Effects of Bio-rational Insecticides on Diamondback Moth (Plutella xylostella L.) and Cabbage Aphid (Brevicoryne brassicae L.) on Cabbage
Abstract
Background:
Cabbage is a subsistence crop for smallholder farmers in Ethiopia. Diamondback moths and cabbage aphids are among the devastating insects that cause yield losses of 90% and 30%.
Objective:
The aim was to test the efficacy of the bio-rational insecticides against diamondback moth and cabbage aphids, and their effect on cabbage yield and yield loss.
Methods:
A field experiment was conducted at the Gumsalasa micro dam with furrow irrigation during 2019/20 in Northern Ethiopia. Treatments were arranged in RCB design and replicated thrice. The experiment included five bio-rational insecticides; (R. obtusifolius), (P. dodecandra), (N. glauca), (T. minuta), (A. indica), Karate 5% EC (standard test), and control.
Results:
Phytolacca dodecandra aqueous leaf extract showed inspiring results, reducing diamondback moth larvae to 0.13 and aphid colonies to 0.16, 48 hours after the 4th spraying, leading to a higher (40.28 t ha-1) fresh cabbage yield followed by A.indica, which had reduced the invasion of diamondback moth and aphid colonies to 0.20 and 0.40 and the yield was recorded as 27.69 t ha-1 compared with 2.46 and 5.53 diamondback moth and aphid colonies, and 23.86 t ha-1 cabbage yield in the control group. Similarly, aqueous extracts of P. dodecandra showed a commendable yield (68.82%) increment over the control, and an estimated yield loss of 40.76% was recorded from the control plots due to the tested insect pests.
Conclusion:
This study concludes that foliar extracts of P. dodecandra can be used as an alternative management option to replace synthetic insecticides and thereby maintain food security.
1. INTRODUCTION
In Ethiopia, vegetable crops contribute significantly to household budgets and the national economy [1]. Brassica is important because it is an important part of the local diet and is nutritionally essential for people who cannot afford alternative vegetables [2]. Cabbage (Brassica oleracea L.) is an important subsistence crop for smallholder farmers in Ethiopia, Kenya, Zimbabwe, and Mozambique [3]. The nutritional capacity of 100g of cabbage is 5.8g carbohydrates, 2.5g fiber, 1.3g protein, 36.6mg vitamin C (44% daily requirement), and 76mg vitamin K (72% daily requirement) [4]. It is an excellent source of minerals, vitamins, fiber, and medicinal properties [5]. Its area, annual production, and average annual yield are 11,401.87 ha, 83,104.3 tons, and 7.29 t ha-1 respectively [6]. Cabbage is easy to grow and highly nutritious, making it an indispensable vegetable in poverty-prone countries like Ethiopia. Despite the medicinal, economic, and nutritional value of cabbage, its production is hampered by pests [7]. Diamondback moth (DBM) and cabbage aphids are important multicultural insect pests that feed exclusively on cruciferous vegetables, especially cabbage, in all cabbage-growing regions of the world [8]. In tropical and subtropical regions, they are major obstacles for cruciferous crops. Cruciferous crops grow in extremely hot and humid areas, where diamondback moths and cabbage aphids continue to cause severe crop losses [9].
Diamondback moths are the greatest threat to cruciferous production, causing crop losses of over 90% [10]. The destructive power of the diamondback moth is its high ability to rapidly develop chemical resistance [11]. The damage begins immediately after hatching under the leaf epidermis, after which they feed on the outer layer of the plant, but the damage depends on the growth stage of the plant, the density, and the size of the larvae. If the larvae are small, the damage manifests as small irregular holes from leaf 'shot holes'. If there are many larvae, they feed on the entire leaf, leaving only the veins [12]. Cabbage aphids are also important pests [13]. The potential for damage affects the quality and market value of cabbage harvests in Ethiopia [14]. Massive infestation on mature plants also reduces market value through the accumulation of moltings exuviae, honeydew, and sooty mold growing on the honeydew [15]. Cabbage aphids are also capable of transmitting viruses such as tulip mosaic virus that cause many diseases in cruciferous vegetables [16, 17]. Investigated toxicity of aphids to winter oilseed rape and the infestation of plants by at least 100 aphids in early autumn caused losses of 20-30%, while infestation during flowering in spring resulted in complete crop failure. Aphids feed by sucking sap from plants, multiply rapidly causing massive infestation, and leaves curl inwards, discoloration, and stunted growth in young plants [18].
Pest control, especially smallholder cabbage control, still relies heavily on chemical pesticides, the use of which has many undesirable consequences. Similarly, superfluous use of pesticides induces resistant development, killing beneficial insects and natural enemies [19]. A major concern regarding the use of chemical pesticides in vegetable production is the human health effects of ingestion [20, 21]. One of the studies investigated that continuous use of chemical pesticides is costly, because 70% of farmers growing cabbage spend 25-30% of the total production input cost to purchase pesticides [22]. These issues have increased interest in alternative control methods. Therefore, there is an urgent need to develop safe alternatives to conventional pesticides to protect diamondback moths and aphids. Many bio-pesticides derived from commonly available plants effectively meet these criteria for affordability and availability for smallholder farmers, as well as human and environmental safety [23]. Bio-pesticides are generally considered to have low toxicity to mammals, fish, and pollinators [23]. Promising repellent activity of aqueous leaf extracts has been reported against cabbage aphids in the laboratory [14] and against diamondback moths in the field [24]. Therefore, due to the accessibility of botanical plants in the study area and sufficient previous evidence, the study was conducted to test the five bio-rational insecticides for their efficacy against diamondback moth and cabbage aphid, and their effects on cabbage yield and yield loss. For this purpose, a field experiment was conducted using a randomized complete block design with seven treatments replicated three times. The bio-rational insecticides included: Rumex obtusifolius, Phytolacca dodecandra, Nicotiana glauca, Tagetes minuta, and Azadirachta indica. Karate (5% EC) and tap water were used as standard checks and control, respectively. Phytolacca dodecandra followed by Azadirachta indica showed the most promising results to reduce insect invasion and to increase the yield of cabbage, and these biopesticides can replace synthetic pesticides.
2. MATERIALS AND METHODS
2.1. Description of Study Area
This experiment was conducted under field conditions at the Gumsalasa micro dam with furrow irrigation during the off-season of the 2019/20 growing season in the Hintalo Wajerat district of northern Ethiopia. Geographically, it is located at an altitude of 2100 meters, latitude 13°14'N, and longitude 39°32'E, in the Southeastern province of Tigray. The average annual minimum and maximum temperatures are 22°C and 30°C respectively, and the soil texture at depths of 0-50 cm is vertisol with a pH of 8.03. The area has a total of 295 ha of irrigable land [25].
2.2. Experimental Design and Treatments
The experiment was set up in a randomized complete block design (RCBD) with three replicates of seven treatments. This experiment includes five bio-rational insecticides; Bitter Dock (Rumex obtusifolius L.), Endod (Phytolacca dodecandra L.), Tree Tobacco (Nicotiana glauca G.), African marigold (Tagetes minuta L.), Neem (Azadirachta indica L.), a synthetic Chemical - Karate 5% EC (standard test) and untreated control (water only) (Table 1). Cabbage seeds (Dutch variety) were planted in the nursery on 22nd December 2019 and transplanted to the test plots on 2nd February 2020 after 40 days. The experimental plot was 6 m2 (2 * 3 m) and contained a total of 21 plots. The distances between plants, rows, and blocks were 30, 50, and 100 cm, respectively. All cultural practices were followed, as recommended for the commercial production of cabbage.
| Trt Code | Botanical Name | Common Name | Local Name | Family Name | Parts Used | Rate |
|---|---|---|---|---|---|---|
| RLE | Rumex obtusifolius | bitter dock | shembata | Polygonaceae | Leaf extract | 50g/l |
| ELE | Phytolacca dodecandra | Endod | Shibti | Phytolaccaceae | Leaf extract | 50g/l |
| TTLE | Nicotiana glauca | Tree tobacco | Chergid | Solanaceae | Leaf extract | 50g/l |
| TMLE | Tagetes minuta | African marigold | Etsefarse | Asteraceae | Leaf extract | 50g/l |
| NLE | Azadirachta indica | Neem Tree | Limo | Meliaceae | Leaf extract | 50g/l |
| Kar | Karate (5% EC) | - | - | - | - | 1l/ha |
| Con | Control (water) | - | - | - | - | - |
2.3. Methods of Extraction
Mature leaves of each plant were collected from near the study site. All collected leaves were washed, ground, and dried for seven days in the shade without direct sunlight [26]. Place the dried leaves in a juice grinder to make a powder and sieve. Each sieved sample powder was soaked in distilled water for 24 hours at a ratio of 50 grams per liter of water [25, 27]. Water extraction was performed by mixing and stirring the samples. For all bio-pesticide extracts, the mixture was then filtered through a filter cloth (that is, a muslin cloth). A total of 15 ml of soap was added to emulsify the herbal treatments [28]. Stock solutions were ready for spray application. The chemical insecticide (Karate 5% EC) and soaps used were of the highest purity available and purchased from local markets.
2.4. Data Collection and Analysis
Data were collected from 10 plants randomly picked from the middle row of each plot before application, 24 hours, and 48 hours after application at 14 days intervals. Pre-spray counts were made immediately before spraying. Post-application counts were performed 24 and 48 hours after application. A total of 4 sprayings were performed. Diamondback moth larvae and nymphs of the aphid colonies were counted 3 weeks after transplantation. Diamondback moth and aphid colonies were recorded as indicators of the efficacy of each treatment. The percent reduction of insects’ infestation due to treatment was calculated by a modification of Abbott's formula [29].
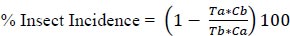 |
Where: Tb is the number of insects collected per sampling before treatment, at number collected after treatment, the Cb number collected from the check plot before treatment, and the Ca number collected from the check plot after treatment of the test plots.
Plant height (cm): measured in cm from the soil level to the top of the longest outer leaf of a single plant and recorded as the average of 10 randomly selected plants.
Head diameter (cm): ten samples of cabbage heads were randomly taken at harvest from each plot, measured using a caliper (model LEG ilex- 250 mm, US patent), and expressed in centimeters.
Head weight: ten samples of cabbage heads were randomly taken at harvest from the middle row of each plot after separating by hand from the straw and measured using a sensitive balance.
Total yield (tha-1): determined after harvesting cabbage from the middle row of the plot after manual separation from the straw.
Avoidable yield losses: they were computed from the yield recorded in untreated check plots and those receiving maximum protection against the tested insect pests. It was computed by:
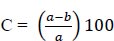 |
Where: C = avoidable loss (%); a = yield in protected plots, and b = yield in unprotected plots.
Data were analyzed using the statistical software GenStat 18th Edition [30]. ANOVA was used when there was a significant difference (P < 0.05) and Fisher's multiple comparison test was applied to mean separation [31].
3. RESULTS AND DISCUSSION
3.1. Effect of Bio-rational Insecticides on Diamondback Moth and Cabbage Aphids
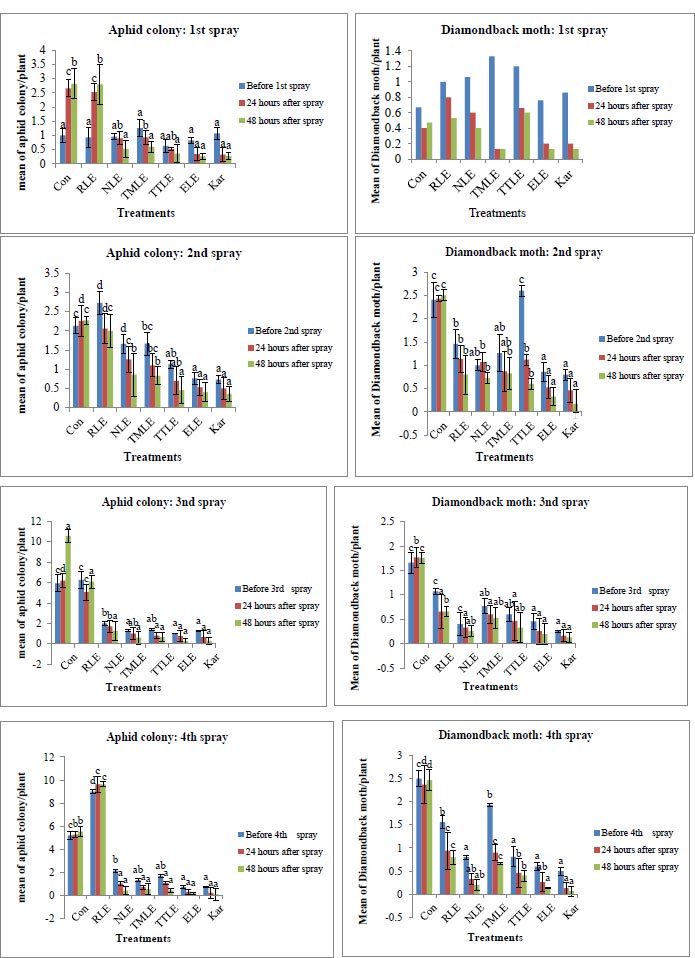
A significant difference (P < 0.05) was observed in the populations of diamondback moth larvae and aphids colonies after foliar spraying, except for the first week of the first spraying (Fig. 1). The highest numbers of diamondback moth larvae and aphid colonies per plant were recorded in control plots and plots treated with R. obtusifolius leaf extract, whereas the lowest numbers of tested insects were noted in P. dodecandra followed by A. indica and N. glauca treated plots after 24 and 48 hours post-treatment applications. Another study reported that insecticides are generally considered the most effective means of protecting plants from pests because they can rapidly control a broad pest complex of major cruciferous pests, and individuals have reported fears of leaf damage from even the slightest puncture, they tend to spray insecticides [32]. However, in the present study, bio-rational insecticides (P. dodecandra, A. indica, and N. glauca) aqueous leaf extracts significantly reduced both diamondback moth and aphid colonies in the four spraying intervals. Similarly, another study reported that P. dodecandra was more effective than neem leaf extract in controlling tomato leaf miners [25]. Moreover, another study registered the lowest number of larvae per plant by applying 5% neem to okra [33]. One of the studies also confirmed that the application of N. glauca and A. indica reduced thrips populations from 23.13 to 6.4 and 25.5 to 2.57, respectively, compared to controls [34]. A study found that treatment with neem extracts to control diamondback moth caused okra to grow vigorously [35]. In addition, another study also pointed out that neem extract plays an important role in transforming the attractive properties of cruciferous vegetables into diamondback moths [36]. It was also reported in a study that aqueous extracts of L. camara and A. indica were effective against diamondback moths and had a significant impact on cabbage invasion [37]. Another study also showed the efficacy of tobacco-based bio-pesticides against whiteflies (70.88%), thrips (57.27%), and aphids (60.40%) [38]. These plant extracts can be applied to cabbage pest control by reducing the use of synthetic insecticide sprays as an important part of an integrated pest management program. Bio-rational insecticides may affect the behavior and development of herbivorous insects that use plants for reproduction because of their low anorexic, non-neurotoxic, and environmental persistence [39].
| S.No | Treatments | Plant Height (cm) | Head Diameter (cm) | Head at Plant-1 (kg) | Total Fresh Head at (t ha-1) | Yield Over Control (%) | Avoidable Yield Loss (%) |
|---|---|---|---|---|---|---|---|
| 1 | Con | 14.67a | 12.77a | 0.47a | 23.86a | 0.00 | 0.00 |
| 2 | RLE | 19.3b | 14.89b | 0.69ab | 25.01a | 4.82 | 4.60 |
| 3 | NLE | 20.41b | 16.51bc | 0.94bc | 27.69a | 16.05 | 13.83 |
| 4 | TMLE | 20.50bc | 16.05bc | 0.93bc | 26.33a | 10.35 | 9.38 |
| 5 | TTLE | 19.66bc | 16.76cd | 0.91bc | 26.72a | 11.99 | 10.70 |
| 6 | ELE | 20.64bc | 18.81e | 1.19c | 40.28b | 68.82 | 40.76 |
| 7 | Kar | 22.61c | 18.45de | 1.17c | 37.52b | 57.25 | 36.41 |
| Grand mean LSD (0.05) CV (%) |
19.68 | 16.17 | 0.89 | 29.49 | 24.18 | 16.53 | |
| 2.93 | 1.82 | 0.41 | 5.46 | - | - | ||
| 8.4 | 6.3 | 25.4 | 10.4 | - | - | ||
3.2. Impact of Bio-rational Insecticides on Cabbage Yield and Yield-related Traits, and Yield Losses
A significant (p<0.05) difference was also recorded in all treatment plots compared to controls. Application of the aqueous extract increased yield and yield-related traits (Table 2). There was a significant difference (P < 0.05) between treatments in affecting plant height (Table 2). Plots treated with P. dodecandra produced the highest (20.64 cm) plants. The mediocre plant height was obtained from A. indica and N. glauca treated plots which are statistically equivalent to the synthetic insecticide Karate 5% EC used as a standard test. However, the control cabbage plot had the shortest (14.67 cm) plants. This is consistent with the results of one of the studies [40] which noted that treating cabbage with insecticides reduced the cabbage insect population and improved crop growth [35]. also reported that okra grows vigorously when treated with botanical insecticides. The head diameter was significantly (P < 0.01) affected by bio-pesticide application (Table 2). The maximum head diameter (18.81 cm) was obtained from the P. dodecandra treated plot and the minimum head diameter (12.77 cm) from the control plot. Medium head diameters were recorded from N. glauca, A. indica, and T. minuta treated plots, all of which are significantly equivalent to Karate 5% EC. There was a highly significant difference (P < 0.001) between treatments for head weight and total raw cabbage yield (Table 2). The maximum cabbage weights (1.19 and 1.17 kg plants-1) and fresh yields (40.28 and 37.52 tons ha-1) were obtained from P. dodecandra and Karate 5% EC while the untreated plots had the lowest head weight (0.47 kg plant-1) and fresh yield (23.86 tons ha-1). Moreover, cabbage plots treated with A. indica, N. glauca, and T. minuta produced comparable head weights and total fresh yields. This indicates that controlling diamondback moth and cabbage aphids with bio-rational insecticides can double cabbage yields, although reducing the tested pest populations. They were also as effective as chemical pesticides in reducing losses. Similar results were reported by [41] who found the highest yield (7540 kg ha-1) from 2.5% of neem extract and the lowest (0.4%) invasion of tomato fruit worm larvae [42]. reported that the maximum numbers of marketable head cabbages were obtained from sprayed cabbage and the greatest numbers of non-marketable cabbages were found in untreated cabbage plots. In the current study, the plant extract was highly effective in inhibiting the tested insect pests and improved cabbage yield compared to controls. This may be due to the pungent odor of the soaked plant extract, which deters pests from eating the plant [43].
Avoidable yield loss and yield increment over control are presented in (Table 2). The highest avoidable yield loss was recorded from P. dodecandra (40.76%) compared to the other aqueous extracts, even more than the standard test karate 5% EC (36.41) due to diamondback moth and aphids. Congruently [44], reported a maximum of (57.60%) avoidable yield loss caused by insect pests in sesame. In other ways, a maximum estimated yield loss of 40.76% was registered from the untreated control plots due to diamondback moths and aphids. Similarly, a study reported yield losses of 17% - 99% due to diamondback moth, 69% due to cabbage caterpillar, and 28% - 51% due to cabbage leaf webber. Moreover, the highest (68.82%) yield advantage was obtained from P. dodecandra-treated plots whereas the overall yield increment ranged from 4.82% to 68.82% due to the various aqueous leaf extracts used in this experiment [45].
CONCLUSION
Among the bio-rational insecticides tested, Phytolacca dodecandra aqueous leaf extract is one of the most promising botanicals with bio-insecticidal activity against diamondback moths and cabbage aphids. It is equally as effective as synthetic pesticides to reduce insect populations and has doubled the yield of fresh cabbage. The maximum estimated yield loss of 40.76% was also protected due to the application of Phytolacca dodecandra aqueous leaf extract. Azadirachta indica leaf extract can also be used as a potential candidate for controlling the pests mentioned, thus growers may use Phytolacca dodecandra leaf extract in cabbage patches, which is recommended to reduce the invasion of cabbage pests and ensure yield.
LIST OF ABBREVIATIONS
| DBM | = Diamondback Moth |
| RCBD | = Randomized Complete Block Design |
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author [A.A], on a special request.
FUNDING
This study was funded by Mekelle University, Ecological Organic Agriculture, Funder ID: MU; EOA.
CONFLICT OF INTEREST
The authors declare no conflict of interest financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thank the DCHS Plant Protection Laboratory for providing them with the necessary facilities required for drying, grinding, and extracting the plants.


