All published articles of this journal are available on ScienceDirect.
Palm Dates Protect Memory Formation in Diabetes Mellitus: Neutralization of Oxidative Stress
Abstract
Background:
Diabetes Mellitus (DM) is associated with spatial memory impairment that is attributed to the oxidative imbalance in the brain. Palm dates were reported to have neuroprotective and antioxidant effects. This investigation examined palm date consumption for its impact on the decline in cognitive function and oxidative imbalance associated with DM using the streptozotocin (STZ) rat model.
Methods:
The palm dates extract was administered to rats orally (3.2 g per kg of body weight) for eight weeks. Memory assessment was performed using the Radial Arm Water Maze (RAWM). Hippocampal biomarkers of oxidative stress were evaluated.
Results:
STZ-treated animals revealed significant spatial memory impairment (short-and long-term) (P<0.05). Date consumption for eight weeks prevented the decline in spatial memory induced by STZ (P<0.05). STZ administration induced oxidation imbalance in the hippocampus as marked by the significant reduction in the activity of glutathione peroxidase (GPx), reduced glutathione (GSH) levels, and GSH/GSSG ratio as well as raised levels of oxidized glutathione (GSSG) (P<0.05). Date consumption for eight weeks prevented oxidative imbalance induced by STZ in the hippocampus (P<0.05).
Conclusion:
This study has verified the beneficial effect of palm dates on cognitive impairment and oxidative imbalance associated with DM.
1. INTRODUCTION
Diabetes Mellitus (DM) is an age-associated disease affecting hundreds of millions worldwide [1]. The hallmark of this disease is hyperglycemia due to insufficient insulin production, insulin resistance, or increased secretion of glucagon [2]. Uncontrolled DM leads to several complications, such as retinopathy, nephropathy, neuropathy, and cardiovascular complications [3]. Chronic DM is shown to negatively impact cognitive function, impair learning and memory, alter brain structure, and induce dementia [4-6]. These alterations showed symptoms similar to accelerated brain aging [6]. The contribution of oxidative stress to DM pathophysiology is well-established. It has been shown that oxidative stress induces insulin resistance, impairs glucose tolerance, and leads to β-cell dysfunction [7-9].
Dates, Phoenix dactylifera L. (Arecaceae), are rich in energy nutrients, vitamins, and minerals, including iron, potassium, and calcium [10, 11]. Besides that, dates are also rich in antioxidants, such as phenolic compounds and potent free radicals scavenging molecules [12]. In animal models of Alzheimer's disease, palm date-rich diets have improved cognitive function and lower beta-amyloid and neuroinflammation in the brain [13, 14]. Similar findings were observed using palm fruit seed extracts [15]. Therefore, in this study, palm dates were examined for their impact on the decline in cognitive function and oxidative imbalance associated with DM. Both the behavioral approach, using the radial arm water maze (RAWM) to test learning and memory functions, and the molecular approach, using oxidative biomarkers assays, were employed in the present study.
2. MATERIALS AND METHODS
2.1. Plant Collection
The ripe fruits of Ajwa date were bought from the local market. Identity confirmation of Ajwa dates was done by Dr. Mohammad Al-Gharaibeh, Plant Taxonomist, Department of Plant Production, Faculty of Agriculture, Jordan University of Science and Technology (JUST), Irbid, Jordan. A voucher specimen (PHS-125) was placed in the Herbarium of the Faculty of Pharmacy, JUST, Irbid.
2.2. Plant Extraction
Pits of Ajwa dates were manually removed from the pulps. Pulps were prepared by adding water in a 1:1 ratio before being mixed in a blender (Moulinex Blender LM207127 500W) until a homogeneous mixture was obtained. The aqueous Ajwa dates mix was prepared daily and administered by oral gavage to the animals at a dosage of 3.2 g/Kg body weight.
2.3. Animals and Interventional Treatments
Adult Wistar rats (all male and each weighing between 250 and 300 g) were kept in cages as 4-6 animals per cage with bedding and good animal health conditions alternating between 12 hours of daylight (light on at 8:00 am) and 12 hours of darkness with temperature control (24±1 °C), and freely accessible food and water. All experiments were performed during the daylight cycle. The Animal Care and Use Committee (ACUC) at JUST, Irbid, Jordan, approved the study.
Rats were allowed to adapt for one week before being randomly allocated into four groups of 12 animals each. The groups were non-diabetic (control), non-diabetic with palm dates (Dates), diabetic (STZ), and diabetic with palm dates (STZ+Dates). The STZ and STZ+Dates groups were administered STZ on day 7 of the procedure (Fig. 1). The dates and STZ+Dates groups were administered the Ajwa dates mix starting from the same day (day 7). An aqueous vehicle solution of the same volume as the Ajwa dates mix was administered via oral gavage to the control and STZ groups. Treatments continued for eight weeks.
2.4. Diabetes Induction and Blood Glucose Measurement
Induction of DM was performed using intraperitoneal streptozotocin (STZ) at a dosage of 35 mg/kg of body weight [16-19]. STZ solution was freshly prepared in citrate buffer (pH 4.5) obtained from Sigma-Aldrich Corp, Mi, USA. All non-diabetic groups were administered the same amount of the buffer solution but without STZ. Following STZ administration, fasting blood glucose (FBG) monitoring was done at 24-hours after the injection and after that, every seven days. DM was confirmed in STZ-treated animals using a glucose analyzer (Accu-Chek Performa Blood Glucose Meter) when blood glucose level reached ≥ 300 mg/dL. Blood was sampled from the tail of the animal. To prevent hypoglycemia, a 10% sucrose solution was administered to rats in drinking water for 24 hours post-injection with STZ.
2.5. The Radial Arm Water Maze (RAWM)
As reported before, spatial learning and memory were assessed using the RAWM [20-22]. The RAWM comprises a black water-filled tank with six stainless-steel panels arranged around the circular tank in a V-shape to make a central space with six radiant arms for animals to swim, where the water temperature is 24 ± 1°C. Two images were placed on the wall at specified locations to give rats visual clues. Experiments were conducted in a room using dim lighting except for the acclimatization phase that preceded starting the investigation by day one.
2.6. Hippocampus Dissection and Biochemical Testing
Dissection of the hippocampus was carried out immediately after the experimental rats’ decapitation; then, the hippocampi were kept at - 80°C until homogenization. Using a plastic pestle, hippocampal tissue was manually homogenized in a cocktail of protease inhibitors (Sigma-Aldrich Corp, MI, USA) solution in phosphate buffer [23]. A commercially available kit was used to measure the total protein concentration in the samples (BioRAD, Hercules, California, USA). To determine total glutathione levels, 5-sulfosalicylic acid (5%) was used to deproteinize the homogenized tissue. The homogenized tissue was centrifuged at 1000x for 110 minutes at 4ºC (Sigma-Aldrich, MI, USA). After that, spectrophotometry was used to quantify the total glutathione level. The GSSG levels were assessed using 1 mL of the supernatant combined with 10 µL of 2-vinyl pyridine, then the Glutathione Assay Kit was used (Sigma- Aldrich CO., MI, USA). The prior measures used to determine total glutathione were followed. Reduced glutathione level was calculated by GSSG level subtraction from the total glutathione. As per the instructions of the manufacturer kit, GPx activity was determined by a Glutathione Peroxidase Cellular Activity Assay Kit, catalog: CGP1, Sigma-Aldrich, MI, USA. The microplates of ELISA were scanned at predetermined wavelengths via a microplate reader (ELx800, Bio Tek Instruments, plate reader, Highland Park, Winooski, USA).
2.7. Determination of the Total Content of Phenolic Compounds, Flavonoids, and Antioxidant Activity
Three extraction replicates of Ajwa dates were prepared as outlined above. The aqueous Ajwa dates suspensions were centrifuged. The supernatants were transferred into screw caps vials and stored at 4°C for in vitro phytochemical analysis, including the total content of phenolic compounds and flavonoids and antioxidant capacity.
Ajwa date extracts were assayed for their total content of phenolic compounds using the Folin–Ciocalteu colorimetric approach as reported in the literature [24]. Gallic acid was the reference standard phenolic compound used. In brief, about 50 µL of each Ajwa date extract, 450 µL of dH2O, and 2.5 mL of Folin–Ciocalteu reagent (0.2 N) were added together before allowing them to stand for 5 minutes. Afterward, the mixture was completed with about 2 mL of sodium carbonate (75 g/L) and left to incubate for 1.5 hours at ambient temperature with frequent agitation. The resulting blue solution absorbance was then measured at a wavelength of 765 nm. Using 80% methanol, six gallic acid calibration curve points were prepared at the following concentration levels: 20, 100, 200, 300, 400, and 500 mg/L. Gallic acid equivalents (GAE) in mg/g of fruit material have been used to represent the Ajwa dates’ total content of phenolic compounds. Three extraction replicates were prepared, and each was run in triplicate (n=9).
The total content of flavonoid compounds in Ajwa date extract was determined by a colorimetric test of aluminum chloride [25]. A mixture of 1 mL of each Ajwa date extract in addition to 4 mL of dH2O and 300 μL of 5% sodium nitrate solution was prepared before being left to stand for 5 minutes. Afterward, the resulting solution was mixed with about 300 μL of 10% aluminum chloride solution and allowed to stand for 6 minutes before adding about 2 mL of 1 M sodium hydroxide. Finally, the total solution volume was completed to 10 mL with dH2O and then mixed vigorously by a vortex mixer resulting in an orange-yellowish colored solution with a measured absorbance at 510 nm wavelength. The reference standard used was quercetin, which was prepared at the following concentration levels: 100, 200, 400, 600, 800, and 1000 µg/mL. Ajwa date extracts were analyzed in triplicates. The total content of the flavonoid compounds was represented by mg of quercetin equivalents/ weight of Ajwa dates dried material. Three extraction replicates were prepared, and each was run in triplicate (n=9).
The antioxidant capacity of Ajwa dates was estimated using the improved ABTS•+ as reported in the literature [26]. Briefly, the ABTS•+ was generated by reacting at room temperature in darkness for about 12- to 16- hours with a solution of 7 mM ABTS and 2.45 mM potassium persulfate. The solution was diluted to reach an absorbance of 0.700 ± 0.040 at a wavelength of 734 nm with 80% ethanol and left to equilibrate at 30°C. To about 3 mL of the resulting diluted ABTS•+ solution, about 30 µL of each Ajwa date extract solution was added and vortexed, and then the solution was left for 6 minutes. The absorbance was then recorded at 734 nm wavelength. The reference standard compound used was Trolox, prepared in a concentration range of 0 to 2.5 mM using 80% ethanol using the same procedure. The antioxidant capacity was represented by Trolox Equivalent Antioxidant Capacity (TEAC, l mol Trolox equivalents/g Ajwa fruit material dry weight). Three extraction replicates were prepared, and each was run in triplicate (n=9).
2.8. Statistical Analysis
Graph Pad Prism for statistical analysis (version 6.0, GraphPad Software, LA Jolla, CA) was used for statistical analysis. A two-way ANOVA test with multiple comparison post-tests (Bonferroni) was performed to compare the error numbers of the learning trials of RAWM. In addition, the One-way ANOVA test, followed by Bonferroni’s post-test, was used to compare the error numbers of the memory and biochemical tests of the RAWM. Results were considered statistically significant at a P <0.05. Data were displayed as mean ± standard error of means (SEM).
3. RESULTS
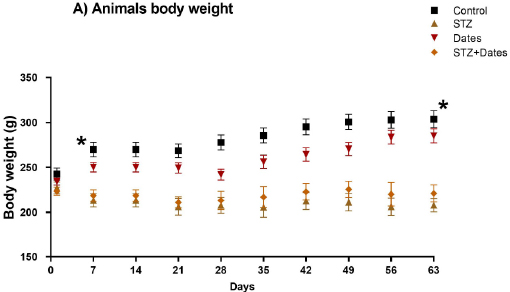
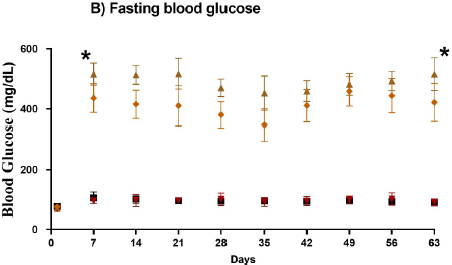
3.1. Measurements of Body Weight and Blood Glucose
The experimental animals’ body weight (BWT) was determined initially and during the study. The interaction between time and STZ treatment significantly changed BWT and FBG. In the two diabetic groups (STZ and STZ+Dates), a reduction in BWT and an increase in FBG were observed after DM induction, as shown in Fig. (1A and B), respectively. BWT was significantly higher in the control and dates groups for all points from day seven than in STZ and STZ+Dates groups (P< 0.05).
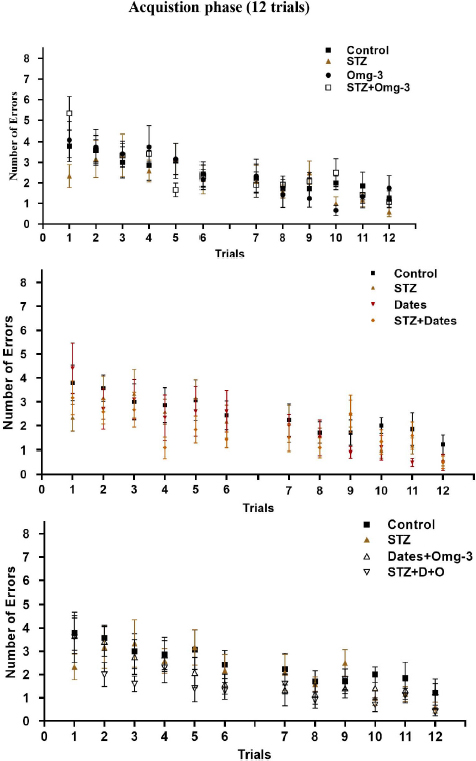
3.2. The Effect of Palm Dates on Memory Impairment-induced by DM
The four experimental groups showed similar and increased errors during the early learning phase compared to the latter period. With training, the number of errors decreased (P<0.05) gradually in all groups without differences (P>0.05) between them (Fig. 2).
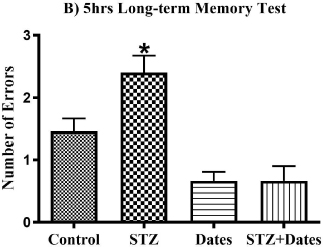
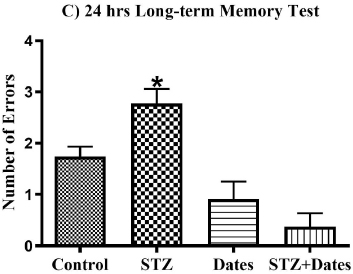
The ANOVA showed a main effect of study treatments in the short-term, 5-hour, and 24-hour memory tests. As shown in Fig. (3), additional post-hoc analysis showed fewer errors in the control, Dates, and STZ+Dates groups versus the STZ group. However, no differences were noticed in the control, Dates, and STZ+Dates groups. These results demonstrated that STZ was associated with increased errors throughout the short- and long-term memory tests mitigated with date treatment.
3.3. The Effect of Dates on Hippocampal Oxidative Stress-induced by DM
3.3.1. Levels of GSH and GSSG, GSH/GSSG Ratio, and GPx activity
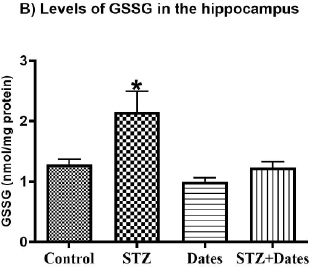
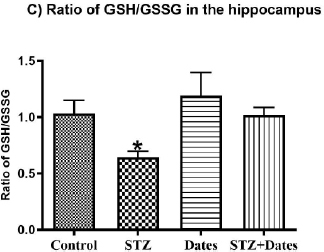
According to the ANOVA tests (Fig. 4), administered treatments significantly affected GSH and GSSG levels, GSH/GSS ratio, and GPx activity. Subsequent group comparisons showed that STZ treatment increased GSSG and decreased GSH, GSH/GSS, and GPx activity. However, no differences were found between the other treatment groups. These results indicated that STZ treatment increased oxidative stress that was ameliorated after the date treatment.
3.4. Determination of the Total Content of Phenolic Compounds, Flavonoids, and Antioxidant Activity
The total content of phenolic and flavonoid compounds and antioxidant capacity of aqueous Ajwa dates were 2.5 ± 0.15 mg GAE/g, 0.47 ± 0.02 mg QE/g, and 7.302 ± 0.47 µmol TE/g, respectively, of dry weight.
4. DISCUSSION
The current study revealed that consuming palm dates for eight weeks prevented memory impairment in STZ-induced DM in a rat model. In addition, the palm dates also prevented hippocampal oxidative stress in the same model.
Previous studies showed that DM is linked to problems in learning and memory [27-30]. In line with previous studies, the current study showed increased errors in short- and long-term memory assessments after eight weeks of DM induction, pointing toward reduced memory. Previous studies also reported cognitive impairment after 31 days to 12 weeks of DM induction by STZ [29, 31, 32]. Furthermore, this study showed that consuming palm dates for eight weeks reduced the number of rats’ errors in both memory assessments, which might indicate a protective role against STZ-induced impaired memory.
Cell dysfunction and tissue injury can be caused by the poor balance between reactive oxygen species (ROS) formation and cellular defense mechanisms as enzymatic and non-enzymatic antioxidants [21, 33, 34]. Diabetes, by itself, promotes ROS formation while reducing the activity and level of antioxidants, thus leading to the development of oxidative stress [35]. Oxidative stress has been shown to contribute to the pathogenesis of DM-induced memory impairment [36, 37].
The brain is susceptible to the alterations associated with oxidative stress due to its lipid composition [38]. Hence, lipid peroxidation results from increased oxidative stress that eventually inactivates receptors, enzymes, and neuro- transmitters in the brain [39]. Furthermore, the increased glucose level enhances the generation of ROS and reactive nitrogen molecules. The formation of ROS plays a significant function in the pathological process of many neurodegenerative diseases associated with memory impairment [30, 40].
In the current study, STZ administration induced oxidation imbalance in the hippocampus as marked by the significant reductions in glutathione peroxidase (GPx) activity, glutathione (GSH) levels, and GSH/GSSG ratio as well as raised GSSG levels (P<0.05). Date consumption for eight weeks prevented oxidative imbalance induced by STZ in the hippocampus (P<0.05). This may explain the involvement of oxidative stress in STZ-meditated impaired memory. The hippocampus is the leading player in spatial learning and memory development. Palm date consumption normalized the disturbed hippocampal antioxidants by STZ. Future research should assess the effects of palm dates on other sets of oxidative stress biomarkers, such as catalase and superoxide dismutase, among others.



The antioxidant activity of palm dates is well-documented [41, 42]. For example, about 22 compounds, such as 5-Hydroxymethylfurfural and 4H-Pyran-4-one,2,3-dihydro-3,5-dihydroxy-6-methyl with strong antioxidant properties, have been identified in palm date [43]. In addition, the induction of oxidative stress and nephrotoxicity by dimethoate in rats was prevented by palm dates [44]. Similarly, date extracts prevented nephrotoxicity and gentamycin-induced oxidative stress in a rat model [45]. The ability of palm date to normalize oxidative stress associated with DM suggests its potential therapeutic use in the management of diabetes.
CONCLUSION
Palm dates prevented STZ-induced memory impairment in an animal model of DM by adjusting the biomarkers of hippocampal oxidative stress to the normal, thus, neutralizing the imbalance between ROS and antioxidants. These results suggested the beneficial effect of palm dates in enhancing cognitive function and reducing oxidative stress among people with diabetes.
LIST OF ABBREVIATIONS
| DM | = Diabetes Mellitus |
| STZ | = Streptozotocin |
| RAWM | = Radial Arm Water Maze |
| GPx | = Glutathione peroxidase |
| GSH | = Reduced Glutathione |
| GSSG | = Oxidized Glutathione |
| JUST | = Jordan University of Science and Technology |
| FBG | = Fasting Blood Glucose |
| SEM | = Standard Error of Means |
| BWT | = Body weight |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Animal Use and Care Committee and the Deanship of Research Committee at JUST (approval number: 508/2018).
HUMAN AND ANIMAL RIGHTS
No humans were used in this study. All the procedures involving animals were performed according to the “Guide for the Care and Use of Laboratory Animals.”
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Data will be available upon request via e-mailing the corresponding author [K.H.A].
FUNDING
This research was funded by the Deanship of Research at JUST, Irbid, Jordan (Project number 508/2018).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


