All published articles of this journal are available on ScienceDirect.
Weeds as A Potential Host of Xanthomonas Oryzae Inoculum in Irrigated Rice Systems in Burkina Faso
Abstract
Background:
Bacterial Leaf blight and bacterial leaf streak caused, respectively, by Xanthomonas oryzae pv. oryzae and X. oryzae pv. oryzicola are two rice diseases that occur in rice-growing areas in Burkina Faso. For better management of both pathogens, it is essential to integrate the use of resistant varieties with good agronomic practices including weed management which plays a role in their survival and dissemination.
Objective:
This study aims to identify the main weeds that can act as reservoirs of Xanthomonas oryzae strains.
Materials and Methods:
Soil was sampled on three rice-growing sites. In the laboratory, we sifted the soil to collect the seeds of the weeds which were put in pots in the greenhouse for germination. After emergence, all weed species were inventoried and then inoculated to determine their host capacity.
Results:
We identified 14 species belonging to dicotyledons against 10 species from the Poaceae and three species from the Cyperaceae genus. This presence is effective in all plots sampled but at a variable level of prevalence. Our results showed that some Poaceae and Cyperaceae species were the main reservoirs of X. oryzae pv. oryzae and X. oryzae pv. oryzicola and behave sometimes like epiphytes. We detected a latent infection of X. oryzae pv. oryzicola from Oryza sativa, Echinochloa colona, Eleusine indica, Digitaria horizontalis, and Rottboellia cochinchinensis. Except Oryza longistaminata, Sacciolepis Africana, Paspalum vaginatum, Paspalum polystachyum, and Echinochloa colona which manifested typical symptoms of bacterial leaf streak, the other species showed yellowing, whitish spots and necrosis at the inoculation site.
Conclusion:
The high prevalence of weeds in rice fields, especially Poaceae and Cyperaceae, is one of the main causes of the importance of bacterial leaf streak disease in the irrigated rice system in Burkina Faso.
1. INTRODUCTION
In Burkina Faso, cereals (sorghum, millet, and maize) constitute the staple food of the population and rice is the fourth cereal cultivated in terms of area and production [1]. Despite the enormous potential to increase rice productivity, Burkina Faso imports large quantities of rice each year, which accentuates the country's food dependency. Unfortunately, rice productivity is still very low due to several biotic and abiotic constraints, including pests and the effects of climate change that affect field yields. It has been reported that over 47% of crop production losses are caused by pathogens. Indeed, more than fifteen pathogens have been identified in rice in Burkina Faso [2-5]. The most damaging of these are blast (Magnaporthe oryzae), Rice yellow mottle virus, and bacterial diseases caused by Xanthomonas oryzae (Xo). It causes two important diseases of rice; Bacterial Leaf Blight (BLB) due to X. oryzae pv. oryzae (Xoo) that colonizes xylem vessels and Bacterial Leaf Streak (BLS) caused by X. oryzae pv. oryzicola (Xoc) that colonizes spaces between leaf parenchyma. BLB and BLS are occurring in the tropical and subtropical areas of Asia and Australia. In Africa, BLB was reported in several West African countries and Madagascar in the 1980s; whereas Xoc was described in these areas in the 2000s [6, 4]. BLS symptoms occur as small, translucent, and typically yellow lesions along the leaf between the veins that expand lengthwise. At later stages, leaves turn grayish-white and die. Xoo induces pale-yellow leaf syndrome and seedling blight or “kresek” at an early stage. In Burkina Faso, BLB induced important yield losses in 1998 and 2004, respectively, in the Bagre area. However, BLS is recurrent on the major rice sites in most rice-cultivated varieties [7, 8]. To control both bacterial diseases, it is needed to combine the use of resistant varieties with good cultural practices including weed control. Weeds are at present the major biotic constraint to increase rice production worldwide. In Africa, weeds induce important yield losses from 28 to 74% in the irrigated rice sites and 48 to 100% in the upland rice systems. The importance of their control has been emphasized in the past by various authors [9-11]. Among several weeds affecting rice production, two major weed complexes have been identified which require an integrated approach for their control. They are the complex formed by various Echinochloa species and the red/weedy rice complex. Wild rice, Oryza barthi and O. longistaminata, are among the most important weeds in West Africa and the Sahel but total control is not evident. Unfortunately, chemical control is used today against weeds with its harmful effects on human health and the environment. In addition to weed control, the deployment of resistant varieties is one of the best ways to manage bacterial diseases as well as weeds.
In rice, there were 29 major genes for resistance to bacterial blight, but so far only a few quantitative resistance loci for bacterial leaf streak [12]. However, while it is well known that rice is the main host of both pathogens, it has been reported that a number of weeds, notably Poaceae and Cyperaceae [13], played the role of inoculum reservoir. Indeed, weeds can significantly influence disease incidence and should be considered when endeavoring to manage and control plant pathogens of cultivated plants [14]. It is essential to know all factors which contribute to the BLB and BLS epidemic for better management of pathogens. Hence, the aim of our study is to identify the weed hosts of Xoc and Xoo in the irrigated rice growing system in Burkina Faso.
2. MATERIALS AND METHODS
2.1. Weed Samples
Soil sampling was performed on the rice-growing sites of Karfiguela, Banzon, and Vallée du Kou based on five plots per site. Five samples of three (03) kg of soil were taken in the first 10-20 cm along the two diagonals of each plot. The samples were then pooled to form a composite sample of 15 kg per plot. The samples are bagged and then labeled by assigning them a code, the name of the site, the date of collection, and the GPS coordinates of the plot. In the laboratory, we sifted the soil to collect the seeds of the weeds which were put in pots in the greenhouse for germination.To determine their host capacity, all weed species were inventoried after emergence and then inoculated.
In addition, during the period of a high prevalence of BLB and BLS in the field (September- October), we performed observations on weeds in the plots on the main irrigated sites: Vallée du Kou, Bazon, Karfiguela, Douna, Di, Niassan, Mogtédo, and Bagre. Symptomatic and asymptomatic samples were collected to determine the presence/absence of Xoo and Xoc strains.
2.2. Rice Varieties and Xo Strains Tested
Two improved rice varieties, TS2 and FKR62, from National Research Institute were used to test the pathogenicity of the strains on weeds. These varieties were previously identified by Wonni et al [8]. are susceptible to Xoo and Xoc strains representing Xo diversity in Burkina Faso. The Xoo strains BAI3 (Race A1) and Xoc Strain BAI10 [4, 14] were used to inoculate weed and rice varieties, respectively.
2.3. Weed Cultivation for Greenhouse Tests
To obtain the weed seeds contained in each sample, we sieved them using two (02) sieves of different mesh (5 and 0.2 mm). The grains of sand passed through the sieves and the particles retained by the mesh, called refusals, usually contain weed seeds. The refusals obtained were dried in the shade and then weighed.
The seeds were grown in soil (2/3 soil and 1/3 sand) previously sterilized at 100 °C to avoid any development of unsampled weeds. The refusals of each sample were divided into four batches (or four replicates), and were mixed in the soil of each pot. Fifteen days after weed germination, to avoid competition between species for mineral elements, we applied 0.4 g of NPK in each pot in accordance with the recommended dose per hectare (200 kg/Ha) in rice fertilizing. Before the inoculation tests, we applied the same dose again, to eliminate the doubt that the symptoms would be due to a deficiency in mineral elements.
2.4. Weed Population by Sample
We carried out a visual observation and a count of each species. The inventory is updated every 15 days after sowing for three (03) months. We used the Adventrop guide (Weeds of African rice fields and Weeds of subtropics) to identify the species even in the juvenile state. abundance index [15] was used to assess the frequency of individuals of a species within the same family. The frequency of occurrence (number of pots where the species is present) and the maximum abundance per station (maximum score obtained by the species) were then recorded.
Weed diversity is assessed using three diversity indices namely: the Shannon-Weave Index (Ish) which is an indicator of diversity taking into account not only the species richness but also the proportion of each species represented within the community, it varies between 1 and 5,
Ish = -Σ (ni/N) log_2 (ni/N), where ni is the number of species i and N the total number of species;
Simpson's index (SI), expresses the probability that two (02) individuals chosen at random in an infinite population, belong to the same species, it varies between 0 and 1,
IS = Σ (ni/N)2, where ni is the number of species i and N the total number of species;
Piélou Equitability Index (Eq), measures the degree of diversity achieved by a stand in relation to its maximum value and allows comparison between two groups that do not have the same number of species. It is between 0 and 1,
Eq =ISh/(log_2 N), where N is the total number of species.
2.5. Evaluation of Weed Epiphytic Capacity
The asymptomatic leaves of the weeds after growing, were collected for Xo strain isolation on Peptone-Sucrose Agar medium. Then, Multiplex PCR specific to both pathogens, described by Lang et al. [16] was achieved from the leaf’s suspension.
Multiplex PCR combines four pairs of primers. It can be performed directly on bacterial colonies or from genomic DNA. The reaction volume of the PCR is 25µl composed of 5µl buffer 5X containing MgCl2, 0.5µl dNTPs, 2.5µl primers, 0.05 µl Taq polymerase, 5 µl of leaf’s suspension. Samples were initially denatured for 5 min at 95 °C, then 35 reaction cycles including (i) denaturation for 30s at 94 °C, (ii) hybridization for 30 s at 60 °C, (iii) elongation for 2mn at 68 °C, followed by a final elongation phase for 10 mn at 68 °C. The amplicons were separated by electrophoresis on 0.8%.
2.6. Identification of Weeds as Hosts for Xo Strains
To determine the capacity of the weed species to harbor Xoo and Xoc strains, we inoculated the weed leaves 30 days after sowing with 108 bacteria/ml suspension. The leaf reactions after inoculation were assessed as follows: (i) presence of typical symptoms after inoculation; (ii) no typical symptoms, but the presence of necrosis at the site of inoculation; (iii) presence of yellowish or whitish lesions at the point of inoculation.
Two weeks after inoculation, the inoculated leaves are collected for the detection of Xoo and Xoc by isolation or multiplex PCR.
2.7. Evaluation of the Survival and Dynamics of Xanthomonas Oryzae Strains after Inoculation
2.7.1. Survival Tests
To assess the survival of Xo strains in the leaves of weeds, we used suspensions obtained from ground leaves inoculated and collected at 7, 14, and 21 JAI; to inoculate the susceptible rice varieties TS2 and FKR62N. Symptom induction on one of these two varieties indicates the presence or survival of the bacteria in the weed’s leaves inoculated.
2.7.2. Assessment of Bacterial Numbers
The ability of the bacterium to multiply in the plant tissues was assessed at 7, 14, 21, and 30 days after inoculation of one-month-old plants following the method described by Wonni et al. (2016). For Xoo, the number of bacterial cells in the leaves was determined within the entire leaf. Xoc cell number was assessed from the portion of the leaf where symptoms or necrosis developed, including the site of inoculation and surrounding tissues. Leaf sections were surface-sterilized in 75% ethanol for 10 s, followed by submersion in sterile water for 30 s, and crushed and resuspended in 1ml sterile distilled water. Leaf homogenates were serially diluted (up to 10-12) and plated onto a PSA medium amended with cephalexin (40 ug/ml), kasugamicyn (20 ug/ml), and cycloheximide (50 ug/ml) to avoid contamination. The plates were incubated at 28 °C until single colonies could be counted. The experiment was repeated independently three times.
2.8. Data Analysis
The data were entered into Excel 2010 software. To compare the level of weed and bacterial populations, histograms were constructed and variance analyzes were performed with SPSS20 software. The phytosociological and ordination analyses were performed with PC-ORD version 5.0 ordination software [17].
3. RESULTS
3.1. Weeds Inventoried from Soil
The inventories performed in the three prospected sites identified 24 species including the three (03) major families of weeds, namely, Poaceae, Cyperaceae, and dicotyledons. In fact, we have counted 12 species belonging to the broad leaf weed family with nine (09) species of the Poaceae family and three (03) species of the Cyperaceae family. The Poaceae were represented by Pennisetum pedicellatum Trin, Setaria pallide-fusca (Schumach.) Stapf & C.E. Hubbard, Paspalum scrobilatum L., Echinochloa colonum (L.) Link, Echinochloa stagnina (Retz.) P. Beauv., Rottboellia cochinchinensis (Lour.) W.D. Clayton, Oryza longistaminata A. Chev. & Roehr, Eleusine indica (L.) Gaertn., Eragrostis tenela (L.) Roem. & Schult., and Digitaria horizontalis Willd. As for Cyperaceae, we identified Cyperus esculentus L., Cyperus difformis L., and Fimbristylis littoralis Gand.
The broad-leaved species inventoried were Phyllanthus amarus Schum. & Thonn., Corchorus tridens, L., Portulaca oleracea L., Melochia corchorifolia, L., Physalis angulate L., Cleome viscosa L., Euphorbia hirta L., Stachytarpheta angustifolia (Mill.) Vahl, Triumfetta rhomboidea Jacq., Ammania prieureana Guill. & Perr., Ludwigia hyssopifolia (G. Don) Exell, Mollugo nudicaulus Lam., Sphenoclea zeylanica Gaertn.and Hyptis spicigera Lam..
3.2. Weed Frequency and Dynamics
Three major families of weeds, namely, Poaceae, Cyperaceae, and Dicotyledonous, were inventoried at the sites of Bama, Karfiguèla, and Banzon (Fig. 1). However, broadleaf (dicotyledonous) weeds were most abundant at all three sites with 51% at Banzon, followed by Cyperaceae (41.02%) at the Karfiguèla site and Poaceae Bama (33.51%).
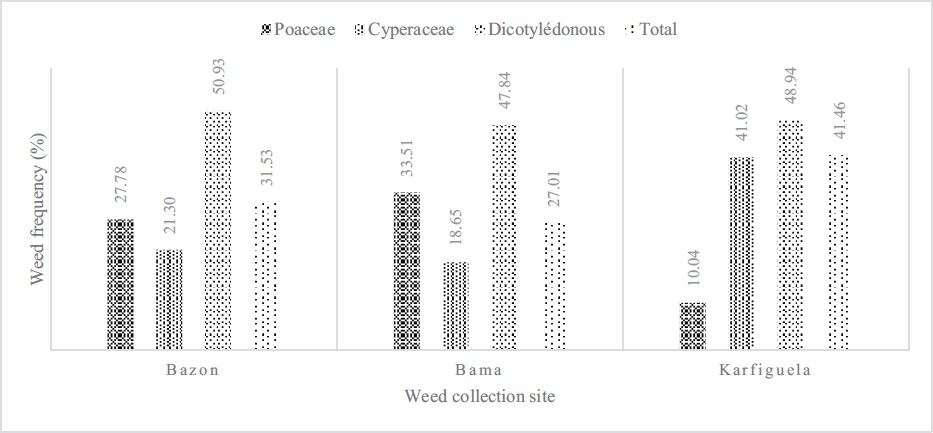
GV4: Group of weeds where the effect of the treatments is the most significant;
1; 13; 14; 18: Sub-group of species according to the effect of the most significant treatments.
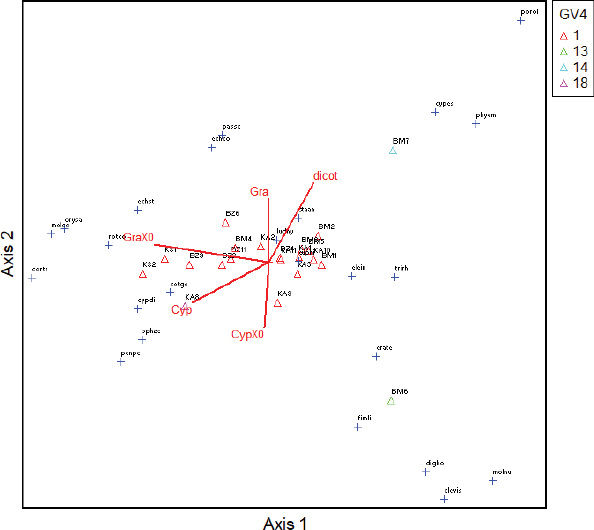
Concerning the dynamics of weeds, the ordination diagram (Fig. 2) shows that there is a slight link (13%) between the classes, the capacity to be a reservoir of Xoo and Xoc of weeds, and the distribution of collection sites. Indeed, regarding the capacity to be a Xo reservoir, it appears that dicotylodonous (Dicot) species do not host Xo strains at any site. However, Cyperaceae (Cyp), reservoirs of Xo are mainly present in Karfiguèla. As for the Poaceae (Gra) reservoirs of Xo, they are more present in Banzon and Karfiguèla.
The Cyperaceae genus are dominated by two species, Cyperus difformis L. with a sum of 99.4 individuals per sample, and Fimbristylis littoralis Gand. (Sum = 24.3) (Table 1). The two most important species of Poaceae are Echinochloa colona (L.) Link (sum=16.3) and Eleusine indica (L.) (sum= 4.9). Regarding the broad-leaved species, the most inventoried species are Stachytarpheta angustifolia (Mill.) Vahl (sum=28.2), and Ludwigia hyssopifolia (G. Don) Exell (sum=22.8).
The Cyperaceae genus obtained the highest specific diversity with F. littoralis (Shannon`s diversity index: H = 1.03) but also the lowest for C. difformis (H =0.41).
3.3. Latent Infections due to Xanthomonas Oryzae Strains
In order to determine the possible transmission of BLB and BLS by grasses and Cyperaceae seeds, we evaluated by PCR and biological tests, the presence/absence of Xoo and Xoc strains in the weed’s leaves. Of all Poaceae and Cyperaceae leaves collected, no weed species exhibited symptoms of BLB and BLS after seed emergence and plant development. PCR analysis of the leaf suspension collected from each species of Poaceae and Cyperaceae revealed the absence of Xoo and Xoc strains.
Surprisingly, foliar infections performed with foliar suspensions from Poaceae and Cyperaceae species collected in experimental conditions, on the two susceptible rice varieties, induced apparent BLS symptoms. These are samples of Echinochloa colona and Rottboellia cochinchinensis from Bazon; Eleusine indica from Bama and Digitaria horizontalis from Karfiguela. PCR analysis of infected rice leaf samples after inoculation of foliar suspensions of weeds revealed the presence of Xoc.
During our surveys, we collected weeds showing typical symptoms of BLS on Oryza longistaminata, Sacciolepis Africana, Paspalum vaginatum, Paspalum polystachyum, and Echinochloa colona. However, some weeds exhibited non-specific symptoms of BLB and BLS, which are leaf blight-like. Diagnosis by isolation and multiplex PCR tests has been made to identify mainly Xoc strains from these samples which are Paspalum vaginatum, Brachiaria lata, and Pennisetum pedicellatum sp.
Under the infiltration tests in the greenhouse, all species of Poaceae and Cyperaceae have the capacity to harbor Xo strains, without exhibiting typical symptoms of BLB or BLS. These are identified as hosts following reactions:
(i) typical symptoms of BLS (Fig. 3A-C) on Oryza longistaminata, Sacciolepis africana, Paspalum vaginatum, and Echinochloa colona;

(A): Echinochloa colona; (B): Paspalum vaginatum; (C): Paspalum vaginatum; (D): Pennisetum alopecuroides; (E): Brachiaria lata.

(A): Xathomonas oryzae pv. oryzae; (B): Xathomonas oryzae pv. oryzicola.
(ii) No specific symptoms such as necrosis, whitish and yellowish lesion, or total absence of reaction at the inoculation point were induced by the two pathovars. However, the presence of Xoo and Xoc has been detected by multiplex PCR or after infiltration of left suspension from infected weeds with FKR 62N and TS2.
Furthermore, to assess the bacteria's ability to multiply in weeds and leaves, we quantified the bacterial populations at 7, 14, and 21 days after inoculation. These quantifications made it possible to confirm the host nature of some weed species.
The results showed that Xoo and Xoc strains survive in the asymptomatic leaves beyond one (01) month after inoculation. Indeed, high bacterial growth is observed during the first week of inoculation and decreases gradually from 14 days after inoculation (Fig. 3A and B). In general, bacterial growth is greater in grasses than in Cyperaceae. On the other hand, Xoo grows more abundantly in the leaves of O. sativa than those of weeds. Dicotyledons inoculated with both strains show necrosis and bleaching on the inoculation.
4. DISCUSSION
4.1. Importance and Dynamics of Weeds in the Studied Sites
Three major families of weeds, namely, Poaceae, Cyperaceae, and Dicotyoledons, have been inventoried on the sites of Bama, Karfiguela, and Banzon. However, broadleaf (dicotyledon) weeds were the most abundant, followed by Cyperaceae (28%) and Poaceae (23%) at all three sites. These results agree with those [18] who showed that broadleaf weeds constitute the most important group of weeds in irrigated rice and lowland areas followed by Cyperaceae and Poaceae.
Similar results are obtained in irrigated rice with the same weed species by [19], where the species such as Cyperus difformis, L., Fimbristylis littoralis Gand., Echinochloa colona (L.) Link, Oryza longistiminata A. Chev. & Roehr. Ludwigia abyssinica A. Rich., Spilanthes uliginosa Sw. and are some major species found in the rice plains of Bama and Karfiguela. Fifty weed species was inventoried in the rice fields in Ivory Coast, that half of them belong to dicotyledons, the rest are distributed equally between Cyperaceae and Poaceae [20]. Studies achieved possible after it [20-22], in the valley of the Senegal river and in Wianga made it to inventory 90 species of weeds, distributed in 27 families where Poaceae and Cyperaceae are the most important with respectively 29% and 16%. Given the importance of Poaceae and Cyperaceae, which represent 50% of the weeds present in the rice fields surveyed in our study, they could constitute potential reservoirs for the two pathovars of X. oryzae. These results agree with studies by Sanou et al. [23]. which showed that farmers consider Poaceae and Cyperaceae to be the most harmful weeds in the irrigated rice system.
The ability of weeds to be a reservoir of X. oryzae pv. oryzae and X. oryzae pv. oryzicola would depend on the shape of the leaves, stems, and root system. Indeed, from the distribution of species according to these parameters, it appears that broad-leaved weeds (dicotylodones) are not the host of Xoo and Xoc. This classification of weeds by Defoer et al. [24], allowed rice farmers to identify Cyperaceae and Poaceae as the most damaging weed weeds [23].
Cyperaceae and Poaceae have been identified as the host group of Xoo and Xoc. In addition, they contained the greatest specific diversity, in particular with the species Fimbristylis littoralis. Their ability to host Xoo and Xoc would be explained by their morphology; but also their proliferation capacity.
| Class | Name | Mean | Stand. Dev. | Sum | Minimum | Maximum | E | H | D` |
|---|---|---|---|---|---|---|---|---|---|
| cyp | cypdi | 33.15 | 46.93 | 99.44 | 1 | 87 | 0.375 | 0.412 | 0.2213 |
| Cyp | fimli | 8.11 | 3.27 | 24.33 | 4.333 | 10 | 0.945 | 1.038 | 0.6305 |
| dicot | staan | 9.43 | 7.65 | 28.28 | 2 | 17.278 | 0.776 | 0.853 | 0.5204 |
| dicot | ludhy | 7.61 | 4.43 | 22.83 | 3 | 11.833 | 0.887 | 0.975 | 0.5914 |
| Gra | elein | 4.44 | 4.23 | 13.33 | 2 | 9.333 | 0.745 | 0.819 | 0.465 |
| Gra | echco | 1.63 | 2.02 | 4.89 | 0 | 3.889 | 0.731 | 0.507 | 0.3254 |
4.2. Poaceae Seeds are likely to Transmit Xoc
We identified latent infections due to Xoc strains from the leaves of Oryza sativa, Echinochloa colona, Eleusine Indica, Digitaria horizontalis, and Rottboellia cochinchinensis following inoculation of leaf suspensions of these species on two susceptible rice varieties including FKR62N and TS2.
However, PCR analysis of the leaf’s suspension from asymptomatic weeds did not reveal the presence of Xoo and Xoc. This result could be explained by a low concentration of inoculum in the leaves, therefore, a small amount of amplifiable DNA. Indeed, rice seeds are the main source of Xoc inoculum [25-28], which can be transmitted to the plant. Xoc may also infect Leersia sp (p)., Leptochloa filiformis, wild and cultivated species of Oryza, Paspalum orbiculare, Zizania palustris, and Zoysia japonica [29]. Bracharia lata, Oryza longistaminata, Pennissetum sp, and Paspalum vaginatum have been reported as possible Xoc hosts [4, 15]. Consequently, the offending species weeds are Poaceae such as rice, which could support the hypothesis of Xoc transmission by the seeds of these weeds.
All species of Poaceae and Cyperaceae retain Xoc after inoculation except for Fimbristylis littoralis. Indeed, this species is characterized by hard and thin leaves, difficult to infiltrate. Our results confirm those [30], who indicated that Xoo and Xoc strains survive on wild or low-cultivated Poaceae. Analysis of the bacterial population dynamics in the leaves reveals Xoc strain's presence of 21 JAI with or without induced necrosis at the inoculation site [31]. Reported epiphytic survival of Xoo on wild grass leaves for 140 days. In our study, we noticed a larger Xoc population in the leaves of Poaceae than that of Cyperaceae. Indeed, Cyperaceae by their leaf structure can limit the penetration of bacteria and consequently their growth. They have a cylindrical shape at the level of their rachis, which gives them resistance to attacks from both external and internal factors.
CONCLUSION
Our study revealed that 50% of the weeds present in the plains of Bama, Banzon, and Karfiguela are Poaceae and Cyperaceae. Evaluation of their ability to harbor pathogens shows that all of them retain the bacteria without typical symptoms of BLB and BLS.
Very interestingly, we were able to highlight latent infections due to Xoc from Echinochloa colona, Eleusine indica, Digitaria horizontalis, and Rottboellia cochinchinensis, proof that weed seeds are potential reservoirs of Xoc.
LIST OF ABBREVIATIONS
| Xo | = Xanthomonas oryzae |
| BLB | = Bacterial Leaf Blight |
| BLS | = Bacterial Leaf Streak |
CONSENT FOR PUBLICATION
Not applicable.
RESEARCH INVOLVING PLANTS
The plant species used in this research were not endangered.
AVAILABILITY OF DATA AND MATERIALS
All the data and supporting information are provided within the article.
FUNDING
IFS (International Foundation for Science) and IRD (Institut de Recherche pour le Développement) have supported this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We thank IFS and IRD which supported this work.


