All published articles of this journal are available on ScienceDirect.
Root Silicon Amendment Enhances Barley’s Resistance to Fusarium Head Blight in the Field
Abstract
Background:
Silicon (Si) amendment plays an important role in enhancing the resistance of several plant species to diverse pathogens. To date, a few studies have focused on how Si application helps barley, a higher Si absorber and accumulator monocot, to resist fungal diseases, including Fusarium head blight (FHB), which reduces the quality and safety of harvested products worldwide. However, no study has ever been conducted to demonstrate Si's ability to suppress FHB development in barley heads under uncontrolled climatic conditions.
Materials and Methods:
This 2-year field study elucidated the effect of multiple Si applications at 1.7 mM via roots in two barley cultivars, Arabi Aswad (AS moderately resistant) and Arabi Abiad (AB moderately susceptible), to control four Fusarium species with diverse pathogenicity. The incidence of FHB (DI type I resistance), severity of FHB (DS type II), and Fusarium-damaged kernels (FDK type III) were also tested to describe the nature of the Si-enhanced barley resistance.
Results:
Si treatment at 1.7 mM under soil culture decreased FHB development by enhancing all resistance types measured in the present research. DI, DS, and FDK were reduced by 18.7%, 20.3%, and 20.2%, respectively, in Si-Fusarium-inoculated treatments relative to fungal-inoculated controls. Si absorption in barley strengthened the defense system measured by type I and type II on AB to a level comparable to AS not amended with Si. Irrespective of the barley cultivar, however, Si resulted in a quasi-similar reduction of FDK. Importantly, Si treatment at 1.7 mM decreased the damage of FHB in previous analyses conducted on AS and AB under in vitro and growth chamber environments, showing that Si enhanced the expression of resistance to FHB infection in seedlings and adult barley plants.
Conclusion:
All of these results are promising outcomes for the application of Si as a safe and effective method against Fusarium species. This study provides new insights into the potential of multiple Si applications at 1.7 mM via roots for boosting barley’s resistance to FHB with a bright prospect for Si use in barley cultivation under field conditions.
1. INTRODUCTION
Barley (Hordeum vulgare L.) is a major cereal crop, ranking fourth in terms of both quantity produced and area under cultivation in the world. The production of malt (27%) and animal feed (70%) is critical for humans [1]. Unfortunately, H. vulgare is vulnerable to a broad array of damaging fungal pathogens that reduce the harvest quality and quantity. Fusarium head blight (FHB) is one of the most destructive and difficult fungal diseases of small-grain cereals worldwide, including barley [2]. FHB is caused by more than 17 Fusarium species, of which F. graminearum and F. culmorum are of greatest relevance and considered the strongest pathogenic pathogens [3] due to their strong viability and wide range of hosts [4]. Barley is most susceptible to FHB during warm and wet conditions at anthesis; infection can be recognized by necrotic patches and bleaching of the florets and discoloured kernels (tan, orange, brown, pink, or red) scattered throughout the head. Fusarium infection results in reduced yield, quality of the harvested kernels, and the percentage of seed germination [5]. In addition, Fusarium-damaged kernels (FDK) can also decrease in weight by 20% compared to healthy kernels [6]. As barley can be invaded by many Fusarium species [7], a wide range of toxins, such as deoxynivalenol (DON), make the harvested grains unacceptable for the malting and brewing industry. Moreover, DON can cause adverse health effects in humans and animals upon consumption [8].
Genetic improvement of barley’s resistance to FHB is considered the best economical and effective option for disease management [7]. Barley’s resistance to FHB is quantitative in nature [4], and the mechanisms are either structural or biochemical, where the latter can be associated with particular proteins or metabolites [6, 9]. Quantitative FHB resistance documented in barley is associated with resistance to spikelet infection (type I), spreading of the infection within the spike (type II), and resistance to kernel infection (type III). These three main types of resistance to FHB are considered to contribute to “field barley FHB resistance” [2]. In general, barley exhibits high type II resistance; therefore, the focus of breeding programs has been on improving type I resistance [4]. However, the progress of FHB-resistant breeding in barley is relatively slow and difficult, which can not cater to the demand for resistant germplasm resources in agricultural production [9]. For this, the application of fungicides in barley heads is currently the most popular method and important measure to control FHB, but the enhancement of fungicide resistance in fungi, along with environmental concerns, has brought a great threat to human health and environmental sustainability [8]. Therefore, it is a hot topic in recent years to find a pollution-free and effective method to prevent FHB damage [5, 7].
The most promising method in this context is the use of silicon (Si). Although the essentiality of Si for plant growth and development has not been proven yet because concrete evidence is lacking on the biochemical and physiological role of Si in plant biology [10], both laboratory experiments and field trials have documented Si to have beneficial effects on not only plant growth and productivity, but also against biotic and abiotic stresses [11, 12]. Si could ameliorate the detrimental effects of diverse fungal pathogens by increasing cell wall strength, activating host defense response through increasing antioxidant enzyme activities or antifungal compounds, and regulating the signal transduction network [13]. In essence, the beneficial effects of Si are greatly hampered by the differences in the Si uptake mechanism of the plant [10]; therefore, the concentrations of Si in various plants differ depending on genotype and species [11]. For instance, barley is a Si accumulator species, absorbs Si at higher rates from the soil solution, and accumulates it in its shoots [14]. To date, a few studies have focused on how Si application helps barley to resist fungal diseases, i.e., powdery mildew (Blumeria graminis F. sp. hordei) and spot blotch (Bipolaris sorokiniana) [15, 16]. The reduction of powdery mildew and spot blotch intensity by Si results in better performance of the primary metabolism of barley plants [15, 16].
Until recently, only one report [17] has noted that a single application incorporated into soil and foliar spraying of Si under controlled conditions could reduce the infection rate and pathogen spreading in barley head tissues by enhancing type I and type II resistance. However, no study has ever been conducted to demonstrate silicon's ability to suppress FHB development in barley heads under uncontrolled climatic conditions. In the FHB-wheat pathosystem, the positive effect of multiple Si root applications supplied continuously into wheat plants relative to a single application incorporated into soil has been shown by enhancing the main types of host resistance to Fusarium infection in the growth chamber and in the field [18, 19]. Although all cited FHB-cereal literature studies have drawn conclusions for small-grain cereals based on wheat data [5, 9], Fusarium infection and mycotoxin contamination in barley have been specifically shown [8]. In view of the need for a better understanding of Si mechanisms to control FHB damage in barley, we hypothesize that any increase of Si in the shoots, as theoretically observed in wheat [18, 19], would improve barley’s defense against Fusarium. If the hypothesis of this study is correct, it will provide first knowledge on the effectiveness of multiple Si root applications as an optimal Si application method to enhance barley’s resistance to FHB. This benefit is important for the sustainable cultivation of barley, given the worldwide prevalence of FHB species.
This research has evaluated the effect of Si supply on enhancing the three “field barley FHB resistance” components in two barley cultivars with contrasting susceptibility to disease challenged with four Fusarium species, addressing the decrease for DI (type I resistance), DS (type II), as well as FDK (type III). The ability of Si to enhance resistance measured by DI, DS, and FDK from moderately susceptible to a level comparable to moderately resistant not amended with Si was also investigated. In addition, the relationships were examined between the current findings and the damage of FHB in previous analyses conducted on AS and AB under in vitro and growth chamber environments to check whether Si could increase the expression of resistance to FHB infection at the earliest and latest barley development stages during FHB infection.
2. MATERIALS AND METHODS
2.1. Study Site, Barley Materials, and Growth Conditions
The same pot-field experiment was performed during the two consecutive growing seasons of 2021 and 2022 under uncontrolled climatic conditions at Deir Al-Hajar Agricultural Experiment Station, Damascus countryside, Syria (longitude 36°26′, latitude 33°20′, and altitude of 600 m above the sea level). The area of the study was characterized by a dry and arid climate; the yearly potential evapotranspiration exceeded 2,000 mm and the average annual precipitation was about 120 mm. In each season, the field trial was established in plastic pots carrying non-used soil. Table 1 shows the monthly mean weather factors at the experiment site for the 2021 and 2022 seasons.
Table 1.
| Growing season | Variable | November | December | January | February | March | April | May |
|---|---|---|---|---|---|---|---|---|
| 2020/21 | Tmin (oC) | 9.8 | 5.6 | 3.7 | 3.7 | 6.9 | 10.1 | 13.0 |
| Tmax (oC) | 20.4 | 16.6 | 15.8 | 17.8 | 21.0 | 36.0 | 31.0 | |
| RH (%) | 73 | 72 | 82 | 71 | 74 | 46 | 50 | |
| Rainfall (mm) | 70 | 8.1 | 22.3 | 11.7 | 2.8 | 6.0 | 5.0 | |
| 2021/22 | Tmin (oC) | 7.9 | 4.7 | 3.2 | 4.5 | 6.5 | 8.9 | 14.2 |
| Tmax (oC) | 20.9 | 15.2 | 12.6 | 15.1 | 19.0 | 23.3 | 32.9 | |
| RH (%) | 72.0 | 70.5 | 82.0 | 74.0 | 68.0 | 59.5 | 45.0 | |
| Rainfall (mm) | 28.5 | 55.9 | 39.7 | 28.5 | 31.0 | 9.2 | 1.5 |
Throughout this study, two barley (Hordeum vulgare) cultivars of Syrian origin with different backgrounds and resistance levels to display a progressive range of Fusarium susceptibility were used as plant material. Arabi Aswad (AS), a moderately resistant cultivar and a moderately susceptible cultivar, Arabi Abiad (AB), based on previous FHB resistance assays by inoculation of detached leaves, seedlings, spikes and spikelets [20-22], were selected due to their similar cycle length, flowering period, and wide use in the market [23].
Barely seeds were surface sterilized with 5% NaOCl for 10 min, followed by rinsing three times with ultra-pure water. Eight sterilized AS and AB seeds were sown in autumn in plastic pots (20 × 15 cm) containing 2 kg of pasteurized soil. Using a gamma irradiator (ROBO, Russia), the pasteurization of the soil was carried out at 25 k Gy of gamma rays with a Co60 source. The soil used was air-dried and sieved (3 mm). The experimental soil was clay in texture (57% clay, 39% loam, 2% sand) with organic matter of 1.25%; K, Na, Ca, Mg = 1.81, 2.99, and 33.1, and 14 mg/100 g soil, respectively (P = 13.4 mM and pH 7.8). Ten days following emergence, each pot was thinned to five seedlings, and nitrogen, in the form of urea, was applied at 0.173 g/pot at two events: emergence and tillering. To decrease year impacts on the results, it appeared to be important in these arid conditions to help the growth development at roughly weekly intervals afterward by irrigation of barley pots.
2.2. Inoculation Procedure
Till now, the recovery of head blight pathogens has not been achieved from Syria barley plants [7]. Nevertheless, Fusarium species are frequently recovered from naturally infected wheat fields [22]. In 2021 and 2022, six F. solani, five F. culmorum, and four F. verticillioides (F. moniliforme) isolates were used, and one F. equiseti isolate was added. The 16 isolates were monosporic-derived cultures of the original wild-type isolates and were selected for their contrasting pathogenicity based on previous several experimental observations [21-23]. On Petri dishes with potato dextrose agar (PDA) with 13 mg/L kanamycin sulphate added after autoclaving, the isolates were morphologically identified with the aid of the Leslie and Summerell [24] manual on the basis of microscopic studies of the shape and size of macro- and micro-conidia, and were molecularly distinguished by RAPD markers. Each isolate was used independently (not mixed). FHB isolates were sampled from kernels of FHB symptomatic spikes through the 2015 growing season over several locations in Ghab Plain with FHB history, one of the principal Syrian wheat production areas. These 16 Fusarium isolates were used in previous experiments conducted under in vitro growth chamber and field trials to assess the resistance levels of AS and AB, and resistance levels were correctly and precisely distinguished [21, 22].
The 16 Fusarium isolates were maintained by freezing at -16°C or in sterile distilled water at 4°C, and fresh cultures were produced on PDA medium. Two-week-old cultures were used to collect macroconidia, and then fungal spores were suspended in 10 mL of sterile distilled water (SDW). The suspensions were filtered through two layers of sterile cheesecloth to remove agar and adhering mycelia. The spore concentration was adjusted prior to use with the aid of a Neubauer chamber under an optical microscope and diluted to 5 × 104 conidia/ml as inoculum sources.
2.3. Disease Evaluation
AS and AB were separately inoculated with 16 Fusarium cultures to evaluate the incidence of FHB (DI, type I resistance), severity of FHB (DS, type II), and Fusarium-damaged kernels (FDK, type III) as indicators of the cultivar’s resistance. At full flowering (GS=65) in the early morning, inoculation of barley plants was by foliar spraying of the spore suspension or SDW for non-inoculated barley plants. Uniformly spraying of the inoculum onto barley heads was carried out in one day. The inoculated barley heads were kept inside plastic bags, the inner surfaces of which had been sprayed with SDW. AS and AB plants were maintained under these conditions for 48 h in order to provide humid conditions favorable for the initial phase of pathogenesis.
DI, DS, and FDK were determined to decide the degree of Fusarium infection in light of visual damage in head tissues. Assessment of disease development rates (DDRs) was achieved at the beginning of heads with discolored spikelets that were demonstrative of head blight about 1 week after inoculation. Thereafter, the gradual blighting of heads was assessed at 14, 21, and 28 days post inoculation (dpi), when wheat heads were at the soft dough stage. At 21 dpi for each Fusarium isolate/AS and AB, DI for type I resistance was evaluated by calculating the number of heads with representative head blight symptoms (i.e., partially or fully bleached heads). The estimates of DDRs determined at 7, 14, 21, and 28 days during the evaluation period were considered as an indicator to determine DS for type II resistance, as reported earlier by Sakr [22]. After harvesting, mature infected heads from each replication were taken for further evaluation; the heads were threshed to save infected and shrivelled grains. The percentage of scabby (tombstone) kernels was rated visually on one hundred kernels for each replication to assess FDK for type III [25].
2.4. Silicon Application
The silicon source was an analytically pure SiO2 powder (H4SiO4, Kieselsaure, Carl Roth GmbH + Co. KG, composed of a minimum silicon content of 99% Si), which was supplied along with the irrigation solution. In a previous study conducted on barley [17], a SiO2 powder decreased FHB damage in head tissues expressed by DI and DS of pathogenic Fusarium isolates, following artificial spike and spikelet inoculation under controlled conditions [17]. Therefore, SiO2 powder was preferred as a Si source in the current research. Si as an irrigation solution was prepared by dissolving a SiO2 powder in demineralized water. From the sowing to the fungal spraying time, the barley plants were weekly irrigated with 300 ml (per pot) with a concentration of 1.7 mM Si. The control was irrigated with 300 ml of sterilized water. AS and AB were watered with a similar volume of irrigation solution in the existence of Si (1.7 mM Si) or not.
2.5. Experimental Design
The trials were carried out to correctly and precisely assess the influence of multiple Si applications supplied continuously via the root system into AS and AB on head blight DI, DS, and FDK (Fig. 1). A 2 × 2 × 16 factorial experiment, consisting of two Si concentrations (0 and 1.7 mM, referred to as −Si and +Si plants thereafter); two barley cultivars with different resistance levels, AS and AB; and 16 Fusarium isolates causing FHB were arranged in a randomized design with three replications. The experiment that established a one-week difference to observe repeatability was repeated twice.
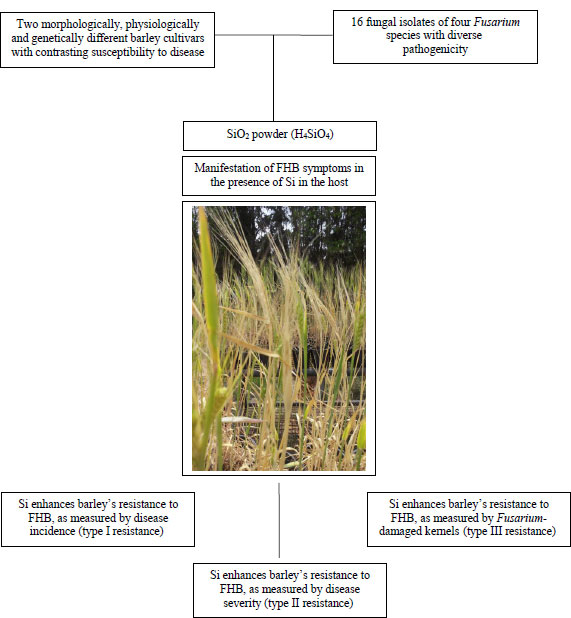
2.6. Silicon’s Ability to Enhance Barley Resistance under Several Experimental Conditions
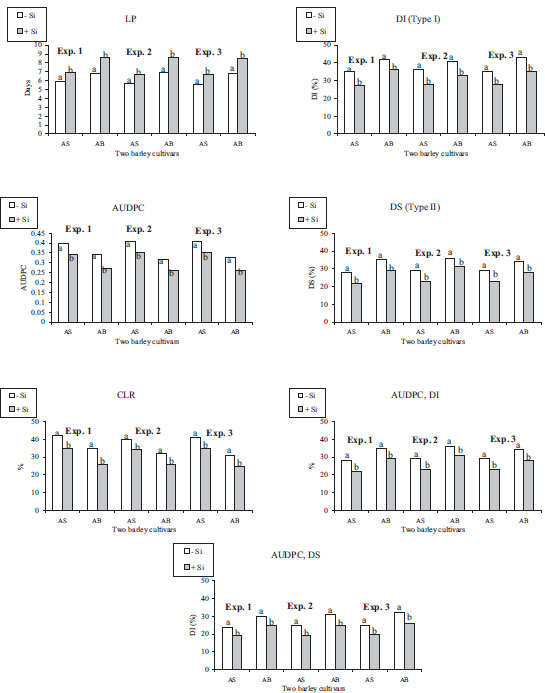
The bio-efficacy of Si to increase AS and AB barleys’ resistance to FHB disease was measured by the latent period (LP) of detached leaf inoculation, the area under the disease progress curve (AUDPC) of Petri-dish inoculation, and the coleoptile length reduction (CLR) of a coleoptile infection under in vitro conditions [26] (Fig. 2). The reduction in head blight symptoms due to the effect of Si was also expressed under growth conditions as DI (type I resistance), DS (type II resistance), and the area under the disease progressive curve (AUDPC) calculated on the basis of DI, type I and DS, type II (Fig. 2). Methods to clarify the nature of the Si-enhanced barley resistance under several experimental conditions were earlier described by Sakr [27] on Si-wheat-FHB interactions. Therefore, we were able to examine the relationship between the current findings and the previous results of in vitro and growth chamber environments to check whether Si could increase the expression of resistance to FHB infection at the earliest and latest barley development stages during FHB infection.

2.7. Statistical Analyses
Data were evaluated by analyses of variance using the DSAASTAT add-in version 2011. Before statistical analysis, the percentages of DI, DS, and FDK were arcsine angular transformed to stabilize the variances. The differences were determined by Fisher’s least significant difference test. Differences at p<0.05 were considered significant. A single degree-of-freedom contrast test was used to make comparisons between AB supplied with Si and AS non-supplied with Si.
3. RESULTS
3.1. Si-application Reduced the Disease Development of Fusarium Pathogens in Barley
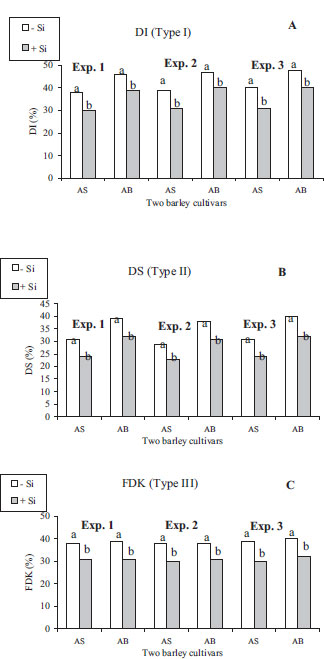
While temperature and rainfall during the barley cycle were favorable to FHB disease in the two seasons, the meteorological conditions across AS and AB cycles for 2021 and 2022 years in the station were somewhat similar (Table 1). For AS and AB, Si resulted in lower disease intensity based on the percentage of diseased florets and smaller necrotic patches, and less bleaching of the florets and discoloured kernels compared to fungal-inoculated controls (Fig. 3). The analysis of variance (ANOVA) indicated no significant interaction/year × treatment (p>0.05). Therefore, the effects were presented by cultivars as the averages of both tested years, and shown in Fig. (4). Moreover, ANOVA showed that Si cultivar, cultivar × Si interaction had highly significant effects (p<0.05) on DI and DS. Regardless of the botanical and pathogenic background of the host and fungal materials in the present research, DI, DS, and FDK (Fig. 4) were significantly decreased in +Si plants AS and AB barley plants inoculated with all analyzed Fusarium isolates relative to –Si plants over the two consecutive growing seasons; this indicated that multiple Si (1.7 mM) application via root system protected any tested barley cultivar whatever its quantitative resistance levels from infestation with any FHB pathogen whatever its pathogenic level under a broader range of environmental conditions. –Si barley plants supplied with SDW did not show diseased symptoms. In plants supplied with Si, DI, DS, and FDK were 18.7%, 20.3%, and 20.2%, respectively, being smaller than in –Si barley plants.
Si (1.7 mM)-supplied cultivars, i.e., AS being moderately resistant and AB being moderately susceptible, had a DI that was lower by 21 and 15%, respectively, in experiment 1; 22 and 15%, respectively, in experiment 2, and 23 and 17%, respectively, in experiment 3 than plants without Si (Fig. 4a).
DS was lower by 23 and 18%, respectively, in experiment 1; 21 and 18%, respectively, in experiment 2, and 22 and 20%, respectively, in experiment 3, in moderately resistant and moderately susceptible +Si cultivars than wheat plants amended with SDW (Fig. 4b).
Supply of Si concentration at 1.7 mM significantly reduced FDK on AS being moderately resistant and on the moderately susceptible cultivar AB by 21 and 18%, respectively, in experiment 1; 21 and 18%, respectively, in experiment 2, and 23 and 20%, respectively, in experiment 3, compared to these cultivars without Si (Fig. 4c); this suggests that Si resulted in a quasi-similar reduction of FDK irrespective of the barley cultivar.
To check whether Si feeding strengthens the defense system measured by type I and type II in AB to a level comparable to AS not amended with Si, a single-degree of freedom contrast test was used to compare the suppressive ability of the FHB damage expressed by DI and DS between both cultivars: –Si AS and + Si AB. In all experiments, Si feeding in AB resulted in decreasing the head blight damage assessed by DI and DS to the same statistical level as that for the cultivar AS, being moderately resistant without Si feeding.
3.2. Si-enhanced Barley Resistance under Several Experimental Conditions
When Si was applied to Fusarium-inoculated treatments under field conditions, all indicators of Si-enhanced barley resistance were enhanced relative to fungal-inoculated treatments. Type I, type II, and type III were enhanced by 18.7%, 20.3%, and 20.2%, respectively. Thus, these values of enhancement in resistance between −Si and +Si plants have been found to be comparable to those obtained under several experimental conditions; barley’s resistance components to FHB disease under in vitro and growth chamber conditions as measured by LP, AUDPC, CLR, type I, type II, type I AUDPC, and type II AUDPC were enhanced by 17.7%, 17.5%, 17.7*%, 19.3%, 19.8%, 18.7%, and 20.0%, respectively.
4. DISCUSSION
Since the FHB burst has seriously threatened the development of barley production around the world in recent years [7], extensive efforts have been conducted in many aspects to defeat this disease [5], however, with limited success in the control [8]. Due to the non-toxic behavior [11] and great ability of a higher Si absorber and accumulator monocot to enhance the resistance of barley crop [14] to some diverse destructive fungal pathogens [15, 16], Si has received attention in the FHB-barley pathosystem [17]. Despite the promising findings generated under controlled conditions in the only study investigating the effect of Si to enhance host resistance to disease [17], it is generally accepted that FHB reactions should be assessed over years under a broader range of climatic conditions due to cultivar-by-environment interactions [4]. For the first time, we have provided novel observations on how multiple Si applications via the root system can enhance the three main types of resistance to Fusarium infection occurring in the reproductive organs, type I, type II, and type III, thus contributing to “field barley-FHB resistance”.
In the current research, we have tried to solve the question of the extent and conditions in which multiple Si applications can enhance barley’s resistance to FHB infection. From a pathogenic standpoint, evaluation of an answer to this question has demonstrated that Si can improve barley’s defense against Fusarium development as observed in Si-wheat-Fusarium associations in the growth chamber and the field [18, 19]; however, main differences can present between barley and wheat in terms of Fusarium infection and mycotoxin accumulation [8]. In wheat, type II resistance is more important than type I as plant defense is inhibited by mycotoxins [5, 9]. Fusarium fungi can penetrate the rachis and spread via direct floret-floret contamination in barley [2]; however, disease spreading to adjacent spikelets is prevented by the rachis node and rachilla, although movement can still proceed through the phloem and rachis surface [6], indicating that barley has natural type II resistance, and type I is predominate [4]. Most Fusarium species are able to produce mycotoxins [7]. It is suggested that these toxins may act as virulence factors and increase the aggressiveness of Fusarium fungi in small-grain cereals [9]. For barley, results of the correlation among disease severity, mycotoxin levels, and other symptoms are not consistent [5]. Regardless of mycotoxins, Fusarium infection can activate the barley defense system [7]. Type I resistant barley cultivars synergistically inhibited the expansion of Fusarium pathogens by forming papillae, reinforcing cell wall deposits, and increasing the biosynthesis of lignin, thionine, hydroxyproline-rich glycoprotein, and hydrolase [8].
Barley is a silicaceous plant [14], and Si is available for plant uptake via the active transport mechanisms inherent to the roots [11]. The influx transporter (Lsi1) takes silicic acid, H4SiO4, from the soil solution up to the exodermis, followed by the efflux transporter (Lsi2), which takes it further across the aerenchyma [10]. The radical transport of H4SiO4 mediated by Lsi1 and Lsi2 in roots has been identified in barley (HvLsi1, HvLsi2) [28, 29]. These genes provide a novel insight for optimizing Si absorption in barley [10]. Since the expression of HvLsi1and HvLsi2 [28, 29] in barley challenged with Fusarium species causing head blight disease has not been analyzed, it can be hypothesized that multiple Si applications via the root are important to augment the availability of Si in the shoots of AS and AB and improve barley’s resistance to the four tested Fusarium species with diverse pathogenicity. Taking into account that Si does not act directly on the pathogen [30] and 90% of absorbed silicon by the plant is deposited approximately in the shoots [11], our results theoretically hypothesize that the presence of soluble silicon in the cytoplasm can stimulate the expression of disease-resistant genes through complex signaling pathways and thereby resist spikelet infection and enhance type I resistance. However, Si has been reported to inhibit toxin biosynthesis and thus reinforce type II resistance in wheat [18, 19].
A description of the nature of the Si-enhanced barley resistance can shed light on the complex interactions among Fusarium, host, and Si that could lead to improved control strategies. As shown in the current phenotyping study, smaller chlorotic/necrotic lesions confined to the infection points of the head were noted in the two tested barley cultivars that received multiple Si applications via the root system in the field, AS and AB, than non-Si-treated plants. AS and AB supplied with Si showed less damage caused by several Fusarium species with diverse pathogenicity, as indicated by the smallest DI, DS, as well as FDK. These smallest values indicate that the advance of the disease in the head tissues of AS and AB was slower relative to fungal-inoculated-controls, leading to lower levels of diseased kernels, which thus reduced the progress of head blight in the field, resulting in lower disease intensity at the end of the barley cycle. A similar finding was also observed in +Si barley, which defeated several destructive fungal pathogens, like B. graminis f. sp. hordei and Bipolaris sorokiniana [15, 16], through alteration of their monocyclic components, such as incubation period, infection efficiency, lesion expansion rate, lesion size, and number of lesions per unit leaf area [15, 16].
The results of this research allow us to accept the hypothesis that the benefit of Si in decreasing the infection of FHB pathogens in barley plants can be due only to an increase of Si in the shoots, as theoretically observed in wheat treated with multiple Si root applications under uncontrolled climatic conditions to defeat Fusarium damage [18, 19]. Our data have been substantiated by Rodrigues et al. [31], Sakr [17, 18], and Sakr and Kurdali [19] through their studies on small-grain cereals. Rice, barley, and wheat cultivars grown at suitable Si rates had sheath blight and head blight intensities that were greatly reduced in comparison to cultivars not supplied with Si [17-19, 31]. This shows the need to expand sustainable measures to control these destructive fungal pathogens by using Si. It is important to highlight the capacity of barley crops to absorb this beneficial element [14], which can lead to promising results and benefits for plants. AS and AB cultivation with nutrient solution containing Si (1.7 mM) was sufficient to theoretically increase the uptake and consequently the accumulation of this element in the shoots to enhance host resistance to FHB infection. The protective capacity of Si at 1.7 mM might be correlated with the adjustment of the homeostatic network of mineral elements [10]. Solution concentration of 1.7 mM Si, which is the maximal solubility of Si in water [11], was reported to produce optimum disease reduction, i.e., Blumeria graminis F. sp. tritici causing powdery mildew in wheat [32], Mycosphaerella fijiensis causing black sigatoka in banana [33], and Phakopsora pachyrhizi causing Asian rust in soybean [34]. Such Si concentration has been found to be realistic in field studies to prevent plant diseases in which Si concentration generally does not exceed 1.67 mM [11].
Si absorption in barley strengthened the defense system, as measured by type I and type II resistance in AB to a level comparable to AS not amended with Si, suggesting that Si absorption by the roots is necessary to avoid the negative impact of Fusarium infection. In accordance with our findings related to wheat challenged with the same tested FHB isolates under several experimental conditions [18, 19, 27], Rodrigues et al. [31] demonstrated that Si reduced sheath blight development in susceptible and moderately susceptible US rice cultivars to levels comparable to those observed in cultivars high in partial resistance to sheath blight but not fertilized with Si. However, Xiao et al. [35] found that although Si application reduced the powdery mildew severity in strawberries, susceptible cultivars treated with Si had a disease severity score four times that of the resistant cultivar. Our results showed that AS showed the greatest control over FHB than AB. This observation suggests that in AS, Si induced slower development of the FHB pathogens and it might be due to Si feeding interacting with the complex resistance mechanisms expressed by differential responses conferred by quantitative trait loci mediated during FHB infection, governing more resistance in AS. However, in this study, we demonstrated that Si reduced head blight development in AB, showing a moderate susceptibility to FHB. This suggests that enhanced head blight resistance by Si is not limited to a moderately resistant cultivar, AS. Thus, the FHB severity was associated with the Si application and barley cultivar. Irrespective of the barley cultivar, however, Si resulted in a quasi-similar reduction of FDK because FDK components did not differ between AS and AB having contrast susceptibility to Fusarium, as previously reported [22].
Importantly, Si treatment at 1.7 mM decreased the damage of FHB in previous analyses conducted on AS and AB under in vitro [26] and growth chamber environments, showing that Si enhanced the expression of resistance to FHB infection in seedlings and adult barley plants. It seems that Si regulates multiple similar signaling pathways involved in spike and seedlings' [36] response to Fusarium infection. Increasing evidence has also shown Si to play a role in numerous key components of plant signaling systems [10, 11].
CONCLUSION
Most of our knowledge regarding the benefits of Si has been gained from cereals, i.e., barley, which are high-Si accumulators. However, research on the application of Si for barley disease suppression is in its infancy. For the first time, pathogenic evidence has shown the positive effect of multiple Si applications at 1.7 mM via roots on enhancing barley’s resistance against Fusarium infection occurring in the reproductive organs in the field, showing the three main types of resistance, type I, type II and type III, to contribute to “field barley-FHB resistance”. Si absorption in a moderately susceptible AB cultivar improved the defense system, as measured by type I and type II resistance, to a level comparable to a moderately resistant cultivar not fertilized with Si. Taking into consideration that pathogenic differences can present between barley and wheat in terms of Fusarium infection and mycotoxins, our results theoretically conclude that Si acts in two different ways to control FHB in barley and wheat depending on the complex interactions between host and Fusarium species. Importantly, Si has been found to enhance barley’s resistance to FHB invasion under dry and arid climate, which highlights the multiple roles of Si in increasing host resistance to biotic and abiotic stresses. All of these findings are promising outcomes for the treatment of Si as a safe and effective method against FHB damage. More biochemical, cytological, and physiological analyses would be required to elucidate how Si can enhance cereals’ defenses to FHB attack.
ABBREVIATIONS
| FDK | = Fusarium-damaged kernels |
| SDW | = Sterile distilled water |
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
The Atomic Energy Commission of Syria provided financial support for this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thank the Atomic Energy Commission of Syria for providing financial assistance to this research.


