All published articles of this journal are available on ScienceDirect.
Flower Initiation Pattern, Developmental Stages, and Seed Morphology of Paraphalaenopsis Labukensis P.S. Shim, A. Lamb & C.L. Chan, An Endangered Orchid in Sabah
Abstract
Background
Paraphalaenopsis labukensis P.S. Shim, A. Lamb & C.L. Chan is a monopodial epiphytic species that can only be found in Sabah. P. labukensis orchids have unique characteristics in that it has a long floral lifespan as compared to other orchid species. The flower developmental pattern of P. labukensis greatly influenced capsule formation and seed maturation.
Objective
The present research was conducted to record the initiation of flower initiation, and floral morphology, and to observe the flowering and capsule development, as well as the effect of different capsule ages on asymbiotic seed germination.
Methods
A total of three individual plants of P. labukensis were observed. The flowering stages were characterized by quantitative parameters such as length of inflorescence, diameter, and length of buds, the number of flowers produced, and the length of the capsule formed. All the data were recorded through direct observation.
Results
Overall, twelve morphological landmark that define each stage of floral development was recorded. Based on the observation, P. labukensis inflorescence was asymmetric and in the shape of a panicle. The number of flowers varied among inflorescences, ranging from 3–5, that blossomed at different times. Furthermore, early capsules appeared 40–90 days after pollination (DAP). Then, 120 DAP of the capsule was selected as the most suitable capsule age for germination as it had reached its maturation period.
Conclusion
Identifying the duration of the whole flowering developmental process will aid in the production of capsules to attain a reliable and adequate seed source for in vitro seed germination.
1. INTRODUCTION
Orchidaceae is one of the largest flowering plants and a major contributor to the floriculture industry as cut flowers and potted plants [1]. The island of Borneo is one of the centres of orchid diversity [2]. The estimated number of orchid species in Borneo varied widely. The species richness of orchids in Borneo has been partially related to the presence of Mount Kinabalu, the highest mountain in the Malay Archipelago [3].
Paraphalaenopsis labukensis is one of Sabah’s endangered and endemic [4, 5]. The species was originally discovered in the Labuk River basin and grow from 500 to 1000 meters altitude. The species is also known as the most spectacular pendant epiphytic orchid in Kinabalu Park. The plant grows suspended from small trees 3 to 5 meters above ground or 12 to 20 meters up on the trunks and branches of larger trees [6]. The rich diversity and their distinctive flowers make them popular among orchid collectors and prone to threats such as over-collecting, habitat loss, and disturbance [7].
The study of the lifespan of orchids as well as their flower morphological development is an interesting topic to explore because it involves various stages of flower development starting from bud formation until senescence. However, nowadays there is not much documentation on the flower development of most orchid species, and in some species, it has not yet been done. The flower development stages, or their flowering pattern however have a crucial role in the in vitro seed germination process.
Due to the wide variety of floral forms, flowering is one of the most intensively studied processes in plant development. In plant physiology the formation and development of floral organs are critical. This is due to the fact that plant biology can be studied through the process of floral development which provides a fundamental understanding of how flower organs emerge in the plant body [8]. However, outlining efficient methods of in vitro germinating seed culture without the study of orchid biology is not sufficient. Understanding the biology of orchids helps in identifying the specific factors that influence seed germination and growth, such as nutritional requirements, light conditions, and hormonal interactions [9]. In the past decade, analyses of flower initiation and development have been carried out primarily in flowering fruit strawberry (F. × ananassa) [10], Mindi (Melia azedarach L) [11], Malus x domestica Borkh [12] as well as in almonds [13]. Seed capsule development and maturation times vary according to species, hybrid, and growing conditions. After estimating capsule development time by allowing the capsule to dehisce, the hand pollination method was repeated by Kauth et al. [14] and Johnson et al. [15]. Determining the optimal harvesting time and capsule age is critical for avoiding seed dormancy and developing an efficient germination strategy [16]. In turn, these studies have resulted in the refinement of the original ideas of how flowers develop occurs in P. labukensis and have indicated the new knowledge of developmental stages, and flower patterns that need to be addressed. In addition, this study aids in observing the most appropriate time for capsule harvesting which is important for in vitro seed germination and the propagation process.
2. MATERIALS AND METHODS
2.1. Plant Material
A total of three plants of P. labukensis were collected from Poring Orchid Conservation Centre, Ranau, Sabah (06 02’20.7” N, 116 06’38.2” E) and kept in the greenhouse located at Institute for Tropical Biology and Conservation, Universiti Malaysia Sabah (06003ʹ 33ʺ N, 116 012ʹ 29ʺE). Orchid plants were re-potted with mosses and coconut husk medium, rinsed twice a day under natural daylight, 80% relative humidity, with an average temperature of 29 ± 2 °C, day and night as well as fertilised using commercial fertiliser (FT Grow) once for every three weeks to promote good growth and to induce flowering.
2.2. Observation of Flower Bud Initiation and Inflorescence Development
Data observation was performed every morning from 9.00 a.m to 11.00 a.m according to Mohammad and Rusdi [17] with slight modifications. From the beginning of the spike through the end of flower senescence, the plant's longevity was observed. Parameters involved and examined during the observations as the size of the flower (from dorsal sepal to lateral sepal), number of flowers produced per plant, and length of inflorescence, by using a ruler and Vernier calliper. All the data collected were determined by calculating their mean and standard deviation. As there is still no information on the flower's lifespan, two blossoms were left undisturbed on a plant to track their longevity and morphological changes. From the beginning of the spike through the end of flower senescence, the plant's longevity was observed.
2.3. Flowering Morphology and Capsule Development
Flower morphology and developmental stages including flower initiation, bud development, flower structure, and capsule development were examined in individual inflorescences (from fruit initiation until maturation). Time (day and period), shape, and colour were used to record any changes in flower bud structure blossoming [18]. The number of flowers and capsule fruit per inflorescence was counted throughout developmental stage observation to determine the plant's reproductive success.
The development of the capsule was observed daily from pollination to full maturity for any changes that occur. Capsule length and diameter were observed, and data were recorded. The length of the capsule was taken by using a ruler while its diameter was taken by using a Vernier calliper. Mature capsules were harvested before dehisced by cutting the capsule from the inflorescence [19]. For P. labukensis, mature capsules were harvested 120 days after pollination.
| Stages | Landmark Events |
Period (days) |
Remarks |
|---|---|---|---|
| 1 | Immature bud | 5 | Bud appeared in purple, while the inflorescence stalk is in light green |
| 2 | Bud swollen | 10 | Bud changes from purple to dark green and gradually changes to light red at the end |
| 3 | Bud multiplication | 12 | Multiplication of floral buds appeared |
| 4 | Bud elongates to form a mature bud | 20 | - |
| 5 | Mature bud occurrence | 28 | Petals are brownish-white in colour with lip is white with purple stripes. |
| 6 | Partially opened flower | 34 | Flower wilt on day 34 after hand pollination |
| 7 | Fully bloomed flower | 58 | The capsule was dark green |
| 8 | Flower wily (end tip) | 65 | The mature capsule appeared to be dark brown red |
| 9 | Wilted of petal and sepal | 73 | Senescence |
| 10 | Wilted petal and sepal | 78 | Senescence |
| 11 | An immature capsule/pod formed | 90 | Dark green |
| 12 | Mature capsule/pod | 125 | Dark red |
3. RESULTS AND DISCUSSION
3.1. Description of Flower Initiation Development Event
To date, there has been no comprehensive analysis of early flower development in P. labukensis. Using quantitative observation, a series of morphological landmarks that define each stage of flower development is presented (Table 1).
Twelve morphological character stages, with the early stages (stages 1-5) characterised as growth stages in which the formation and development of bud occur to generate bloom. Floral bud initiation began in early February and the flower reached the full bloom stage by the end of April. At this stage, a whole bud took around 14 to 20 days to reach full form. When a bud matured, it changed its colour from dark green to dark red. The second phase of the cycle is the maturation stage, which began at the stage 6 to 10 with the appearance of mature buds that multiplied into full-blown flowers with entire petals and sepals. The flowering of P. labukensis lasts for 3 weeks if pollination does not occur.
The last phase was the senescence stage (stages 10 to 12). At the full bloom stage, flowers' petals started to wilt after three weeks. The end of the flower tip wilted first, and it turned in brown and senesce. However, flowers that had pollinated turned into capsules or orchid pods after 28 days. The capsules can mature up to 125 days after pollination.
3.2. Spike Initiation and Flower Bud Formation (Stage 1- 3)
The spike's initial size was an average of 0.50 ± 0.20 cm and it is dark green (Fig. 1A). After five to ten days of initiation, the spike began to swell and appears pinkish red (Fig. 1B). This change is a precursor to the formation of flower buds. The swollen spike took four days to elongate and form a flower bud on the stem's peduncle. After three days, the length of the flower bud increased steadily as it approached the flower bud initiation stages (Fig. 1C). Flower bud initiation occurs after the spike has reached about 5 cm in length if environmental conditions are favourable. Continuous observations of early primordial growth and flowering of P. labukensis were carried out until buds reached maturity (bud opening). Fig. (1E) shows the appearance and multiplication of the floral bud of P. labukensis. It started to initiate on day 12 after the formation and swelling of an early spike.
3.3. Development and Maturation of Flower Bud (Stage 3-5)
After one week of bud swelling, the flower stalk quickly developed and formed branches, reaching a maximum length of 9.50 ± 2.50 cm. Each flower stalk typically had five to ten branches. They were pinkish-red and oval. Florets developed in the axils of the bracts on the flower stalk's branches or main axis (Fig. 2A). The florets were then multiplied and enlarged, as shown in Fig. (2B & C). Fig. (2D) depicts a mature bud that is ready to open and begin the flowering process.
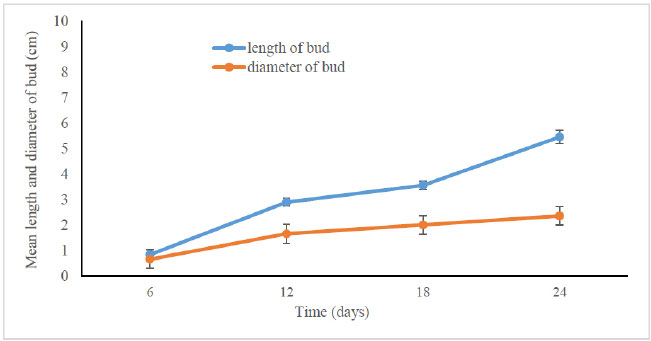
According to Tanaka et al. [20], the florets went through seven developmental stages, beginning with floret primordium formation, then sepal formation, labellum formation, petal formation, column formation, anther cap and stigma formation, and pollen formation. Furthermore, the diameter increased constantly and significantly following the increase in bud length. The length of the bud on day 6 was only 0.83 ± 0.56 cm while the diameter was 0.65 ± 0.33 cm. Subsequently, both the length and bud increased after it multiplied on the flower stalk until it became a mature bud on day 24. (Fig. 3).


3.4. Flower Development Stages and Senescence (Stages 6 to 11)
The observation of the flower blooming phase was recorded from an undisturbed and unpollinated plant. The P. labukensis flower started to bloom on day 28 after spike initiation. On the other hand, the flowering time of other orchids was observed to be between February and April. Meanwhile, P. laycockii was reported to produce flowers in July [21]. The time it takes for plants to form flowers varies greatly depending on the species. Weather, light, and environmental stress all have an impact on the process of bud formation until the formation of blossoming flowers [22].


The formation inflorescence at an average length of 10.50 ± 0.89 cm appeared from the stem node and was slightly erect to pendulous with more than 2-3 flowers as shown in (Fig. 4). According to Kirchoff & Claßen-Bockhoff [23] orchids with more flowers per inflorescence have been shown to attract more pollinators which could increase the chances of capsule development.
Meanwhile, Fig. (5A-D) summarised the progression of flowering stages (Stages 6 to 10). The flowering staged process was first observed when an axial bud appeared and developed into an enclosed floral bud. When the mature bud was fully formed, a partially opened flower (Fig. 5A) formed. The petals and sepals fully opened and bloomed after five days, as shown in Fig. (5B). Fig. (5C & D) depicted the front and side views of a P. labukensis flower, respectively.

| Days (Aged) | 3 | 6 | 9 | 12 | 15 |
|---|---|---|---|---|---|
| Length of the capsule (cm) |
4.40 ± 0.21 | 5.00 ± 0.30 | 8.40 ± 0.35 | 9.50 ± 0.43 | 10.00 ± 0.50 |
| Diameter of the capsule (cm) | 0.55 ± 0.20 | 0.72 ± 0.35 | 0.85 ± 0.55 | 1.08 ± 0.53 | 1.15 ± 0.53 |

The flower arrangement was asymmetric and in the shape of a panicle. The column is a central structure that houses both the male (anther) and female (stigma) parts of the flower. A modified petal, such as a labellum or lip, acts as a landing area for pollinators beneath the column. It took approximately 58 days to complete the whole floral development of P. labukensis (Fig. 5C & D). The flower of P. labukensis was 5.80 ± 0.35 cm in length when measured longitudinally across the sepal from left to right. The size of the flower measured was smaller than that reported by Chan & Cribb [4] where the size of the flower was 6.2 cm. As a result, it took one week for the flowers to wilt signature for the senescence event. As per observations, the wilting of the P. labukensis flower started with the closing of the lateral sepal followed by the dorsal sepal.
3.5. Capsule Development and Seed Morphology
Artificial pollination or hand pollination was used to create capsules. This technique required physically transferring pollen from one flower's stamen to another's pistil. The flower from which the pollen is collected is known as the pollen donor or pollen parent, whereas the bloom from which the pollen is received is known as the seed parent [24]. Hand pollination is frequently performed with a cotton swab or a tiny needle. The most typical reason for utilising this strategy is that there are no or few pollinators because the plants were in a greenhouse. After the flower was pollinated, the length, diameter, and colour change of the capsule were observed. Flowers that were two to three days old and in full bloom were hand-pollinated by depositing pollen on the same flower and began to senesce after three days of pollination. After three days of pollination, the flower petal began to wilt (Fig. 6A-C), and the lateral sepal dorsal sepal closed. The formation of P. labukensis capsules was between 40 to 90 days of pollination (DAP). When capsule formation began, the ovary began to swell (Fig. 6D), and the diameter increased from 0.5 ± 0.2 cm to 0.8 ± 0.3 cm within seven to ten days of pollination. The length and diameter of the capsule remained unchanged after ten days of pollination until capsule maturation, with an average length of 1.0 ± 0.25 cm. The dried petals remain attached to the tip of the ovary until maturation. The capsule's colour changed from dark green to dark red five days after pollination (Fig. 6E). The capsule was pubescent and erect, with thick-walled walls that were rather dry and papery. As P. labukensis capsules take 125 days to mature, the capsules were harvested before the capsule ruptured.
Based on Table 2, capsules aged between 100 to 120 days Among the three different capsules aged tested, 120 days capsule had the longest length of with 21.5 ± 0.50 mm, followed by 90 days capsule (15.2 ± 0.25 mm), and 40 days capsule has the shortest length of seed with only 5.00 ± 0.20 mm.
In this experiment, pollinating young and completely open flowers was employed since pollen is most responsive for 1 to 8 days following bloom opening, and thus, this could increase the chances of capsule formation [25, 26]. In this study, young and fresh flowers of P. labukensis with yellowish pollinia at the age of two to three days were used. Fresh and bright yellow pollinia are more fertile than the orange-brown like in Cymbidium orchids [27, 28]. The success of the capsule formation in this study was increased by the hand-pollination technique with a 95.47% success rate, of which some pollinated flowers did not develop into capsules due to fungi infection or eaten by predators such as ants before maturation [29]. When pollination occurs, the ovary will begin to swell and form a seed capsule filled with millions of seeds [30].
The time taken for an orchid capsule to reach full maturity varies according to species [31]. For P. labukensis, the capsules took 120 days to reach full maturity. Unlike other orchid species such as H., discolour took 25 to 30 days after pollination [32]. Even, there has been no significant effect of different pollen ages on the capsule development of H. discolour [32]. One of the reasons for the long seed maturation times for some species of orchids is the fact that the ovules are either not yet formed or immature at the time of pollination.
3.6. Morphology of Seed
The difference between young and mature capsule seeds can be seen; the seeds in the young capsule are creamy yellow, soft, and sticky (Fig. 7B). Whereas the mature capsule is drier, with only a few sticky seeds (Fig. 7D) which indicates the seeds are matured and ready for germination. The seed of P. labukensis darkened to a dark brown colour as it matured due to an increase in the amount of suberin in the seed coat, which may have contributed to the seed's hydroponic nature (Fig. 7C & D). Capsules and seeds were dried and powdery-like in structure so that they could be easily spread on the culture medium. It is important to note the maturity of seed capsules can vary depending on factors such as temperature, humidity, light, and nutrition. Additionally, some orchid species may have seed capsules that take longer to mature, while others may develop more quickly [31, 33].
The seed morphology of P. labukensis was found to be oblong or slightly fusiform (Fig. 7E). Swamy et al. [34] discovered that the seeds of Bulbophyllum mysorense are transparent, short, spindle to oblong, and have blunt ends. Augustine et al. [35] discovered fusiform, spindle, and narrowly ellipsoidal seeds in Bulbophyllum species. Seed shape variations can be used as an additional taxonomic marker for species identification.
CONCLUSION
The study was focused on the flowering development patterns of P. labukensis starting from bud initiation until the senescence stage. It was found that the P. labukensis took a month to develop a complete set of inflorescences with twelve sequences of flower developmental stages. The appropriate time to pollinate the flower of P. labukensis was between the third and fourth days of blooming while capsules harvested between days 110 till 120 days of pollination was the most appropriate period to harvest and culture for seed germination. Hence, these results could help to estimate the best capsule age for an efficient and successful in vitro propagation of this endangered orchid species.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available within the article.
CONFLICT OF INTEREST
The authors declare no conflict of interest financial or otherwise.
FUNDING
None.
ACKNOWLEDGEMENTS
The author would like to thank Poring Orchid Centre, Ranau, Sabah, for the supply of P. labukensis sample and Sabah Biodiversity Center (SaBC) for providing access license ref. no. JKM/MBS.1000-2/2JLD10(6).


