 8 to 196 mg kg-1 with a mean of 51 mg kg-1. The presence of Zn in all sample locations could be attributed to both natural occurrences in soil, with about 70 mg kg-1 in rocks, and anthropogenic pollution resulting from gold mining activities.
The study revealed that heavy metal concentrations in soil decreased with distance from the mining sites (0 m) up to 900 m away. This trend indicates that heavy metal contamination of soil could extend up to 900 m from the mining site, suggesting that the gold mine was the main source of pollution.
Furthermore, the results indicated higher concentrations of heavy metals in soils at Epe and Ijana compared to Igun. This difference can be attributed to the textural classes of the three locations. The higher clay content in the Epe and Ijana sites likely created strong adsorptive sites for the metals, allowing the soil to immobilize the introduced heavy metal ions. The slightly higher soil pH at the Epe and Ijana sites compared to Igun could also be responsible for the higher values of heavy metals in those locations. Previous studies have reported that the mobility and availability of Cr tend to increase with soil pH, leading to the reduction of soluble Cr (VI) to dissoluble Cr (III) under acidic conditions [28]. Additionally, a positive correlation between available Cr in soils and pH values has been reported [29].
This experiment is limited by the fact it only looked at a limited number of heavy metals. It is possible that other heavy metals could also be present in the soil at elevated levels. Furthermore, a potential bias could be suspected in this study due to the fact that it only looked at 30 sites in the Ilesha gold mine area, which may not be representative of all the sites in the area. It is possible that the sites that were selected had higher levels of heavy metals than other sites in the area. Again the study used soil samples to measure heavy metal levels. However, soil samples can be contaminated by other factors, such as agricultural runoff or industrial pollution. It is possible that the soil samples in this study were contaminated by factors other than mining.
CONCLUSION
A study examined heavy metal levels (Arsenic - As, Cadmium - Cd, Cobalt - Co, Chromium - Cr, Copper - Cu, Nickel - Ni, Lead - Pb, Zinc - Zn) at 30 sites in the Ilesha gold mine area, Osun State, Nigeria (Epe, Igun, Ijana). Soil samples were compared to controls and environmental guidelines. Soil particle size (clay) and pH influenced metal concentration. The nearest areas had the highest concentrations, decreasing with distance, indicating the mine's role in pollution. The study revealed heavy metal contamination in soils, mostly within limits set by WHO, except Cadmium. In conclusion, mining significantly impacted metal pollution in tested sites. Most metal levels met WHO and FAO limits, except Cadmium. The importance of managing mining-induced metal pollution is highlighted for environmental and human health protection. However, further studies are suggested for other similar locations in other to affirm or otherwise the findings in this work.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


