All published articles of this journal are available on ScienceDirect.
The Use of PGPB-based Bioformulations to Control Bacterial Diseases of Vegetable Crops in Ukraine
Abstract
Ukraine is one of the largest producers of vegetable products in the world. The sustainable development of this industry in Ukraine is vital for the food security of many countries worldwide. Recently, farmers in Ukraine are facing the challenge of increasing the number and severity of bacterial diseases. This problem is getting particularly harsh in the production of vegetables. The changing climatic conditions in many regions contribute to the increase of the aggressiveness of bacterial pathogens. Ukraine is also experiencing the negative consequences of rising temperatures, changes in the amount and quality of precipitation, and stronger winds. These factors facilitate the changes of stable regions of the spread of bacterial pathogens. In Ukraine, they result in the emergence, successful acclimatization and spread of new bacterial pathogens of vegetable crops, in particular, Ralstonia solanacearum. The growing risk of bacterial diseases in vegetable production requires the development of new strategies to control their causative agents, which should, at the same time, meet the requirements of environmental safety. This paper is aimed to analyse the potential of plant growth-promoting bacteria (PGPB) based bioformulations to control bacterial diseases of vegetable crops in Ukraine. Farmers in Ukraine, who are engaged in growing vegetables, feel lack of biocontrol compositions against the causative agents of bacterial diseases because the range of biocontrol agents with antibacterial activity approved for use in the country is limited. The most commonly used plant protection products in Ukraine are represented by the preparations based on bacteria of the genera Bacillus and Pseudomonas. Most of such products on the market are those developed and manufactured in Ukraine. Under given circumstances, the use of inoculants based on PGPB to control bacterial diseases of vegetable crops in Ukraine, as well as globally, seems very promising. Such biocontrol agents prevent contamination of plants with phytopathogens by inducing acquired systemic resistance and stimulating their growth and better productivity. Seed inoculation is a promising way of using PGPB in crop production. The market of bioformulations for vegetable farming in Ukraine is represented by products with a limited shelf life. It still experiences a shortage of up-to-date preparation forms that would ensure the long-term viability of PGPB and a prolonged activity of the compositions based on them. Thus, the development and introduction of encapsulated PGPB nanopreparations may contribute to solving the problem of biological control of pathogens of bacterial diseases of vegetable crops in Ukraine.
1. INTRODUCTION
According to the State Statistics Service of Ukraine, UBTA (https://www.ukrstat.gov.ua), Ukraine has a leading position in the world in terms of natural resources and agricultural potential. Vegetables account for 0.5% of Ukrainian agricultural exports. Over the recent years, Ukraine exported vegetables to 96 foreign markets. The total planted area under the vegetables in 2022 was 3320.1 thousand ha, of which 1283.1 thousand ha were allocated for potatoes, 452.8 thousand ha were used to grow open ground vegetables (tomatoes, cabbage, cucumbers, onions etc.), 46.2 thousand ha were allocated for melon crops and 1538 thousand ha for fodder vegetables. In terms of regions, the largest planted areas with vegetables were in the Polissia and forest-steppe zones: Zhytomyr, Vinnytsia, Lviv, Chernihiv, Kyiv, Poltava, Rivne, Khmelnytskyi, Kharkiv and Volyn regions. Vinnytsia, Zhytomyr, and Lviv regions have been the three leading regions in terms of potato acreage (23% of the total acreage). It is noteworthy that western regions of Ukraine predominate in terms of potato-planted areas, which indicates a trend of territorial shift of vegetable production over the recent decades. Lviv, Kherson, Dnipropetrovsk, Kyiv and Kharkiv regions took the leading positions in terms of the total area under open ground vegetables (40.6% of the total volume in the category). The traditional leader in the production of watermelons and melons was the Kherson region (19.9 tons or 43.1% of the total melon production in Ukraine before the war) (https://www.ukrstat.gov.ua).
Bacterial diseases are among the factors restraining the production of vegetable crops, especially in the regions of Ukraine with warm, moderate weather conditions. Vegetable crops are affected by fungal, viral, phytoplasmic, bacterial, and nematode diseases, causing tangible economic losses. Pathogenic bacteria disrupt the physiological processes in plants, causing necrosis, spotting, wilting and rotting, which leads to partial or complete death of the plants. Plants affected by phytopathogenic bacteria produce fewer fruits of reduced quality and a lower overall yield.
Recently, in Ukraine, there have been significant changes in the species composition of bacterial pathogens of vegetable crops and an increase in their harmfulness. Firstly, it is connected with the arrival of infected seed and planting materials in the country; secondly, with climate change, which makes weather conditions more favourable for the spread, and wintering of pathogens and their vectors (insects, ticks, nematodes), and thirdly, with the absence of drugs with a strong bactericidal effect. Insufficient awareness of the diversity and properties of these pathogens often prevents adequate assessment of potential losses from the diseases and choosing the correct pathogen control strategy [1].
Environmentally friendly technologies for growing vegetables are based on the use of biofertilizers, antagonistic bacteria and rhizobacteria that stimulate plant growth, as well as substances that activate protection [2]. Technologies for the production and use of bacterial inoculants are constantly developing and improving, and the market for bacterial biofertilizers is steadily growing. Various types of plant growth-promoting rhizobacteria (PGPB) are used worldwide as effective biofertilizers to improve soil yields and fertility and thus potentially contribute to more sustainable agriculture [3, 4]. PGPB is an important cluster of beneficial bacteria that colonize roots and grow in the rhizosphere of plants and soil. They enter into both synergistic and antagonistic interactions with the soil microbiota and get involved in ecologically important biological processes. They promote plant growth by strengthening their resistance to biotic and abiotic stresses and support the nutrition of their host plants. Due to the active stimulation of plant growth, PGPBs are considered an environmentally friendly alternative to hazardous chemical fertilizers [5].
The bioformulations have a number of advantages over their chemical equivalents. The former are environmentally friendly sources of renewable nutrients essential for maintaining health and soil biology. In addition, they are antagonists of various phytopathogens and protect plants from biotic and abiotic stresses [3, 6, 7]. Thus, the purpose of this review is to analyse the market of bioformulations used in Ukraine to combat bacterial pathogens of vegetable crops, outline progress in obtaining bioformulations, their direct use for phytocontrol of phytopathogenic prokaryotes and increase the productivity of vegetable crops, as well as potential prospects for application of such PGPB in sustainable agriculture.
2. BACTERIAL DISEASES OF VEGETABLE CROPS IN UKRAINE AND PROSPECTS FOR THEIR ELIMINATION
In Ukraine, vegetable crops are cultivated in the open ground, mainly in the southern, southeastern and partially central black-earth regions, where the weather conditions are most favourable for the cultivation of agricultural crops as well as for spread of bacterial pathogens. The harmfulness of bacterial diseases varies widely depending on the time and degree of damage to the plant, approaches to its treatment, characteristics of the causative agent, and the meteorological and environmental conditions that affect the relationship between plants and parasites [8].
The weather conditions over the recent years have been favourable for the spread of bacterial diseases to new territories with a more continental climate and increase their pathogenicity. The optimal temperature range for growth and development of most phytopathogens is from 28 to 36°C. When the lowest temperature is above 28°C, the specific resistance of plants against bacterial diseases is inhibited. In fact, even a slight increase in the temperature leads to a sharp acceleration of the spread and development of vegetable crop diseases [8]. The phytosanitary survey of diseases of vegetable crops established that in the spring-summer periods, the phytopathological complex in all growing zones may include various combinations of bacterial diseases, the prevalence of which varies by year (Fig. 1).
Based on the analysis of phytosanitary inspection reports for most regions of Ukraine, in particular, Zhytomyr, Ternopil, and Chernihiv regions, one of the pressing problems of growing vegetable crops is the brown rot pathogen Ralstonia solanacearum (https://dpss. gov.ua) (Fig. 2).
Among all bacterial diseases, the greatest damage to potatoes is caused by wet (soft) rot. At the same time, the harmfulness of rot is significant both during the cultivation of potatoes and during their storage. The main causative agents of bacterial potato rot are gram-negative bacteria of the genera Pectobacterium and Dickeya. In particular, in 2021, the development of black leg (the causative agent of the genus Pectobacterium (P. sarotovorum subsp. Atrosepticum and P. sarotovorum subsp. sarotovorum) and the genus Diskeya (D. сhrysantemi, D. dianthicola and D. solani)) was manifested in only two regions: In the Volyn region, 1% of the area was affected and 0.1% of the plants were damaged; in the Ternopil region, 75% of early-ripening plants were affected, 90% of the mid-ripening varieties, and 88.9% of late-ripening plants (damaged 0, 2, 0.4 and 0.3% of plants were damaged, respectively) (https://dpss.gov.ua).
The causative agent of potato ring rot is the gram-positive bacterium Clavibacter michiganensis subsp. sepedonicum, which causes substantial losses, especially during crop storage. Ring rot is widespread throughout Ukraine and appears on tubers in two forms: ring and pitted. The causative agent of the disease is the bacterium Corynebacterium sepedonicum. The harmfulness of ring rot during storage is manifested in the rotting of tubers. In some cases, such losses can amount to up to 15-20%. In almost all European countries, this disease is a quarantine object. In the period of widespread development of the disease, this is the main reason for the culling of seed crops. The development of the disease in 2022 was at the level of 0.1-33.3% (in 2021, 0.2-33%). A low level of damage (0.1-0.6%) was recorded in Vinnytsia, Volyn, Sumy and Kherson regions (https://dpss.gov.ua).
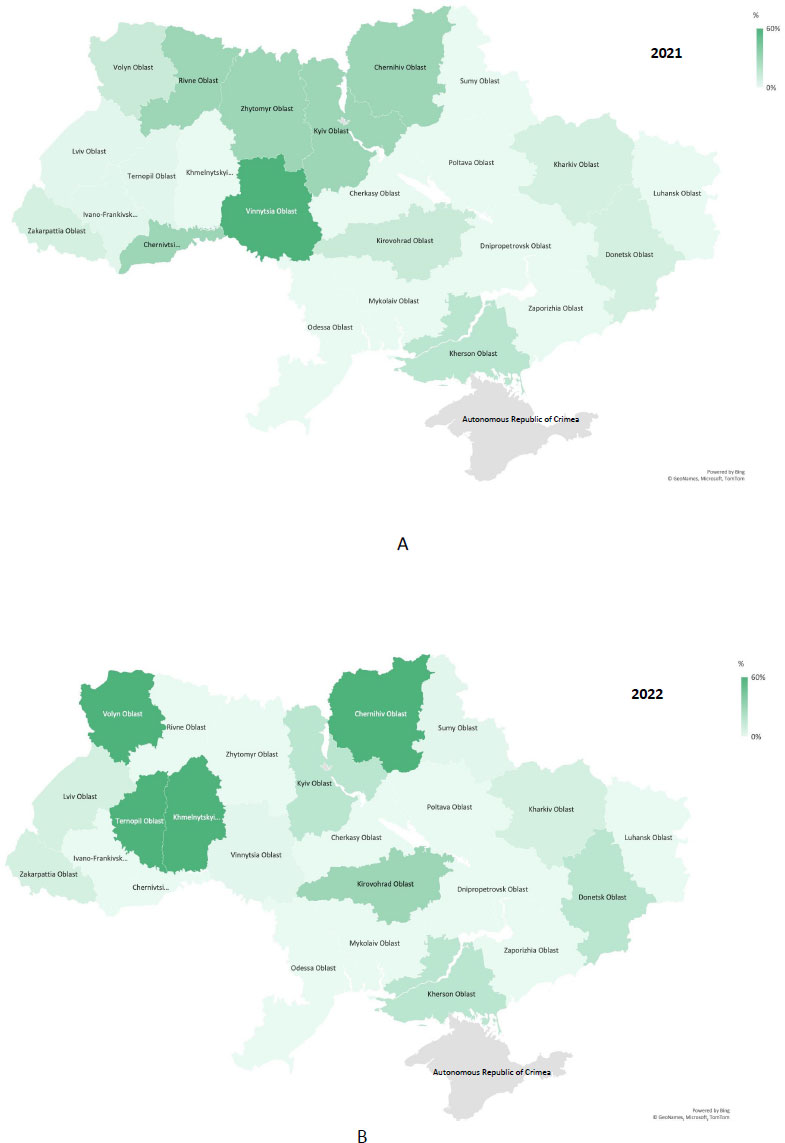
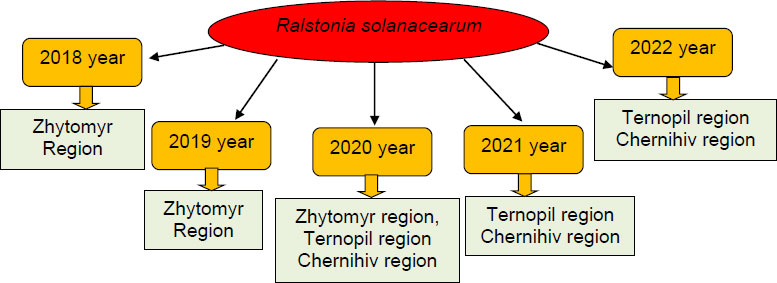
In recent years, the State Service of Ukraine on Food Safety and Consumer Protection (SSUFSCP) has regularly reported the detection, in some farms, of an extremely harmful potato brown rot pathogen, R. solanacearum, which is included in the A2 list of the European and Mediterranean Plant Protection Organisation (EMPPO) and in the quarantine lists of many countries of the world. This pathogen has never been detected in the climatic zone of our country before. In particular, in 2022, the brown potato rot was detected in Chernihiv and Ternopil regions. Initially, it didn’t develop and was not actively manifested, but in the second half of July, against the background of alternating rainy and hot periods, its prevalence increased. Apart from potatoes, the pathogen infects a wide range of economically important vegetable host plants, including, pumpkins, eggplants, tobacco, tomatoes and many ornamental plants. Bacterial cancer of potato (C. michiganensis subsp. michiganensis) in 2022 appeared on individual potato plantings in the Volyn, Zakarpattia, Ivano-Frankivsk, Lviv, and Chernivtsi regions in the first half of July (https://dpss.gov.ua).
The main bacterial pathogens of tomatoes, which cause significant damage to plantings of this crop in Ukraine, are C. michiganensis subsp. michiganensis, Pseudomonas syringae pv. tomato, Хanthomonas sp., which cause bacterial spots (BSX) and the causative agent of soft rot P. carotovorum subsp. carotovorum. C. michiganensis subsp. michiganensis and BSX are quarantine organisms in the European Union (European Plant Quarantine and Protection Organization (EPPO List A2)) and are subject to international phytosanitary control. In particular, black bacterial spot (X. vesicatoria) in the first decade of September 2021 was detected on 1-12, max. 20% of tomato plants (Kyiv, Kherson regions). Natural infection to tomatoes is caused by the potato ring rot pathogen C. michiganensis subsp. sepedonicum, which is included in the EPPO A2 list and in the A3 list (regulated non-quarantine organisms) of the List of regulated harmful organisms (No. 879/33850 dated 08.08.2019).
The hot weather in July-August restrained the development of bacterial diseases in cabbage. However, before harvesting, 1-7, max. 15% of cabbage plants in Donetsk and Kherson regions were affected by vascular bacteriosis (Х. campestris pv. campestris), the intensity of development was 2%. Besides, on average, 1-5%, max. 10%, of cabbage plants in Kherson region were affected by mucous bacteriosis (Erwinia carоtovora ssp. carоtovora, E. aroideae). Bacteriosis of cucumbers (angular spot) (P. syringae pv. lachrymans) was detected in 35-100% of the surveyed areas. It became widespread with a lesion of 3-16%, max. 45-60% of plants in Volyn, Ternopil and Chernihiv regions, with the development of symptoms in 1.5-5% and 2-5% of affected fruits (https://dpss.gov.ua).
The growing prevalence and harmfulness of bacterial diseases makes it urgent to develop new and improve existing diagnostic methods since bacterial diseases often have external manifestations similar to the symptoms of diseases caused by micromycetes and mycoplasmas. Incorrect laboratory diagnosis leads to improper or even harmful use of pathogen combating agents. Current plant protection is based on the complex use of multifactorial methods of combating pathogens. However, for their effective application, it is necessary to be well aware of the target groups of pathogens, and to have information about the nature of the pathogen infecting plants, the ways it spreads and the sources of infection. Since bacteria that cause diseases in vegetable crops have characteristic biological properties that distinguish them from other groups of pathogens, the measures to combat phytopathogenic bacteria also have their own characteristics. According to the existing classifications, all measures to combat bacterial pathogens in vegetable crops can be divided into agrotechnical, chemical, the use of bioinoculants, antibiotics, the cultivation of resistant varieties, and the production of genetically modified plants [9-11] (Fig. 3).
3. APPLICATION OF PGPB IN BIOCONTROL OF PLANT PATHOGENS
Biological control of plant pathogens, based on the use of living microorganisms and biologically active substances, synthesized by them, is a topical trend aimed at improving the adaptation, productivity and resistance to pathogens of plants grown in compliance with environmental standards [12, 13]. PGPB are soil bacteria capable of stimulating plant growth and increasing their resistance to pathogens of various etiologies and abiotic stresses through a variety of mechanisms. PGPB, being the biocontrol agents, have certain advantages over conventional chemical control methods due to their apparent environmental safety and low human toxicity. These biocontrol agents not only prevent plant infection, but also stimulate plant growth and increase plant productivity [14-16]. Depending on the degree of integration with the plant, PGPB can be divided into two groups: extracellular plant growth-promoting rhizobacteria (ePGPB), which colonize the rhizosphere and rhizoplane or live in the intercellular space of the cortical layer of the root, and intracellular plant growth-promoting rhizobacteria (iPGPB) which are localised inside root cells, usually in specialised nodular structures [17, 18]. The ePGPB includes representatives of the genera Agrobacterium, Arthrobacter, Azotobacter, Azospirillum, Bacillus, Burkholderia, Caulobacter, Chromobacterium, Erwinia, Flavobacterium, Micrococcous, Pseudomonas and Serratia and other soil bacteria. The iPGPB includes representatives of the family Rhizobiaceae, that enter into close symbiotic relationships with plants: Allorhizobium, Azorhizobium, Bradyrhizobium, Mesorhizobium, Rhizobium [19] and Frankia [18].

The mechanisms by which growth-promoting rhizobacteria can affect plant growth vary among species and strains, and at different stages of the host plant's life cycle, so their effects on the plant cannot usually be explained by a single mechanism. The most common mechanisms by which PGPB stimulate plant growth are: biological nitrogen fixation; increasing the availability of nutrients in the rhizosphere (solubilization of phosphorus, facilitating the assimilation of iron due to the production of siderophores); formation of such phytohormones as auxins, cytokinins, gibberellins [5, 20]. PGPB have been shown to accelerate seed germination, stimulate root growth, increase leaf area, increase chlorophyll content, accelerate nutrient absorption, increase protein content, increase shoot and root mass, stimulate root hydraulic activity, increase yields, increase plant resistance to biotic and abiotic stresses, and slow down the aging process (Fig. 4) [21, 22].
A significant success in the use of PGPB inoculants in crop production is the provision of a reliable mechanism of plant protection against phytopathogens. The implementation of the protective functions of PGPB bioformulations is carried out through direct, indirect and combined mechanisms. The direct mechanism involves the synthesis by PGPB of active metabolites, phytohormones, and antioxidants that help plants protect themselves from damage by phytopathogens. The indirect defense mechanism of PGPB includes stimulation of plant growth and induction of acquired systemic resistance in plants [23].
The success of implementing the protective action of PGPB inoculants is determined by the ability of their components to inoculate and actively live in the rhizosphere, rhizoplane, or in the inner space of the plants for which the inoculant was used. Many PGPB bacteria are known to form microcolonies or biofilm-like structures on roots that enable successful colonization of these plants and play an important role in plant defense [24]. For example, Paenibacillus polymyxa has been shown to colonize the root extremities of peanut plants, forming biofilms, and to protect the plants from rot [25]. Mutants of P. fluorescens strain CHA0 are distinguished by enhanced biofilm formation due to a high mucoid content, show a markedly enhanced ability to colonize carrot, cucumber and tomato roots and are better biological control agents compared to the wild type [26, 27].

The strain ATCC 6051 of B. subtilis is able to form biofilms on the roots of Arabidopsis plants and protect them from P. syringae infections [29]. It was shown the population of Azospirillum brasilense in the rhizosphere of inoculated tomatoes exceeded the population of pathogenic bacteria P. syringae pv. tomato by two orders of magnitude (107 vs. 105 CFU/g [dry mass] of root). When tomato seeds were treated with a mixture of these cultures, a decrease in the population of the pathogen and an increase in the population of A. brasilense were noted, which prevented the development of bacterial spotting and improved plant growth. The displacement mechanism is based on the ability of A. brasilense to obtain nutrients more quickly and to colonize plant surfaces [29].
The manifestation of induced systemic resistance (ISR) depends on the combination of host plant and PGPB bacterial strain [30]. Most of the reported cases of PGPB-mediated ISR have been associated with free-living strains of rhizobacteria. However, endophytic bacteria also activate the ISR. When elucidating the mechanisms of the plant-PGPB-pathogen interaction, it was established that several bacterial determinants (e.g., flagella, siderophores and lipopolysaccharides) trigger ISR [30]. Volatile organic compounds (VOCs) play a key role in the induction of ISR [31]. Several species of bacteria from different genera, including Bacillus, Pseudomonas, Serratia, Arthrobacter, and Stenotrophomonas, produce VOCs that affect plant growth. Acetoin and 2,3-butanediol, synthesized by Bacillus, are the best-known VOCs responsible for the significant improvement of plant growth. Some other PGPB strains release VOCs that may directly and/or indirectly cause increased plant biomass, and disease resistance. For example, the volatiles 2R, 3R-butanediol and acetoin released by B. subtilis GBO3 and B. amyloliquefaciens IN937a were able to activate the ISR pathway in Arabidopsis seedlings infected with the soft rot pathogen P. carotovorum subsp. carotovorum [32].
PGPB-triggered ISR enhances plant cell wall strength and alters host physiology and metabolic responses, leading to enhanced synthesis of defense chemicals in the plant following the phytopathogen challenge [33]. After inoculation of tomato seeds with the endophyte P. fluorescens WCS417r showed thickening of the outer tangential and outer radial sides of the first layer of the cortical cell walls during colonization of epidermal or hypodermal cells [34]. Therefore, the most common PGPB inoculants that protect plants from various phytopathogens are preparations based on bacteria of the genera Pseudomonas and Bacillus.
3.1. Bacteria of the Genus Pseudomonas in Biocontrol
Bacteria of the genus Pseudomonas are one of the best-studied groups of microorganisms for the biological control of bacterial and fungal diseases. Among the wide spectrum of fluorescent Pseudomonas, specific strains belonging to P. fluorescens, P. putida, P. aeruginosa, and P. chlororapis have enormous potential for biological control due to their inherent ability to produce metabolites with antifungal and antibacterial activities [35] and enzymes [36]. Fluorescent pseudomonas can grow rapidly in vitro and in vivo, use seed and root exudates, form biofilms for colonization and reproduction in the rhizosphere, and produce a wide range of bioactive metabolites. Among the various antimicrobial metabolites of Pseudomonas, the following have been studied and characterized: phenazine-1-carboxylic acid (PCA), 1-aminocyclopropane-1-carboxylate deaminase (ACCD), 2,4-diacetylfluoroglucinol (DAPG), and hydrogen cyanide (HCN) [35]. Research by Lanteigne et al. [38] confirmed that DAPG and HCN are involved in the biocontrol of tomato bacterial cancer. It was shown that in open ground the Pseudomonas sp. LBUM300 strain reduced the development of bacterial cancer symptoms, while separate inoculation with mutant strains of P. sp. LBUM300 phlD- (which does not synthesize DAPG) or hcnC- (which does not synthesize HCN) did not reduce the development of bacterial cancer symptoms [37].
The ability of Pseudomonas sp. LBUM 223 to produce PCA for the biological control of potato scab by inhibiting the growth of Streptomyces scabies and the repression of taxomin biosynthesis genes of (txtA and txtC) was investigated using a defect mutant in PCA production (LBUM). Growth of S. scabies was inhibited by LBUM 223 phzC to a significantly lesser extent than by the wild-type LBUM 223. Pseudomonas also significantly suppressed the expression of txtA and txtC in S. scabies and protected potato from disease [38]. Boudyach et al. showed that strains of fluorescent Pseudomonas, isolated from the rhizosphere, were able to significantly reduce the manifestations of bacterial cancer on tomato seedlings after pretreatment of seeds and roots [39]. Kavitha et al. [40] reported that P. fluorescens is one of the main antagonists of X. oryzae pv. oryzae in rice, R. solanacearum in Chili, Fusarium in cucumber, C. michiganensis ssp. michiganensis and Xanthomonas vesicatoria in tomato. A significant number of PGPB Pseudomonas strains produce antibiotics that inhibit or slow down the growth and development of phytopathogenic bacteria and fungi [41]. Antibiotics produced by fluorescent pseudomonads include phenols, pyrrole-type compounds, polyketides, and peptides [42].
3.2. Bacteria of the Genus Bacillus in Biocontrol
Many works have shown the prospects of creating biological plant protection products based on bacteria of the genus Bacillus. Special interest in this topic arose in connection with the established fact of endophytic bacillus-antagonists. It has been shown that bacteria of the genus Bacillus are able to colonize plant tissues from the seedling phase and persist in them for a long time. Coexistence of antagonistic bacteria with a plant turns out to be beneficial for the latter, as it prevents the penetration of pathogens into it and, thus, protects it from pathogenic microorganisms [23]. The ability of bacilli to form spores and withstand extreme conditions is their advantage in soil microbiocenoses under adverse environmental factors [43].
The high level of antagonism of bacilli against phytopathogenic bacteria is due to the fact that they synthesize a wide range of exometabolites, which in turn are able to induce systemic resistance of the host plant [44]. However, some of the Bacillus can suppress the growth and development of plants in the course of ontogenesis. Therefore, when creating inoculants, not only the antagonistic activity of a particular strain of Bacillus, but also its safety and lack of phytotoxic effect should be taken into account. It has been shown that bacteria from the genus Bacillus are capable of producing chitinases, RNases, DNases, proteases, amylases, phosphatases, and nitrogenases, as well as microbiologically transforming hard-to-reach organic and inorganic compounds into a form available to plants and to enriching the soil with biological nitrogen [5].
Utkhede and Koch reported that treatment with a suspension of B. subtilis Quadra 136 and 137 and Trichoderma harzianum R, Rhodosporidium diobovatum S33 at a concentration of 0.3, 0.6, 10 g/l prevents the development of bacterial cancer caused by C. michiganensis subsp. michiganensis under the greenhouse conditions [45]. Similarly, treatment of tomato seeds with PGPB strains B. subtilis GBO3, B. amyloliquefaciens IN937a and Brevibacillus brevis IPC11 recorded maximum protection against bacterial cancer under greenhouse conditions [46]. Cui et al. showed that the extent of soft rot, as well as the transmission of Pcc and its survival in the rhizosphere, were reduced after inoculation with B. amyloliquefaciens KC-1. The B. amyloliquefaciens KC-1 population persisted in Chinese cabbage stems after germination. These results showed that B. amyloliquefaciens KC-1 was able to survive and inhibit the growth of Pcc in Chinese cabbage and its rhizosphere, thereby, protecting the host against the pathogen.
The presence of a high density of B. amyloliquefaciens KC-1 in the rhizosphere soil is a necessary condition to suppress infection caused by the soil-borne pathogen Pcc. The use of B. amyloliquefaciens KC-1 throughout the period of plant growth can be an effective strategy for the prevention of soft rot of Beijing cabbage. A recent study showed that the pathogen population and symptoms in the rhizospheric soil of R. solanacearum were significantly reduced after the application of Bacillus sp [47].
As mentioned above, PGPB bacteria cause induced systemic resistance (ISR), similar to systemic acquired resistance (SAR). ISR in plants is associated with the activation of ion currents, phosphorylation/dephosphory lation of proteins and the formation of signal molecules, in particular, salicylic and jasmonic acids, ethylene and ROS under the conditions of contact with pathogenic micro organisms. Such rearrangements in plant metabolism cause a change in the regulation of gene expression and biosynthesis of protective substances, strengthening of the cell wall and accumulation of phytoalexins and pathogen-dependent proteins (PR) [23]. The ability to induce ISR against a wide range of phytopathogens by activating salicylate, jasmonate and/or ethylene-dependent pathways has been established in Bacillus spp., Serratia liquefaciens, Penicillium spp. and Trichoderma spp.
4. BIOFORMULATIONS USED IN UKRAINE TO COMBAT BACTERIAL DISEASES OF VEGETABLES
The competitiveness of agricultural products on the world, European and domestic markets and the environmental protection are determined by the state of biologisation of plant protection in Ukraine. This is especially relevant today, when Ukraine joined the World Trade Organization (WTO) and is taking a course towards EU integration and developing the market for organic plant products, grown with the predominant use of biotechnology and a minimal use of agrochemicals. The market share of biological preparations for the protection of vegetable crops in Ukraine so far is less than a tenth of the volume of the domestic market of plant protection products. However, the growing trend of this share indicates positive prospects for the development of this business. According to the State Statistics Service, the segment of biological preparations in real terms has grown to 8.3%. The reason for replacing chemical preparations with biological ones is the growing demand for organic products as part of the progressive trend of improving the environmental friendliness of food products and human living space.
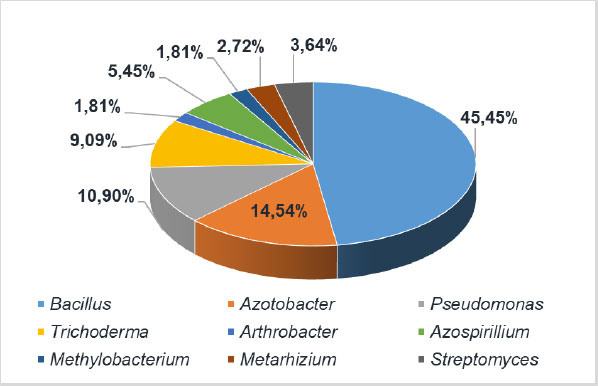
Biotechnological solutions for the production and practical application of an impressive range of biological agents for traditional and organic agriculture have been developed to address the challenges of vegetable protection. The effectiveness of bioinoculants depends on the forms of release and the conditions under which the biological preparation comes into contact with phytopathogens. Various preparative forms of bioformulations are manufactured for vegetable crops in Ukraine, such as suspension concentrate, soluble concentrate, aqueous solution, water-soluble powder, wettable powder, powder, granules, water-soluble gel. Most of the bioformulations are released in the form of a suspension concentrate with a short shelf life (Fig. 5) (https://data.gov.ua). The most recent preparations in the form of granules and gel constitute a small percentage of the total. Such bioformulations ensure long-term viability of the biological agent and have better prospects for future. The most popular bionoculants on the market of Ukraine (https://data.gov.ua) are preparations based on Bacillus bacteria. Their market share has grown up to 45.45%. Preparations based on Azotobacter and Pseudomonas are also quite common (Fig. 6).

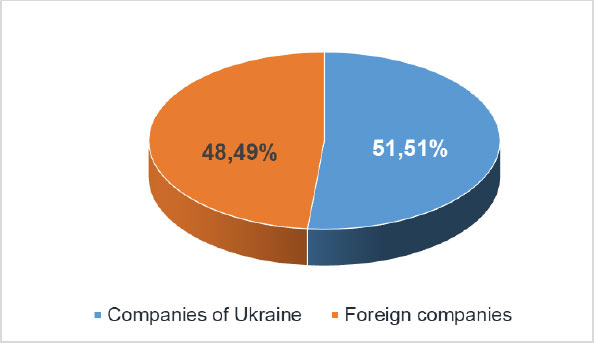
These bioformulations can stimulate plant growth and control bacterial pathogens. To increase and maintain the level of biological control, multi-strain mixtures of PGPB have also been successfully used in vegetable crops. Therefore, it is not by chance that the interest in bioformulations is steadily growing all over the world, and their use is expanding. Manufacturing companies are distributed on the Ukrainian market (https://data.gov.ua) in a parity ratio (Fig. 7). It is noteworthy, that technologically more advanced preparations with an extended shelf life are brought to the market by foreign companies. At the same time, Ukrainian companies produce cheaper liquid preparations with a shelf life of up to 1 year.
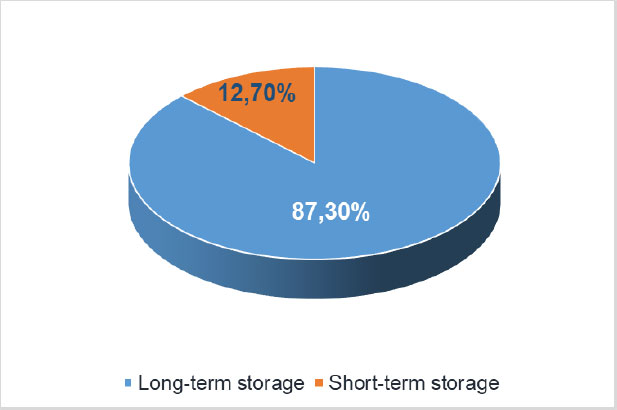
Bioformulations are designed as products suitable for a long-term storage. The validity terms regarding their shelf life range from 2-12 months at room temperature to 2-3 years. Increasing the initial number of viable cells in the bioformulations is the way to compensate for any rapid spoilage [48-50]. In any case, storage conditions must be optimized to maintain the long-term cell viability [51-53]. A clear correlation has been reported between the amount of bacteria applied to plants and the subsequent yield [54]. An analysis of the assortment of bioinoculators for vegetable crops on the market of Ukraine (https://data.gov .ua) shows that the vast majority of preparations had a short shelf life, which could be explained by the pricing policy, since such preparations are an order of magnitude cheaper (Fig. 8).
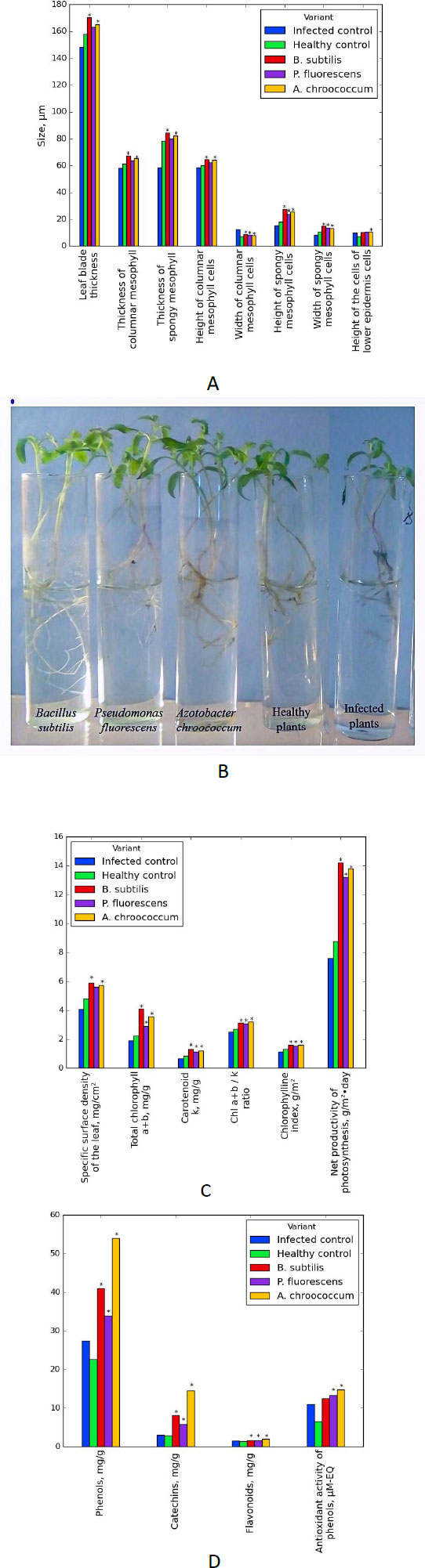
According to our recent data, tomato plants (Chaika variety) affected by the causative agent of bacterial cancer were treated with bioformulations based on bacteria of the genus B. subtilis (109 CFU/cm3), P. fluorescens (109 CFU/ml3) and A. chroococcum (109 CFU/cm3). It was established that the bioformulations provide structural and functional rearrangements at the cellular level, as a result of which the upper and lower epidermis thickens and is permeated with biopolymers that have protective properties and create non-specific barriers for the spread of the pathogen. Treatment with a suspension of cells of growth-stimulating bacteria led to an increase in the content of chlorophylls and carotenoids and increased the activity of the components of the antioxidant system [1] (Fig. 9).
The use of PGPB B. subtilis cell suspension of increases the resistance of spring wheat plants of the Grenny variety against the causative agent of basal bacteriosis (P. syringae pv. atrofaciens) by 25%. The initiation of the synthesis of cell wall biopolymers, in particular, cellulose, lignin, and suberin, and the accumulation of oxycinnamic and oxybenzoic acids in plant leaves were established [55]. It was established that the treatment of tomato plants with a biological preparation based on B. subtilis, raised the enzyme activity in the leaves and was maintained at the maximum level of 94.7–112.7 units/mg/s at pH=4.7 and pH=5.5 for 24 h. This effect indicates the induction of synthesis of its anionic and cationic-anionic forms.
| Bacteria/ Strain(s) | Plant(s) | Protection Against | Mechanism of Action | Refs. |
|---|---|---|---|---|
| Paenibacillus polymyxa | Peanut | Biocontrol against crown rot disease | Biofilm formation | [25] |
| B. subtilis ATCC 6051 | Arabidopsis | P. syringae | Formation of a stable, extensive biofilm and secretion of surfactin | [28] |
| Azospirillum brasilense | Tomato | P. syringae pv. tomato | A. brasilense is better able to obtain nutrients, to colonize plant surfaces | [29] |
|
Pseudomonas sp. LBUM300 Pseudomonas sp.LBUM 223 |
Tomato, potato |
Biological control of bacterial canker | Production of 2,4-diacetyl-phloroglucinol (DAPG), and hydrogen cyanide (HCN), phenazine-1-carboxylic acid (PCA) | [37, 38] |
| Fluorescent Pseudomonas strains | Tomato | Biological control of bacterial canker | Colonization of roots, production of DAPG, HCN, PCA | [39] |
| P. fluorescens | Tomato, rice, cucumber, pepper Chili |
Xanthomonas oryzae pv. oryzae, R. solanacearum, C. michiganensis ssp. michiganensis, X. vesicatoria |
Antagonism | [40] |
|
P. brassicacearum J12, P. protegens RS-9 |
Tomato, potato banana, pepper, eucalyptus |
Bacterial pathogens | Production of antibiotics, DAPG, HCN, pyrrolnitrin and pyoluteorin, siderophore and protease | [41] |
| B. subtilis Quadra 136 and 137. Trichoderma harzianum R, Rhodosporidium diobovatum S33 | Tomato | C. michiganensis subsp. michiganensis | Direct stimulation of plant growth, synthesis of phytohormones | [45] |
| B. subtilis GBO3, B. amyloliquefaciens IN937a, Brevibacillus brevis IPC11 | Tomato | Bacterial canker | Increased of the level of phenylalanine ammonia lyase and total phenol contents | [46] |
|
B. subtilis GBO3, B. amyloliquefaciens IN937a |
Arabidopsis | P. carotovorum subsp. carotovorum | Production the volatiles 2R, 3R-butanediol and acetoin that trigger ISR | [32] |
| P. fluorescens WCS417r | Tomato | Bacterial pathogens | Thickening of the cortical cell walls | [34] |
| B. subtilis, P. fluorescens, A. chroococcum | Tomato | Bacterial canker | Induction an increment of the activity of antioxidant system components and of the content of chlorophylls and carotenoids | [58] |
| B. subtilis | Wheat | P. syringae pv. atrofaciens | Initiation of the synthesis of cell-wall biopolymers, in particular, cellulose, lignin, and suberin, and the accumulation of the content of oxycoric and oxybenzoic acids in plant leaves | [55] |
| B. subtilis Vru1 | Bean | Biological control of R. solani | Enhancements in the number of the bacterium and the high level of metabolite production such as indole-3-acetic acid | [59] |
When treated with a biological preparation based on A. chroococcum, the peroxidase activity increased to 77.7 mg/s. Biological preparations based on B. subtilis and A. chroococcum showed high antibacterial activity against bacterial cancer pathogens C. michiganensis subsp. michiganensis and black bacterial spot Xanthomonas vesicatoria. These preparations significantly affected the mechanism of resistance formation, for example, the synthesis of ROS during the peroxidase signaling pathway [56, 57]. Thus, the analysis of the research findings indicates that growth-stimulating bacteria can be considered effective inducers of plant resistance for and, accordingly, can be used as promising biopesticides to combat phytopathogens (Table 1).
5. POTENTIAL DIRECTIONS FOR THE CREATION OF BIOLOGICAL PREPARATIONS FOR THE MARKET OF UKRAINE
The development of a new generation of biological preparations of complex prolonged action for crop production, which combines the properties of biofertilizers, insecticides, fungicides and bactericides, allows solving a wide range of issues of biological protection of plants, increasing the quality of products and restoring soil fertility [47, 60-68]. PGPB-based inoculants are usually a mixture containing one or more cultures of beneficial bacteria in a carrier material. The carrier materials used in the composition of the biofertilizer should ensure the viability of the PGPB and be convenient in practical use. The carriers can be: clay, talc, peat, vermiculite, perlite, bentonite, zeolite, diatomaceous earth, rice or wheat bran, rock phosphate granules, charcoal, soil, sawdust or compost. Usually, the carrier material is chosen on the basis of a longer viability of bacteria during storage and after its application to the soil, and the desired way of application (liquid, powder, granular or in the form of seed coating) [42].
5.1. Increasing the Effectiveness of Strains
The effectiveness of inoculants is determined by the quality of their production and the quality of their storage. It is very important to select strains that most fully meet the objective of using the inoculant and best interact with the plant species for which they will be used. However, it is equally important to maintain the viability and all the essential properties of the biological agents in the ready-made preparation. The PGPB strain must meet a number of criteria, in particular, it must be highly competent for the rhizosphere and be environmentally safe after inoculation it must colonize plant roots in significant numbers, promote plant growth, exhibit a wide range of activities, be compatible with other rhizosphere bacteria, be resistant to external physical and chemical factors, such as heat, desiccation, radiation and oxidants, and show the best competitive powers compared to the existing communities of rhizobacteria [69].
5.2. Improvement of Carriers
In order to develop a low-cost technology, low-cost formulations should be used for the production and protection of the biomass. After the biomass production process is completed, the preparation must be made in a liquid or solid forms, taking into account the necessary term of storage, the use of protectors/carriers, storage conditions (temperature, humidity, etc.), ease of use and preservation of useful substances [70].
5.3. Application of Nanotechnologies in Biological Preparations
Suman et al. proved the advantage of using nanofertilizers, having shown that controlled-release fertilizers can also improve the soil by reducing the toxic effects associated with the excessive use of traditional chemical fertilizers [71]. The use of PGPB as a fertilizer by traditional methods is not efficient enough, since 90% is lost during application due to the negative effects of external environmental factors (heat, UV radiation, etc.), thus affecting the cost of application. The technology of nanoencapsulation can be used as a versatile tool to protect PGPB, increase their validity term, improve the dispersion in fertilizers, and provide controlled release of PGPB [72, 73]. For example, the use of nanocapsules (alginate with silicon nanoparticles and carbon nanotubes) of P. fluorescens VUPF5 and B. subtilis VRU1 significantly increased the root length and proliferation of the commercial pistachio rootstock UCB-1 [74]. The use of sodium alginate (NaAlg) nanocapsules with Bacillus subtilis Vru1 allowed to effectively colonize beans with this PGPB strain and control such a pathogen as Rhizoctonia solani [59].
Thus, creation of encapsulated bioformulations with PGPB becomes an increasingly popular approach. Encapsulation provides better storage conditions for PGPB and increases the efficiency of their use for plants. Alginate, which is a biodegradable substance and does not harm the environment, is most often used for encapsulating PGPB [75]. However, further research is needed to investigate the effects of encapsulation on bacteria and their targeted release in the organic crop production systems.
A promising tool for improving the productivity of crops is the use of biological preparations with a complex prolonged action, which combine the properties of biofertilizers, fungicides and bactericides, based on PGPB. The most promising are preparations based on bacteria of the genera Bacillus, Azotobacter and Pseudomonas. Nanoparticles produced using such bacteria [76] may also deserve special attention for crop protection and productivity [77].
CONCLUSION
The changing climatic conditions in many regions of Ukraine, as in the whole world, contribute to the increase in the aggressiveness of bacterial pathogens. Adaptation to climate change through preservation and protection of ecosystems is accepted as one of the priority United Nations Sustainable Development Goals (SDGs). Ukraine also feels the negative consequences of rising temperatures, changes in the amount and quality of precipitation, and stronger winds. These factors contribute to changing the stable distribution zones of bacterial pathogens. In Ukraine, they lead to the emergence, successful acclimatization and spread of new bacterial pathogens of vegetable crops. Farmers in Ukraine, who are engaged in growing vegetables, feel a lack of bioformulations to effectively control the pathogens of bacterial diseases. The range of bioformulations with antibacterial activity, approved for use in the country, is limited. The use of bioformulations based on PGPB is a promising method of controlling pathogens of bacterial diseases of vegetable crops and corresponds to the strategies preservation of the environment and development of biodiversity SDGs. Such PGPB prevent contamination of plants with phytopathogens by inducing acquired systemic resistance and stimulating their growth and better productivity. Seed inoculation is a promising way of using PGPB in crop production. The most commonly used plant protection products in Ukraine are the preparations based on bacteria of the genera Bacillus and Pseudomonas. Most of such products on the market are those developed and manufactured in Ukraine. However, the preparations based on bioinoculants for vegetable farming in Ukraine have a limited shelf life. There is still a shortage of modern forms of bioformulations that would ensure the long-term viability of PGPB and the prolonged activity of the bioformulations based on them. Thus, the development and introduction of encapsulated PGPB bioformulations in Ukraine may contribute to solving the problem of biological control of pathogens of bacterial diseases of vegetable crops.
AUTHORS' CONTRIBUTIONS
Yuliia Kolomiiets – Analyzed and interpreted the data; Wrote the paper.
Liudmyla Butsenko – Analyzed and interpreted the data; Wrote the paper.
Yaroslav Blume – Edited the manuscript; Made critical remarks.
Alla Yemets – Analyzed and interpreted the data; Reviewed and analyzed the manuscript contents; Made the manuscript corrections.
All authors have approved the final version of the manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The work was partially financially supported by the Ministry of Education and Science of Ukraine (Biotechnology for the identification and control of pathogens of bacterial diseases of solanaceae to resolve the food crisis in Ukraine, 0123U102105, 2023-2025)
CONFLICT OF INTEREST
Dr. Yaroslav Blume is the Editor in Chief, and Dr. Alla Yemets is the Editorial Board Member of the journal, The Open Agriculture Journal.
ACKNOWLEDGEMENTS
Declared none.


