All published articles of this journal are available on ScienceDirect.
Biobutanol Production Using Non-grain Biomass Sorghum saccharatum as a Substrate
Abstract
Background
The global energy challenge has recently prompted biotechnological research to explore new non-food substrates of plant origin for obtaining liquid biofuels. One of the important areas of research is the process of pretreatment and further use of non-grain biomass (lignocellulose) as a substrate for bioconversion to higher alcohols.
Objective
The aim of this work was to determine the macrocomponent composition and biochemical characteristics of sweet sorghum [Sorghum saccharatum (L.) Moench], select an effective bacterial culture for fermentation of the non-grain part of sorghum biomass as a substrate for obtaining biobutanol, and elaborate the best protective medium and storage temperature for lyophilization of the producer.
Methods
This work was conducted using butanol producing strains Clostridium sp. UCM B-7570, Clostridium acetobutylicum UCM B-7407, and C. tyrobutylicum IFBG C4B from the “Collection of Microorganism Strains and Plant Lines for Agricultural and Industrial Biotechnology” of the Institute of Food Biotechnology and Genomics of the National Academy of Sciences of Ukraine. The bacterial cultures were cultivated on the sweet sorghum biomass provided by the National Botanical Garden named after M. M. Gryshko of the National Academy of Sciences of Ukraine. A gas chromatograph was used to determine the presence of ethanol, acetone, and butanol in the cultural liquid.
Results
It has been established that the proposed improvement of the biobutanol production process made it possible to obtain 8 g/dm3 of the target product from 60 g of dry green biomass of sweet sorghum of the Energodar variety. The composition of the protective medium for drying the Clostridium sp. UCM B-7570 culture and its storage period in the lyophilic form have been optimized.
Conclusion
The obtained results demonstrate the possibility of using the biomass of different varieties of sweet sorghum as a substrate for obtaining biobutanol, and the optimized storage conditions of the Clostridium sp. UCM B-7570 culture can minimize the possibility of its degradation.
1. INTRODUCTION
Liquid biofuel production, primarily ethanol and butanol, has received increasing attention from renewable sources based on plant biomass in the last few years [1]. The industrial production of butanol at the beginning of the 20th century was based on the fermentation of carbohydrate raw materials (corn flour) by Clostridium acetobutylicum bacteria with the production of mainly acetone and butanol [2]. The increasing demand for butanol and the sharp growth of petrochemical production have led to the biotechnological approach for obtaining butanol to become unprofitable and it was replaced by a more efficient chemical synthesis [3]. Recent years have witnessed a renewed interest in the microbiological method of obtaining butanol not only as a raw material for the chemical industry, but also as an alternative fuel [4].
Similarly to bioethanol, biobutanol can be obtained from biomass in several ways: by fermentation of vegetable raw materials that contain sugar or starch (biobutanol of the first generation) and by processing lignocellulosic raw materials (biobutanol of the second generation) [5]. Acetone-butanol-ethanol (ABE) fermentation is a process of biobutanol production from biomass using clostridia that has identical characteristics to butanol obtained by chemical synthesis [6]. The high cost of substrates, such as starch (corn, wheat, millet, etc.) and sugar (molasses, etc.), is the main reason affecting the economics of the ABE process [7]. These circumstances and some characteristics of saccharolytic clostridia to use different carbohydrates have stimulated research into the use of alternative substrates of plant origin [8].
Sweet sorghum [Sorghum saccharatum (L.) Moench] is a fodder, technical, and energy crop, quite undemanding for both climatic conditions (drought) and soil composition (salinity), and is widely distributed in many countries of the world [9-12]. Sweet sorghum can grow in the steppe and forest-steppe zones, and recently, the area sown in the northern region of Ukraine has also increased [10-12]. This plant is capable of producing high yields even in adverse conditions, i.e., up to 40–60 tons of green mass from 1 ha with a sugar content of up to 16–18% [12]. In the process of sweet sorghum processing, juice is obtained, and the stems and leaves are used in fodder or for the production of building materials and paper [13]. Sweet sorghum is used as a grist for various purposes. Firstly, in the food industry, up to 4 tons of sugar syrup can be obtained from one hectare [14]. Secondly, the green mass of plants is used in the preparation of feed: up to 25,000 feed units can be obtained from one hectare [15]. Thirdly, biofuel can be obtained from non-cereal biomass, namely bioethanol (up to 6.0 t/ha ~ 35.7 Gcal/ha), solid biofuel (up to 25 t/ha ~ 95.3 Gcal/ha), biogas (up to 17.6 thousand m3/ha ~ 90.8 Gcal/ha), and high-quality organic fertilizer [12].
This is why, the aim of this work was to investigate the macrocomponent composition and biochemical characteristics of sweet sorghum, select an effective bacterial producer for fermentation of the non-grain part of sorghum biomass, and determine the protective medium and storage terms of the producer's culture under freeze-drying conditions.
2. MATERIALS AND METHODS
2.1. Bacterial Strains, Plant Biomass, and Cultivation
Clostridium sp. UCM B-7570, C. acetobutylicum UCM B-7407, and C. tyrobutylicum IFBG C4B were used for the research as the strains for butanol production. They were obtained from the “Collection of strains of microorganisms and plant lines for agricultural and industrial biotechnology” of the Institute of Food Biotechnology and Genomics of the National Academy of Sciences of Ukraine (IFBG). Juice, bagasse, and non-grain biomass of sweet sorghum (S. saccharatum (L.) of the Energodar variety (NBS, IFBG), hybrid AMBR-1 (National Botanical Garden named after M.M. Gryshko of the National Academy of Sciences of Ukraine (NBS). IFBG, hybrid-720 (Alta Seeds Advanta US), and hybrid ST-207 (RAGT Semences) were obtained from raw materials, collected from plants grown on the introduction and breeding plots of NBS (Fig. 1).

Juice and bagasse of pasty consistency were obtained from the green mass of sugar sorghum using a juicer press (Philips, HR 1945/80, the Netherlands). Sorghum biomass was dried at 30°C for seven days. A weight moisture analyzer RADWAG MA 50/C/1 (Poland) was used to determine the moisture content of the raw materials. A laboratory mill “Zyklon MSh 1” (Ukraine) was used for biomass grinding to a particle size of 200 mesh. An inoculation medium was used, as described earlier by Tigunova et al. [8]. 5% inoculum by volume was used as an initial concentration. Dry biomass with a concentration of 60 g/dm3, bagasse with a concentration of 60 g/dm3, or sorghum juice were used as an enzymatic medium. We used 1 atm pressure for sterilization of biomass in 30 min and 0.5 atm for juice in 30 min. Clostridium sp. UCM B-7570, C. acetobutylicum UCM B-7407, and C. tyrobutylicum IFBG C4B were cultivated in flasks with a liquid medium or on Petri dishes in an aerostat “Crystal” (Germany) at 35±1°C in a nitrogen atmosphere. After cultivation (120 h after the start of fermentation), ultracentrifuge “Labofuge 400R” (Germany) was used for the precipitation of the cells for 30 min at 13,000 rpm. Fermentation was carried out in 100 cm3 flasks using 60 cm3 medium. The flasks were thermostated at 35°C after weighing. Colonies of the Clostridium sp. UCM B-7570 cultures were scanned using a Carl Zeiss 2000-C microscope (Germany). Photos were taken with a Canon PowerShot A640 camera (Japan).
2.2. Biomass Pretreatment
Organosolv preparation of the medium was carried out with acetone (KhimreZ, Ukraine) in 200 cm3 flasks with a reflux condenser. The resulting mass (60 g) was placed in a flask, and 100 cm3 of an aqueous solution of acetone (50 cm3 of water and 50 cm3 of acetone) and 0.1% sulfuric acid as a catalyst were added and boiled for 60 and 90 min. The macrocomponent composition of the non-grain part of sorghum biomass was determined as described by Tigunova et al. [4]. An explosive autohydrolysis of raw materials was performed in a specially prepared laboratory installation. The raw material was treated with saturated steam at a temperature within 180-260°C and a pressure of 2 atm, which was followed by a sharp drop in atmospheric pressure, as a result of which the lignocellulosic raw material was separated (defibered) [4].
Enzymatic hydrolysis of previously prepared biomass was done using the enzyme complex of cellulases from Trichoderma reesei ATCC 26921 (Sigma, USA), cellobiases from Aspergillus niger (Sigma, USA), and β-glucosidases under the conditions recommended by the manufacturer: the temperature 50°C and pH 5.0 (recommended range 50–65°C, pH 4–5). Alkaline hydrolysis was performed using a 1% NaOH solution at 100°C. Acid hydrolysis was carried out at 100°C with 1% H2SO4. After hydrolysis, the neutral pH value of the medium was maintained by adding chalk or HCl [16].
2.3. Solvent Investigation
Gas chromatography with a flame ionization detector (FID) and column with Chromosorb Carbovax (Merk, Germany) 6000 2.4 m × 3 mm were used for the determination of ethanol, butanol, and acetone contents in the culture liquid. The temperature in the column was 80±5°C, and in the evaporator, it was 140±10°C. The ratio of nitrogen-hydrogen-air flow was 1:1:10. To determine the concentration of butanol in the culture liquid, the calibration graph was built using different concentrations of butanol in distilled water.
2.4. Lyophilization Process
The effect of the protective medium on the viability of cells of the butanol-producing strain Clostridium sp. UCM B-7570 in the process of lyophilization was studied using the protective media of the following composition (%): glucose or sucrose - 1.0, 10.0, 30.0; gelatose - 10.0; agar - 0.2. Bacteria were added to the protective medium at a cell concentration of 4x106 cells/cm3, and then a suspension of 5 cm3 was added to vials with a volume of 25 cm3. The obtained samples were frozen in a low-temperature refrigerator “LAB 11/EL19LT” (“Elcold”, Denmark) at -80°C. The frozen samples were transferred in special cassettes to the pre-cooled chamber (condenser temperature –50°С) of the CRUODOS-50 lyophilic dryer (TELSTAR, Spain). The drying lasted for 72 h. To recover the living cells from anabiosis, distilled water was added to the lyophilized material up to the volume of 5 cm3 and kept at room temperature for 0.5 h.
2.5. Statistical Analysis
All experiments were performed in three replications. Statistical processing of experimental data was carried out using the Microsoft Excel program. The authors used statistical tests and algorithms built into the Microsoft Excel program and based on Student's criteria. The difference between the two mean values was considered significant at p<0.05.
3. RESULTS
3.1. Characteristics of Plant Biomass
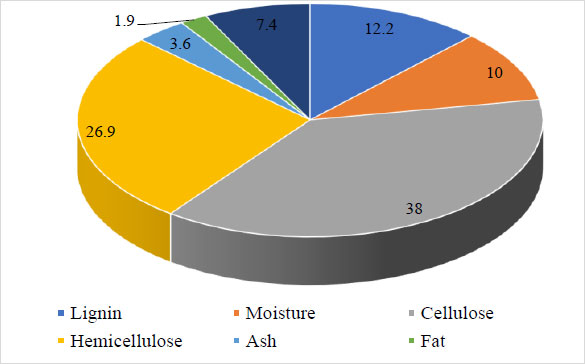
In the first stage, before further use of biomass as a substrate, the macrocomponent composition of sweet sorghum biomass was determined (Fig. 2).
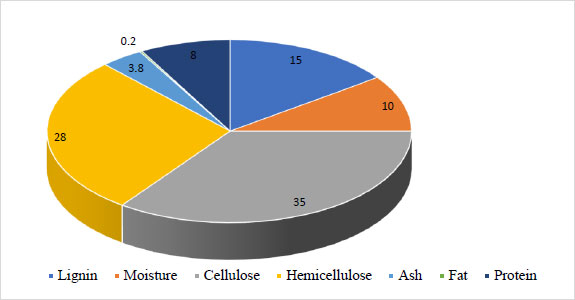
The largest part of sorghum biomass was determined to consist of cellulose (38.0%) and hemicelluloses (26.9%), which can be used as a substrate in the future. The lignin part (12.2%), which cannot be decomposed by microorganisms, was determined. In order to comprehensively study the prospects of using sweet sorghum as a raw material for the production of butanol, the juice was prepared (which is used as a substrate in the microbiological industry), and bagasse was discarded. The macrocomponent composition of sweet sorghum bagasse was also studied (Fig. 3).
It has been established that, unlike biomass, the sweet sorghum bagasse contains more hemicellulose (28.0%) and lignin (15.0%), but less cellulose (35.0%). The chemical composition of sweet sorghum juice obtained by pressing the green mass is presented in Table 1. The obtained results indicated that sorghum juice contains a sufficient amount of trace elements and amino acids essential for microorganisms [17], as well as a certain amount of sugars suitable for bioconversion. Thus, based on its technological characteristics, sweet sorghum juice can be considered a suitable raw material for bioconversion.
| Indicator | Value |
|---|---|
| Concentration of dry substances, % | 17,6 ±0,2 |
| The content of fermentable sugars: in all, % including saccharose, % fructose, % glucose, % other monosaccharides, % |
15,3±0,2 9,4±0,5 1,2±0,2 2,5±0,3 1,9±0,2 |
| Juice рН | 5,0±0,01 |
| Total nitrogen content, % | 0,08±0,01 |
| Phosphorus content (in terms of Р2О5), % | 0,004±0,0005 |

3.2. Strain Screening
The strains of Clostridium sp. UCM B-7570, C. acetobutylicum UCM B-7407, and C. tyrobutylicum IFBG C4B were cultivated using juice, bagasse, and non-grain biomass of sweet sorghum as a substrate. The resulting accumulation of butanol is presented in Fig. (4).
The obtained data showed that the lowest accumulation (0.5 g/dm3) of butanol in the culture liquid was obtained using biomass of sweet sorghum milled to 200 mesh and strain IFBG C4B. The highest yield of butanol (8.2 g/dm3) was obtained using the native juice of sweet sorghum and the strain C. tyrobutylicum UCM B-7407. Pretreatment of the substrate affects the accumulation of the final product [17]; therefore, at the first stage of biomass preparation, sweet sorghum (Energodar variety, hybrid AMBR-1, hybrid-720, and hybrid ST-207) was dried to a constant moisture (7%) and milled.
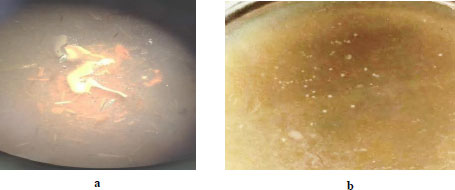
To explore the effectiveness of a culture of Clostridium sp. UCM B-7570 to use sweet sorghum biomass (Energodar variety, hybrid AMBR-1, hybrid-720, and hybrid ST-207) as a substrate, it was cultivated by the deep method [4] in Petri dishes with agar medium (Fig. 5).
The obtained results indicated that the UCM B-7570 strain forms colonies in all agar media. Characteristic lightening zones and accumulation of gas bubbles were formed around the colonies, which indicates that the UCM B-7570 culture can be used for the bioconversion of sweet sorghum biomass. The colonies changed their shape from a biconvex lens [4] to amoeba-like ones when grown in a layer of agar. This may be possibly due to the difficulty in the availability of the substrate (Fig. 5a). However, the colonies remained unchanged (Fig. 5b) under the conditions of cultivation on the agar surface in anaerobic conditions. After transferring the UCM B-7570 culture to a liquid medium, its fermentation activity did not change; therefore, the culture did not degrade and was capable of accumulating solvents in the culture liquid.
3.3. Butanol Production
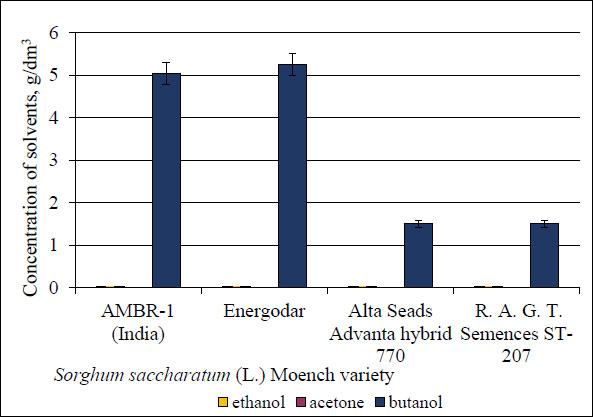
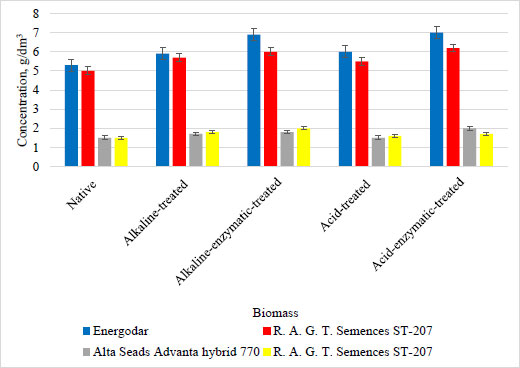
To study the possibility of butanol accumulation in a liquid medium, the colonies were transplanted from the agar medium to the created enzymatic medium, which contained 60 g/dm3 of each sweet sorghum genotype (Energodar variety, hybrid AMBR-1, hybrid-720, and hybrid ST-207) and the culture was planted UCM B-7570 with the subsequent accumulation of solvents in the culture liquid (Fig. 6).
The highest level of butanol accumulation in the culture liquid occurred under the conditions of using sweet sorghum biomass of Energodar variety and hybrid AMBR-1 (5.2 and 5 g/dm3, respectively). However, hybrid-720 and hybrid ST-207 also accumulated butanol, but at lower levels (1.5 g/dm3 in both cases). To increase the bioconversion [18], pretreatment of the non-grain part of sweet sorghum biomass was carried out using alkaline, acid, alkaline-enzymatic, and acid-enzymatic hydrolysis. The obtained results of butanol accumulation under the conditions of cultivation of UCM B-7570 and pretreated non-grain biomass of sweet sorghum (Energodar variety, hybrid AMBR-1, hybrid-720, and hybrid ST-207) are presented in Fig. (7).
It has been established that cultivation of the UCM B-7570 strain on the biomass of four sweet sorghum genotypes, but with different types of pretreatment, can result in higher levels of butanol accumulation when using several types of preliminary treatment. It was determined that the highest accumulation of butanol was obtained during the acid-enzymatic hydrolysis of Energodar sweet sorghum biomass and was 7 g/dm3 (with the initial biomass of 5.3 g/dm3). The obtained results have indicated the Energodar sweet sorghum biomass to be the most promising for obtaining butanol under both the conditions of using the raw material and after its hydrolysis.
3.4. Improvement of Butanol Production using Energodar Biomass
To increase the output of biobutanol, Energodar variety sweet sorghum biomass was pretreated with various methods of hydrolysis: organosolv (for 60 and 90 min) and thermobaric (or explosive autohydrolysis). The resulting component composition of the raw material is presented in Table 2.
| Pretreatment | Component Composition, % | ||||||
|---|---|---|---|---|---|---|---|
| Ash | Fats, Resins | Water Solutions | Cellulose | Lignin | Hemicelluloses | Humidity | |
| Nature | 3,6 | 1,6 | 16,9 | 38 | 13 | 26,9 | 10 |
| Thermobaric | 3,42 | 1,71 | 17,5 | 39,9 | 9,83 | 17,64 | 10 |
| Organosolv 60 min | 2,52 | 1,7 | 11,9 | 42,42 | 10,92 | 20,54 | 10 |
| Organosolv 90 min | 1,65 | 1,6 | 6,8 | 42,54 | 8,19 | 29,22 | 10 |
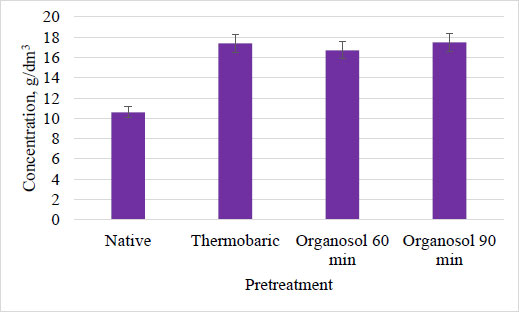
The results of the study have revealed differences in the component composition of sorghum biomass after different types of hydrolysis. The amount of cellulose increased, which can be explained by the degradation of crystallinity under the influence of hydrolytic factors and breaking of lignin-cellulose bonds, as well as hemicellulose complexes, and the decrease in the percentage of residual lignin as a result of destruction during pretreatment. The duration of hydrolysis significantly affected the component composition, reducing the content of water-soluble substances that could not withstand the harsh conditions of hydrolysis and increasing the percentage of hemicelluloses due to the probable release of lignin-hemicellulose complexes. Enzymatic hydrolysis was carried out after different ways of biomass preparations. The total accumulation of sugars is presented in Fig. (8).
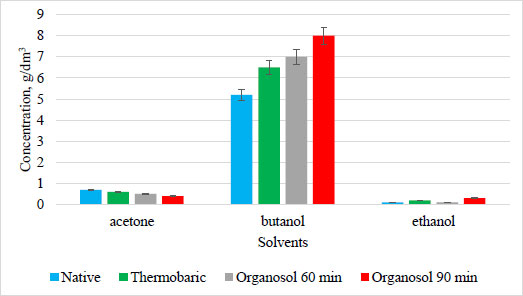
The highest level of accumulation of sugars was obtained under the conditions of enzymatic hydrolysis of sorghum biomass, which was pretreated thermobarically and via organosolv process for 90 min. The obtained results indicated that the availability of the substrate for enzymes increased during the pretreatment of biomass. It was shown that each type of hydrolysis of Energodar sweet sorghum biomass resulted in a sugar concentration of 17 g/dm3 after enzymatic treatment. Cultivation was carried out using organosolv and thermobaric hydrolysis of Energodar biomass, and the accumulation of solvents is shown in Fig. (9).
The highest level of butanol accumulation (8 g/dm3) by strain UCM B-7570 in the culture liquid was obtained using Energodar variety sweet sorghum biomass following the organosolv pretreatment for 90 minutes. Besides, it was established that different types of hydrolysis for the pretreatment of biomass increased the accumulation of butanol.
3.5. Strain Lyophilization
The stability of the strain and its properties are important for the accumulation of butanol. The freeze-drying method was used to store the UCM B-7570 strain. The effect of various factors of the protective medium on the residual moisture of the freeze-dried suspension of UCM B-7570 culture was studied. It was found that the content of residual moisture in lyophilized cultures depends on the type of cryoprotectant and its concentration. The lowest indicators of residual moisture were obtained under the conditions of using glucose or sucrose at a concentration of 10%. The storage time and the temperature are important for freeze-dried samples of the UCM B-7570 strain. It was found that following the storage of lyophilized producers for one month at 4°C, the productivity of crops did not decrease compared to the initial parameter (2.7 g/dm3). The subsequent storage of lyophilized cultures was carried out at 4°C for six months, with monthly testing of the accumulation of butanol by the strain UCM B-7570 using Energodar sweet sorghum biomass. The obtained results indicated that after storage at 4°C for six months, the reconstituted lyophilized culture of UCM B-7570 was viable and the accumulation of butanol in the culture liquid after cultivation practically did not change compared to the accumulation before lyophilization (2.7 g/dm3).
4. DISCUSSION
Sweet sorghum has a large number of different varieties and hybrids that are grown in the world and each differs from the other both morphologically (height and diameter of stems, panicle shape, grain colour) and in terms of grain ripening period and its quality [19]. A whole gene pool of S. saccharatum (L.) Moench has been created in the NBS, which is characterized by early maturity, drought resistance, high yield of phytomass, high content of carbohydrates in the above-ground mass, and yield of bioethanol [12]. The core of the stem of sweet sorghum contains sweet juice with a different composition of sugars: sucrose, glucose, and fructose [20]. For this reason, the most promising varieties and hybrids of the NBS with these parameters were selected: Energodar variety, hybrid AMBR-1, hybrid-720, and hybrid ST-207. Sweet sorghum juice, syrup, or bagasse are used for microbiological production [21]. Therefore, the above-mentioned substances were used in our study.
An integral indicator that characterizes the efficiency of growing sweet sorghum for energy purposes is the output of biofuel (bioethanol, biogas, solid biofuel) and the output of energy [22, 23]. Ethanol is obtained using sweet sorghum juice at an industrial scale [24], and correlative data have been obtained in our study. Juice from sweet sorghum stalks is mainly obtained using a roller press [25]; therefore, we used a juicer with a similar mechanism. Production of butanol using sweet sorghum as a raw material is mainly at the stage of development [26], which shows the relevance of our research.
It is known that pretreatment increases the bioconversion of lignocellulosic raw materials [27], the main stage of which is grinding [28], as also noted in our study. This can be explained by the partial cleavage of cellulose and the formation of an additional amount of fermentable sugars [29].
The next stage is the stage of temperature treatment of lignocellulosic biomass to release sugars [30]. There are various approaches for preliminary biomass preparation [31], such as organosolv [32, 33], using alkalis [34], diluted acids [35, 36], ionic liquids [37-39], high-frequency heating [40], treatment with hot steam [41, 42], lime [43], or ammonia [44]. Our research focused on the use of the most promising methods and their comparison, namely, pretreatment using organosolv treatment or thermobaric autohydrolysis. With the use of such types of pretreatment, the yield of sugars can be increased, the amount of insoluble lignin can be decreased, and the content of cellulose, which is easily split, can be increased. For further bioconversion, a stage of biomass hydrolysis to pentose or hexose sugars is carried out. Hydrolysis, as a rule, occurs with the use of alkalis, acids, or enzymes. Enzyme activity depends on temperature, the concentration of water ions, and the presence of an activator and inhibitor. The detailed mechanism of enzymatic hydrolysis of lignocellulose is still unknown, although significant progress has been made in studying the lignocellulolytic genes of microorganisms involved in enzymatic hydrolysis. The highest level of accumulation of sugars was obtained under the conditions of enzymatic hydrolysis of sorghum biomass, which was pretreated thermobarically and via organosolv technique for 90 min. The obtained results have indicated the availability of the substrate for enzymes to be increased during the pretreatment of biomass.
We have studied the possibility of using domestic producer strains for the production of butanol from different components of sweet sorghum biomass and established that butanol accumulation depends on which sorghum varieties have been used as a substrate. The use of the UCM B-7570 strain and Energodar sweet sorghum biomass as a substrate opens prospects for the development of effective biotechnology for the production of butanol in comparison to the existing ones [45]. Long-term storage of bacterial cultures is a major challenge in the way of microorganism collections and industrial microbiological production. The observed losses in the viability of bacteria and their physiological properties are most likely caused by changes in the composition of the population under the influence of various physicochemical factors [46]. Biological materials are usually stored following lyophilic drying [47]. The main challenge in preserving collections of microorganisms is the problem of instability. When stored on artificial nutrient media, microorganisms quickly dry out, die, reduce, or lose their physiological and enzymatic properties. The necessity for long-term preservation of microorganisms and various materials of biological origin arose at the beginning of the development of microbiological science. In our work, the composition of the protective medium has been optimized, making it possible to store strains with minimal degradation.
The complex process of the metabolic pathway of butanol and degradation of lignocellulose can significantly burden the fermentation of lignocellulosic raw materials, thereby leading to low butanol production using monocultures. In nature, lignocellulosic biomass is completely decomposed and assimilated by microbial consortia containing fungi and bacteria. In addition to sweet sorghum, we have previously investigated other types of non-grain lignocellulosic biomass, which is the most widespread in Ukraine: rye, rapeseed, sunflower, corn, wheat, and barley [47]. It has been shown that pretreatment of vegetable raw materials is extremely important for the production of biofuel. Obviously, this technology is an additional step to the existing processes of preliminary preparation of raw materials with minimal technological changes. Subsequently, components of lignocellulosic biomass can serve as a substrate for the microbiological conversion of microorganisms of the genus Clostridium for the production of biobutanol [16].
CONCLUSION
The conducted studies have shown the biomass, juice, and bagasse of sweet sorghum to be converted by strains producing butanol of Clostridium sp. UCM B-7570, C. acetobutylicum UCM B-7407, and C. tyrobutylicum IFBG C4B, but butanol accumulation has been found to depend on the substrate and its pretreatment. Screening of strains of Clostridium sp. UCM B-7570, C. acetobutylicum UCM B-7407, and C. tyrobutylicum IFBG C4B has shown the greatest accumulation of butanol in the culture liquid using the UCM B-7570 strain and milled sweet sorghum biomass (1.5 g/dm3) as a substrate and strain UCM B-7407 and juice (8.2 g/dm3). It was found that optimizing the conditions of pretreatment of raw materials increased the accumulation of butanol by 25-50%. We have shown that sweet sorghum biomass (Energodar variety, hybrid AMBR-1, hybrid-720, and hybrid ST-207) can be used for butanol accumulation without strain degradation. Under the conditions of using the Energodar variety and hybrid AMBR-1, the producer strain Clostridium sp. UCM B-7570 accumulated the most butanol (5 g/dm3). A combination of acid and enzymatic hydrolysis was determined as the optimal pretreatment for the Energodar sweet sorghum biomass, specifically, hybrid AMBR-1, hybrid-720, and hybrid ST-207. It has also been shown that the optimization of the technological parameters of cultivation, namely, pretreatment with the help of organosolv hydrolysis, made it possible to obtain 8 g/dm3 of biobutanol from 60 g of green biomass of Energodar sweet sorghum. The composition of the protective medium was optimized, and it was observed that the storage of lyophilized bacteria samples for six months at a temperature of 4°C did not affect the productivity of the strains. The obtained results have indicated the possibility of using different varieties of sweet sorghum as a substrate for the production of biobutanol [48].
ABBREVIATION
| FID | = Flame Ionization Detector |
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
All the data and supportive information are provided within the article.
FUNDING
This study was conducted within a project funded by the National Academy of Sciences of Ukraine with No. 0119U101489 and project “Intensification of butanol accumulation using various plant raw materials as a substrate and the domestic producer strains” of the National Academy of Sciences of Ukraine (State registration no. 0120U101706).
CONFLICT OF INTEREST
Dr. Yaroslav B. Blume is the Editor-in-Chief of the journal The Open Agriculture Journal.
ACKNOWLEDGEMENTS
Declared none.


