All published articles of this journal are available on ScienceDirect.
Sorbus Aucuparia L. Fruit Extract and its Cosmetics – As Promising Agents for Prophylactic and Treatment of Pyodermitis: Phytochemical and Microbiological Research
Abstract
Background:
The problem of acne is relevant. The market mostly represents synthesized substances for prevention and treatment, but medical cosmetics based on plant-origin raw material, especially fruits, can be offered. The fruits of Sorbus aucuparia L. are rich in biologically active substances, which turned out to be unique in solving problems of acne.
Objective:
The aim of the research was to study the phenolic composition and microbiological activity of the S. aucuparia fruit extract and the proposed medicinal cosmetics to prove the possibility of their use for the treatment of pyodermitis.
Methods:
The phenolic compounds of the S. aucuparia fruit extract obtained with 70% ethanol solution were studied by HPLC. The microbiological research was carried out by methods of diffusion into agar.
Results:
In the S. aucuparia extract gallic acid, gallocatechin, epigallocatechin, catechin, epicatechin, epicatechin gallate, ellagic acid, hyperoside, rutin, quercetin-3-D-glucoside, chlorogenic, caffeic, ferulic and р-coumaric acids, umbelliferone were identified and quantified. The antimicrobial activity of the S. aucuparia extract has been established. The synergism of antimicrobial activity of 1/4 and 1/64 minimal suppression concentration erythromycin against all tested Staphylococci strains was manifested by the extract. Biological active substances of S. aucuparia fruits more effectively restore sensitivity to erythromycin in Staphylococci with low macrolide resistance of Staphylococci (MLS-resistance) due to blocking reflex mechanisms.
Conclusion:
The S. aucuparia fruit extracts are rich in phenolic compounds and show antimicrobial activity against all used test-strains of microorganisms. The results of the study indicate their high antimicrobial activity against the main pathogens of pyodermitis - S. aureus, S. epidermidis, and Propionibacterium acnes.
1. INTRODUCTION
Medicines based on phenolic compounds are widely used for their antimicrobial, hemostatic, choleretic, diuretic, hypotensive, tonic, and astringent properties. Most phenolic compounds of plant origin are low-toxic and usually do not have side effects [1, 2]. Of particular interest is Sorbus aucuparia L. raw material, which has long been used in folk medicine [3-5]. Brunner (1985) investigated the antifungal properties of sorbic acid isolated from the S. aucuparia fruits [6]. Sorbus fruit extracts stopped the growth of molds on bread and jam, as well as molds and yeasts on grape agar. Further purification and UV studies showed that the antifungal component is identical to sorbic acid [7, 8]. Taking this into account, it is advisable to use Sorbus products for the treatment of skin infections.
S. aucuparia L. (European rowan, Rosaceae) is a widely spread tree, which is commonly grown as an ornamental plant. The berries are traditionally used for manufacturing jams, syrups, and liquors. In folk medicine, they have been used for treating bronchitis and gastrointestinal disorders, as well as anti-inflammatory, diuretic, vasorelaxant, and anti-diabetic agents and vitamin sources [9, 10]. S. aucuparia fruits are rich in organic acids, microelements, ascorbic and sorbic acid, carotenoids, and phenolic compounds, especially phenolic acids [11,12]. The bark of the plant has also been studied: 40 carboxylic acids and 39 components of essential oil were determined [13]. S. aucuparia L. fruit phytochemicals, particularly phenolic compounds, demonstrate various biological activities such as antioxidant, anti-inflammatory, anti-diabetic, anti-cancer, and cell regulatory ones [10, 14-16].
The problem of acne vulgaris is not new, researching natural remedies to alleviate this health problem is relevant [17-19]. The market in most cases represents a range of drugs for prevention and treatment of acne based on synthesized substances. In principle, it is possible to offer medical cosmetics based on fruits of medicinal plants, which are rich in biological active substances (BAS), which turned out to be unique in solving problems of acne-hormone ordinary (S. aucuparia L.) [10, 20-22]. Thus, it is advisable to develop a method of obtaining liquid extracts from Sorbus aucuparia fruits, propose some medicinal cosmetics on their basis, and conduct a study of their antimicrobial activity. For this purpose, museum and skin isolates of microorganisms were used as test cultures: antibiotic sensitive and antibiotic-resistant strains S. aureusi and S. epidermidis, isolated from patients with pyodermitis, antibiotic sensitive E. coli and Bacillus subtilis, collection strains of Propionibacterium acnes ATCC 6919 and ATCC 11827, and the culture of yeast-like fungus Candida albicans.
The aim of the research was to study the phenolic composition and microbiological activity of S. aucuparia L. fruit extract and propose some medicinal cosmetics to prove the possibility of their use for the treatment of pyodermitis. It also was interesting to detect the synergism of antimicrobial activity of erythromycin with Sorbus fruit exacts relative to skin isolates Staphylococcus aureus with various mechanisms of MLS-resistance.
2. MATERIALS AND METHODS
2.1. Materials
For the research, a liquid extract of S. aucuparia fruits was used and developed on their basis a lotion, a cream, and a mask. S. aucuparia fruits were collected in Ivano-Frankivsk region, Halytskyi district, village Tyaziv (49.01487805049442, 24.68280436695995), 2018. The identity of the plant was established with the consulting assistance of Professor A.R. Grytsyk (the Ivano-Frankivsk National Medical University (IFNMU)) according to the botanical catalog [23]. Voucher specimens No. 455–457 were deposited at the Department of Pharmaceutical Management, Drug Technology and Pharmacognosy, Ivano-Frankivsk National Medical University. The raw material was dried for 14 days at room temperature in a well-ventilated area and stored in paper bags.
2.2. The Method of Obtaining Extract and Lotions
The liquid extract from S. aucuparia fruits was obtained by the percolation method. The extraction was carried out in a laboratory percolator, using 70% ethanol as an extractant.
500.0 g of crushed S. aucuparia fruits were placed into a container, and 500 mL of 70% ethanol solution was added, mixed, and left to swell for 4 hours in the closed container. The swollen raw material was loaded into the percolator, compacting tightly. The top was covered with filter paper, pressing down with the load. The raw material was poured with an extractant until a “mirror” was formed and insisted for 24 hours. After the infusion, percolation took place, the essence of which consisted of the simultaneous collection of the liquid extract and the supply of fresh extractant at a rate of 3-4 mL/min. The first portion of the extract was collected in a separate container in the amount of 85% of the weight of the loaded raw material (430 mL). Next, percolation was carried out until the raw material was completely exhausted (with a step of DER 1:1), due to which 8 extracts were obtained. The extraction process was controlled by quantitative determination of polyphenolic compounds in obtained extracts in portions with a DER step of 1:1, by spectrophotometry on an Evolution 60s spectrophotometer (Thermo Fisher Scientific, USA). The obtained extracts were evaporated under vacuum on a rotary evaporator at a temperature of 40-50 oС to 15% of the raw material mass loaded into the percolator (170 mL). Next, the thickened residue was dissolved in the first portion of the extract and kept at a temperature of 10 oC for 48 hours. The finished extract was filtered through a paper filter. The liquid extract was standardized by the content of dry residue, the amount of polyphenolic compounds, and the content of ethanol according to the methods of SPhU 2.0 [13, 24].
The composition of the lotion (100.0) is the liquid S. aucuparia fruit extract 20.0 g, citric acid 0.5 g, miramistin 0.1 g, lavender oil 0.1 g, mint oil 0.1 g, propylene glycol 10.0 g, ethanol 70% 40.0 and purified water 29.2. Making the lotion, the solubility of medicinal substances in the proposed solvents: purified water and 70% ethanol considered. In separate containers, citric acid was dissolved in purified water, and miramistin – in 70% ethanol solution. The resulting solutions were combined. Propylene glycol and the S. aucuparia fruit liquid extract were added to the mixture. Finally, lavender and mint essential oils were added and shaken well. The resulting lotion was kept for settling for 24 hours at 10 oC, after that, it was filtered into a container.
2.3. HPLC Analysis
For the identification, separation, and quantification of phenolic compounds in the S. aucuparia L. fruit extract, HPLC was used [24, 25]. The research was carried out on an Agilent 1200 3 D LC System Technologies chromatograph (USA), which is equipped with a G1322A flow-through vacuum degasser, a G13111A four-channel low-pressure gradient pump, a G1329A autosampler (automatic injector), a G 1316A column thermostat, G1315C diode matrix and G1362A refractometric detectors. [26-28].
In the study of tannin metabolites, methanol (A) and a 0.1% solution of formic acid in water (B) were used as mobile phases. Elution was performed in a gradient mode: 0 min –A (20%): B (80%); 25 min - A (75%): B (25%); 27 min – A (100%): B (0%); 35 min - A (100%): B (0%). Separation was carried out on a Zorbax SB-C18 chromatographic column (3.5 μm, 150 x 4.6 mm) (Agilent Technologies, USA), flow rate through the column 0.25 ml/min, thermostat temperature 35 °C, volume injection container 4 μl. Detection was carried out using a diode-matrix detector with signal registration at 250 and 275 nm and fixation of absorption spectra in the range of 210-700 nm.
To study hydroxycinnamic acids, methanol (A) and a 0.1% solution of formic acid in water (B) were used as mobile phases. Elution was carried out in the gradient mode: 0 min –A (25%): B (75%); 25 min - A (75%): B (25%); 27 min - A (100%): B (0%); 35 min - A (100%): B (0%). The separation was carried out on a Zorbax SB-Aq chromatographic column (4.6 mm ± 150 mm, 3.5 μm) (Agilent Technologies, USA), the flow rate through the column was 0.5 ml/min, the temperature of the thermostat was 30 °C, injection volume 4 μl. Detection was carried out using a diode-matrix detector with signal registration at 250 and 275 nm and fixation of absorption spectra in the range of 210-700 nm.
When studying flavonoids, acetonitrile (A) and a 0.1% solution of formic acid in water (B) were used as mobile phases. Elution was performed in the gradient mode: 0 min –A (5%): B (95%); 20 min - A (30%): B (70%); 30 min - A (60%): B (40%); 50 min – A (100%): B (0%); 60 min - A (100%): B (0%). The separation was carried out on a Zorbax SB-C18 chromatographic column (3.5 μm, 150 x 4.6 mm) (Agilent Technologies, USA), the flow rate through the column was 0.25 ml/min, the temperature of the thermostat was 30 °C, the injection volume 4 μl. Detection was carried out using a diode-matrix detector with signal registration at 280 and 365 nm and fixation of absorption spectra in the range of 210-700 nm.
Identification and quantitative analysis were performed using solutions of standard substances. The measurements were made three times. The results are presented as an average mean and a standard deviation.
2.4. Microbiological Analysis
A first step screening of antimicrobial activity of the obtained extract and the cosmetics was carried out by micro method of diffusion into agar developed at the Department of Microbiology, Virology and Immunology of Ivano-Frankivsk National Medical University [29, 30].
As it is important from a clinical point of view, the next step was the study of the antimicrobial activity of the S. aucuparia cosmetics (lotion, cream, mask) on clinical isolates of antibiosensitive and antibioresistant microorganisms. Bacterial cultures were identified based on biochemical microtests “STAPHYtest 16”, “ENTEROtest 24”, “NEFERMENTtest 24” (Lachema, Czech Republic), as well as considering the complex morphological and cultural properties according to recommendations of the 9-th edition of “Bergey's Manual of Systematic Bacteriology”. Yeast-like mushroom cultures were identified based on 40 biochemical tests with the VITEK 2 system using VITEK 2 YST ID card (Biomerieux, France) [29, 31]. The antimicrobial activity of the S. aucuparia fruit liquid extract and its lotion were studied with the help of a micro method of diffusion into agar [32, 33].
30 ml of agar was poured in Petri cups located on a strictly horizontal and level surface. After the environment had been established, a special probe with equal edges produced wells with a diameter of 4.0 mm. The agar was evenly used by the suspension of the test culture (concentration of 1x107 KUO/ml). In a study, 20 μL of preparations were confirmed in wells, whereas 20 μL 70% ethanol solution was used in control. After cultivation in 24 hours, the diameters of the zones of growth delay of bacterial test cultures were determined. Registration of fungistatic activity was carried out after 2 days, and fungicide - after 4 days of cultivation. Strains of P. acnes were cultivated for 5 days on Brain Heart Infusion Agar (HiMedia Laboratories Pvt. Ltd., India) in gas bags for anaerobes (GasPak™ EZ, Bacton, Dickinson and Co., USA) at 37 °C. Digital images of crops on plates were obtained, which were processed using the Image Tool 2.0 computer program (UTHSCSA ImageTool 2.0, The University of Texas Health Science Center in San Antonio, © 1995-1996) [34, 35]. To study the antimicrobial activity of the cosmetic cream and mask, these agents were introduced in wells with a diameter of 13,8±0,1 mm. To unify the results from the obtained diameter of the growth inhibition zones, removed the diameter of the well filled with the medicines.
The next step of the research was to detect the synergy of antimicrobial activity of the S. aucuparia L. fruit extract and its cosmetics with erythromycin. It was investigated with clinical isolates of MLS-resistant Staphylococci. The antibiotic at a final concentration of 1/4 or 1/64 MIC was added to the nutrient agar. The extract and the cosmetics were introduced into wells of 20 μl. After 24 hours of incubation, the diameters of Staphylococcus growth inhibition zones were compared under the influence of the plant medicines on antibiotic-free medium and on media with sub-bacteriostatic erythromycin concentrations. All measurements were made three times. The results are presented as an average mean and a standard deviation.
3. RESULTS
3.1. Phytochemical Profile
HPLC was used to identify, separate and quantify phenolic compounds in the S. aucuparia fruit extract. The content of tannins (gallic, ellagic acids and catechins) was determined at wavelengths of 280 and 255 nm (Table 1).
| Catechins | Quantitative Content, mg/100 g | Flavonoids | Quantitative Content, mg/100 g | Hydroxycinnamic Acids, Coumarins | Quantitative Content, mg/100 g |
|---|---|---|---|---|---|
| Gallic acid | 63 ± 2 | Hyperoside | 150 ± 4 | Chlorogenic acid | 4200 ± 49 |
| Gallocatechin | 5030 ± 54 | Rutin | 147 ± 4 | Caffeic acid | 60 ± 3 |
| Epigallocatechin | 4730 ± 43 | Quercetin-3-D-glucoside | 60 ± 3 | Ferulic acid | 330 ± 7 |
| Catechin | 610 ± 8 | - | - | р-Coumaric acid | 105 ± 4 |
| Epicatechin | 262 ± 5 | - | - | Umbelliferon | 710 ± 6 |
| Epicatechin gallate | 210 ± 6 | - | - | - | - |
| Ellagic acid | 84 ± 3 | - | - | - | - |
Determination of the flavonoid content was carried out at a wavelength of 255 nm (Table 1). Determination of the content of hydroxycinnamic acids and hydroxycoumarin was conducted at a wavelength of 320 nm (Table 1).
3.2. Antimicrobial Activity
The results of the studies of the antimicrobial activity of the S. aucuparia fruit extract and its lotion are presented in Table 2.
As a serious problem in modern dermatological practice is the resistance of Staphylococci, the clinical samples of Staphylococcus (S. aureus, S. epidermidis) were also used in the experiment and the results are present in Table 3.
Since acne vulgaris takes an important place in the structure of cosmetological pathology occupied by acne, the extract and the lotion have also been tested for antimicrobial activity against two museum strains of Propionibacterium acnes (Table 4).
Considering the pronounced antimicrobial activity of the Sorbus fruit extract against staphylococci and propionibacteria, in addition to the lotion, we also produced other cosmetics (a cream and a mask). The developed cosmetics are complex products that contain the liquid Sorbus fruit extract and some excipients. Therefore, the question of their compatibility with biological active substances of the Sorbus fruit extract naturally arises. In this regard, the antimicrobial activity of excipients and cosmetics were studied (Table 5 and Fig. 1).
| Drugs |
S. aureus MSSA |
S. aAureus MRSA |
E. coli | B. subtils | C. albicans | |
|---|---|---|---|---|---|---|
| Fungistat | Fungicide | |||||
| 70% ethanol (control) | 6.14 ± 0.37 | 7.48 ± 0.39 | 7.25 ± 0.78 | 6.30 ± 0.42 | 5.55 ± 0.31 | 4.25 ± 0.48 |
| LESF | 9.82 ± 0.77* | 11.19 ± 0.56* | 6.11 ± 0.52 | 4.79 ± 0.27 | 4.11 ± 0.32 | 5.81 ± 0.97 |
| LS | 10.19 ± 0.75* | 12.89 ± 0.68* | 4.79 ± 0.44 | 8.09 ± 0.31* | 7.00 ± 0.44* | 6.82 ± 0.14* |
LG – the lotion with the liquid extract of S. aucuparia fruits.
| Drugs |
S. epidermidis Denisenko, R-phenotype |
S. epidermidis "Kryl", D- phenotype (Ind+) |
S. Aureus "Cherepchuk", Neg- phenotype (Ind–) |
|---|---|---|---|
| 70% ethanol (control) | 0 | 0 | 6.14 ± 0.37 |
| LESF | 13.91 ± 1.05* | 7.85 ± 0.41* | 15.00 ± 0.48* |
| LS | 18.9 ± 0.96*/** | 16.15 ± 0.79*/** | 17.14 ± 0.45* |
| Drugs | P. acnes ATCC 6919 | P. aligncnes ATCC 11827 |
|---|---|---|
| 70% ethanol (control) | 0 | 0 |
| LESF | 9.05 ± 0.33* | 10.65 ± 0.5* |
| LS | 17.38 ± 0.07*/** | 19.07 ± 0.17*/** |
| Cosmetic Forms |
S. epidermidis "Kril", D-phenotype (Ind+) |
S. aureus "Cherepchuk", Neg-phenotype (Ind–) |
P. acnes ATCC 6919 |
P. acnes ATCC 11827 |
|---|---|---|---|---|
| Cosmetics based on Sorbus fruit extract | ||||
| REPS | 7.85 ± 0.41 | 15.00 ± 0.48 | 9.05 ± 0.33 | 10.65 ± 0.5 |
| LS | 16.15 ± 0.79 | 17.14 ± 0.45 | 17.38 ± 0.07 | 19.07 ± 0.17 |
| CS | 13.11 ± 0.81 | 16.82 ± 0.87 | 17.03 ± 0.72 | n.і. |
| MS | 10.32 ± 0.57 | 12.77 ± 1.35 | 18.04 ± 0.99 | n.і. |
| Supporting components | ||||
| 70% ethanol (control) | 0 | 0 | 0 | 0 |
| Lavandula essential oil (0.1%) | 0 | 6.57 ± 0.45 | 0 | 0 |
| Mentha essential oil (0.1%) | 0 | 9.4 ± 0.65 | 0 | 0 |
| Miramistin (0,1%) | 5.82 ± 0.28 | 6.98 ± 0.63 | 0 | n.і. |
CS – the cream with the liquid extract of S. aucuparia fruits.
MS – the mask with the liquid extract of S. aucuparia fruits.
| Drugs |
S. epidermidis "Kril", D-phenotype (Ind+) |
S. aureus "Cherepchuk", Neg-phenotype (Ind–) |
||||
|---|---|---|---|---|---|---|
| ERY-free Medium |
1/64 MIC ERY |
1/4 MIC ERY |
ERY-free medium |
1/64 MIC ERY |
1/4 MIC ERY |
|
| REPS | 7.85 ± 0.41 | 12.08 ± 0.22 | 12.80 ± 0.32 | 15.00 ± 0.48 | 17.00 ± 0.26 | 12.53 ± 0.14 |
| LS | 16.15 ± 0.79 | 15.00 ± 0.7 | 14.49 ± 0.48 | 17.14 ± 0.45 | 18.44 ± 1.46 | 20.21 ± 0.07* |
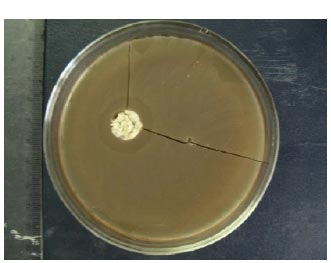
The synergism of the antimicrobial activity of the liquid Sorbus fruit extract, the lotion and erythromycin against skin isolates of staphylococci with different mechanisms of MLS resistance was studied and is presented in Table 6.
4. DISCUSSION
4.1. Phytochemical Profile
The obtained data (Table 1) show that the liquid S. aucuparia L. fruit extract is rich in catechin and epigallocatechin. The results of the phytochemical research show that the dominant flavonoids are rutin and hyperoside; the main hydroxycinnamic acids in the extract are chlorogenic and ferulic acids. The high content of umbelliferone in the extract should also be marked.
The obtained results confirmed that chlorogenic (3-O-caffeoylquinic acid) acid is the main phenolic acid reported in Sorbus spp. [4, 38-40]. Moreover, it has been reported that caffeoylquinic acids constitute 56–80% of the total phenolics in Sorbus fruits, whereas the cultivated berries contain less caffeoylquinic acids than wild rowanberries [3], but in our case, it is about 25% of total phenolic compounds content. The content of chlorogenic acid in the fruits of S. aucuparia was up to 10.01 mg/g dw [40], while in the obtained extract, there is 42 mg/g dw of it, so that is quite a high level. Ferulic acid in methanol and water extracts and jams was also previously reported [41] and is confirmed in our research.
Quercetin, kaempferol, isoquercetin, rutin, hyperoside and isorhamnetin were reported in the samples of Sorbus fruits. The quercetin and its glycosides are the main flavonoids in Sorbus spp., that is consistent with our results. The content of rutin in water and methanol extracts of S. aucuparia was found similar, 82.3 and 80.4 µg/g dw, respectively [41], but in our aqueous-ethanol extract, its concentration is about two times higher.
Epicatechin was also reported in the leaves of many Sorbus spp. [42], as well as in the fruits of S. aucuparia [3], but there is no information about coumarins, so umbelliferon was detected for the first time.
Since Sorbus polyphenols (proanthocyanidins, chlorogenic acid isomers, and flavonols) are recognized as potent antioxidants and health-beneficial phytochemicals, and considering the significant phenolic content in various Sorbus spp., it can be concluded that their products could be excellent sources of natural antioxidants [38], but Sorbus preparations may also be interesting as cosmetics for prophylactic and treatment of piodermitis, due to antimicrobial properties of their phenolic compounds.
4.2. Antimicrobial Activity
According to the results of the antimicrobial studies, it was found that the S. aucuparia fruit extract has an antimicrobial effect mainly only against Gram-positive bacteria, especially Staphylococci. Gram-negative bacteria (in particular, E. coli) are not sensitive to it. The antifungal effect of the extract against Candida is weak. It was found that antibiotic-sensitive (MSSA) and multiple resistant (methicillin-resistant, MRSA) strains of Staphylococcus aureus are equally sensitive to the biologically active substances of the S. aucuparia fruit extract, as well as to its cosmetic lotion (Table 2).
A serious problem in modern dermatological practice is the acquisition of macrolide resistance of Staphylococci (the so-called MLS resistance) since erythromycin and its semi-synthetic derivatives are considered the drugs of choice for the treatment of pyodermitis [42-45]. Resistance of Staphylococci to macrolides is caused by various molecular mechanisms and there are distinct parallels between its genetic determinants and phenotypic manifestation [46, 47]. In this regard, it was important for clinical practice to compare the sensitivity of Staphylococcus (S. aureus, S. epidermidis) with different mechanisms of MLS-resistance to the S. aucuparia fruit extract (Table 3). The results indicate that all 3 main phenotypes of MLS-resistant Staphylococci are highly sensitive to the biologically active compounds of the extract, as well as the lotion. The antistaphylococcal activity of the S. aucuparia fruit extract is higher for S. epidermidis with R- and D-phenotypes of MLS-resistance p < 0.05. For the same strains, the lotion also showed significantly higher antimicrobial activity than the extract.
The liquid S. aucuparia fruit extract also showed high activity against both strains of P. acnes (ATCC 6919 and ATCC 11827). The lotion with the extract caused intense inhibition of both Propionibacteria cultures. The highest activity was shown against P. acnes.
The results presented in Table 5 indicate the high antimicrobial activity of all developed cosmetic forms against the main pathogens of pyodermitis - S. aureus, S. epidermidis and P. acnes. The excipients used showed minimal activity against these microorganisms. Thus, the essential oils of Mentha and Lavandula at a concentration of 0.1%, which corresponds to their content in the lotion, significantly suppressed the growth of S. aureus “Cherepchuk” with a non-inducible type of resistance to macrolides. Their significant influence on the growth of both test strains of P. acnes in the conditions of the performed experiment was not observed. The well-known antiseptic miramistin showed weak activity against S. aureus and S. epidermidis, but also did not act on the growth of P. acnes.
The results of the performed experiment also allow us to draw a conclusion about the good pharmacological compatibility of the selected ingredients. Their combination not only reduces, but even increases the antimicrobial effect of the liquid S. aucuparia fruit extract. It can be assumed that miramistin, in all cosmetic forms, increases the permeability of the bacterial cell wall and membrane for the biologically active components of the extract.
Therefore, correction of the condition of the facial skin in complicated and advanced forms of pyodermitis requires the appointment of patients not only with cosmetic products but also with more intensive treatment, including antibiotics. Antibiotics of the macrolide group are the drugs of choice in the implementation of the antimicrobial treatment of pyoderma [29, 32, 48]. Therefore, it is of great practical importance to clarify the issue of the pharmacological compatibility of the developed cosmetics with the Sorbus fruit extract and macrolides, in particular with erythromycin. For this, it was studied the antimicrobial activity of the extract against skin isolates of staphylococci in the presence of sub-bacteriostatic concentrations of erythromycin. This experimental approach allows us to evaluate the nature of the interaction of antimicrobial drugs (synergistic, antagonistic, indifferent). 2 skin isolates of staphylococci with fundamentally different mechanisms of MLS resistance as test strains were used. The strain of S. epidermidis “Kril” is characterized by a high level of resistance to macrolides (MBcK of erythromycin 2000 μg/ml, MBcC – 4000 μg/ml). In addition, erythromycin could induce resistance of this strain to clindamycin. The inducible type of MLS-resistance of staphylococci is based on the post-transcriptional modification of ribosomal 23S-rRNA by the enzyme adenine-N6-methyltransferase [49, 50]. The strain of S. aureus “Cherepchuk” is characterized by a low level of MLS-resistance (MBsC of erythromycin 125 μg/ml, MBsC - 250 μg/ml), which applies only to 14- and 15-membered macrolides without induction of resistance to other antibiotics of the MLS group (in particular on clindamycin). This MLS-resistance phenotype is ensured by the active efflux of macrolides from microbial cells, mediated by an ATP-dependent membrane pump of the ABC type [34]. The use of microbial strains with fundamentally different mechanisms of MLS resistance makes it possible to assess with high reliability the nature of the interaction of the investigated drugs [51-53]. During the experiment, it was established that the Sorbus fruit extract does not have an antagonistic effect on the antimicrobial activity of erythromycin against both strains of staphylococci. Moreover, the liquid extract of Sorbus fruits often showed a pronounced dose-dependent antimicrobial synergism with erythromycin against S. aureus “Cherepchuk” with an efflux mechanism of MLS-resistance: the diameters of the growth inhibition zones on media with 1/4 MIC and 1/64 MIC of erythromycin were respectively, by 20.8% and 7.7% higher than on the medium without the antibiotic (p<0.05). The lotion with the Sorbus fruit extract on media with sub-bacteriostatic concentrations of erythromycin retained high antimicrobial activity against MLS-resistant staphylococci. The lotion with the liquid extract of Sorbus fruits also showed weak dose-dependent synergism with erythromycin against S. aureus “Cherepchuk”.
So, the liquid extract of S. aucuparia fruits showed greater antibacterial potential against Gram-positive bacteria (including dominant skin pathogens staphylococci and Propionibacterium acnes). Taking into account the chemical composition of the S. aucuparia fruit extract, there is every reason to assume that its antimicrobial effect is due to phenolic compounds: phenol carboxylic acids (mainly chlorogenic acid), catechins and sorbic acid, as well. It could be suspected that these compounds influence on the bacterial cell wall and membrane rigidity, permeability, or integrity with subsequent inactivation of metabolically critical enzymes.
The observed erythromycin potentiating activity of the S. aucuparia fruit extract against MLS-resistant staphylococci presumably involves inhibition of active transmembrane antibiotic efflux, but additional experiments are needed to prove this hypothesis. A number of plant-originated agents (including flavones, isoflavones, epicatechin gallate, and 4′,5′-dicofeilchinic acid) have been identified as bacterial efflux pump inhibitors. Further study of synergy with antibiotics will allow to improve the effectiveness of skin infection treatment caused by resistant strains by medical and cosmetic products created on the basis of S. aucuparia fruit extracts.
CONCLUSION
The quantitative content of hydrolysable tannins (gallic and ellagic acids), simple catechins (gallocatechin, epigallocatechin, catechin, epicatechin) and complex catechin (epicatechin gallate), flavonoids (rutinate hyperoside, quercetin-3-D-glucoside), hydroxycinnamic acids (р-coumaric, caffeic, ferulic, chlorogenic) and coumaric acid lactone (umbelliferone) were identified and determined by HPLC in the S. aucuparia fruit extract.
During the microbiological studies, it was established that the biologically active components of the liquid extract of Sorbus fruits have antimicrobial activity against gram-positive bacteria, especially staphylococci (S. aureus, S. epidermidis) and propionibacteria (P. acnes) - the main causative agents of pyodermitis. It applies equally to both antibiotic-sensitive, MLS- and methicillin-resistant strains of staphylococci of skin origin.
The composition and technology of the cosmetics (lotion, cream and mask) with this extract were developed. They maintain high antimicrobial activity against staphylococci and propionibacteria. Their combination with erythromycin does not lead to the appearance of antagonism of antimicrobial action. The developed therapeutic cosmetics based on the liquid extracts of Sorbus fruits can be used for the treatment of acne, furunculosis, and other forms of staphylodermia, as their active components have an etiotropic effect on the development of these diseases.
LIST OF ABBREVIATIONS
| HPLC | = High Performance liquid Chromatography |
| BAS | = Biological Active Substances |
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
The scientific research are fragments of complex research works of the Department of Pharmaceutical Management, Drug Technology and Pharmacognosy of Ivano-Frankivsk National Medical University “Research of some wild and cultivated medicinal plants of the western region of Ukraine and development of medicines based on them” (state registration number 0110U006205). This work was also supported by the European Union in the MSCA4Ukraine project “Design and development of 3D-printed medicines for bioactive materials of Ukrainian and Estonian medicinal plants origin” [ID number 1232466].
CONFLICT OF INTEREST
Dr. Ain Raal is the Editorial Board Member of the journal The Open Agriculture Journal.
ACKNOWLEDGEMENTS
The authors sincerely thank all the defenders of Ukraine who made the performance of this study possible.


