RESEARCH ARTICLE
Evaluation of Germination and Seedling Growth of Plant Seeds Primed with Cultures of Providencia Sp. and Bacillus Cereus under Varying Conditions
Ayotunde O. Ajinde1, Tolulope O. Ogunnusi1, Olumayowa J. Iyanda2, Oghenerobor B. Akpor1, *
Article Information
Identifiers and Pagination:
Year: 2023Volume: 17
E-location ID: e187433152306011
Publisher ID: e187433152306011
DOI: 10.2174/18743315-v17-230621-2023-10
Article History:
Received Date: 15/02/2022Revision Received Date: 17/04/2023
Acceptance Date: 19/05/2023
Electronic publication date: 18/07/2023
Collection year: 2023

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Seed quality, an important determinant of germination and vigor potential, can be improved through seed priming. This study was therefore aimed at assessing the effects of steeping duration and inoculum concentration on the germination and seedling growth of five seed crops through priming with growth-promoting rhizobacteria.
Methods:
Broth cultures of five bacterial strains, belonging to Providencia vermicola (2 strains), P. rettgeri (2 strains), and Bacillus cereus (1 strain), isolated from rhizosphere were used for priming in the study. Seeds of cowpea (Vigna unguiculata), soybean (Glycine max), sorghum (Sorghum bicolor), sesame (Sesamum indicum), and okra (Abelmoschus esculentus) were used as experimental materials. To determine the effects of steeping duration, viable seeds of the respective crops were primed with broth cultures of the respective isolates and allowed to stand for a known duration (1, 2, 3, 4, or 5 h). Then, another set of viable seeds was steeped in varying concentrations of the bacterial cultures for a period that was determined to be the optimal steeping duration in the first experiment.
Result:
At the expiration of both experiments, final germination, mean germination time, germination index, and vigor index of the respective seeds were estimated. Generally, higher final germination and seedling vigor index values were restricted to shorter steeping periods for cowpea and soybean. With respect to inoculum concentration, there was no consistent pattern with the parameters.
Conclusion:
The study revealed the primacy of steeping duration over inoculum concentration with respect to bacterial priming.
1. INTRODUCTION
Improved agricultural techniques have greatly enhanced food production in the last 50 years as a result of intensive usage of chemical inputs for plant growth promotion and pest control [1]. This current overreliance on synthetic fertilizers and pest control chemicals has resulted in a number of environmental issues, including reductions in soil quality and biodiversity and groundwater contamination [2-4].
Moreover, the contemporary agriculture model is threatened by climate change-induced changes such as increased temperature and CO2 levels and drought [5]. Due to the expected increase in crop production to meet the nutritional and industrial needs of the world’s population that are forecasted to hit around 9 billion by 2050 on the one hand and the increasing awareness about the environmental and health impacts of the current agro-model on the other hand, significant attention is being shifted to more ecofriendly alternatives [1].
Soil microorganisms play important roles in biogeochemical cycling, soil fertility, and plant diversity balance [6]. There are some bacterial species that live in close proximity to plants and have beneficial effects on them. Plant growth-promoting bacteria (PGPB) refers to soil bacteria, occurring freely in the soil or in close association with plant roots, that have beneficial effects on crop productivity. Bacterial species that have been shown to possess plant growth promotion potential include members of the genera Arthrobacter, Azotobacter, Azospirillum, Bacillus, Burkholderia, Enterobacter, Herbaspirillum, Pseudomonas, Rhizobium, and Serratia [7].
Good seed vigor, even under adverse environmental conditions, is a desirable property of seeds [8, 9], but seeds can deteriorate during storage. In the case of soybean, for example, deterioration in seed quality can occur in seeds before and after harvest periods, mostly as a result of adverse environmental conditions and due to disease pathogens [10]. Hence, seed priming represents an attractive strategy to counteract the potential loss in seed quality. Seed priming involves soaking seeds in a solution to allow the initiation of the germination process but not radicle protrusion, the terminal germination event to enhance germination and growth. Priming serves to extend the lag phase to allow for some important pre-germinative physiological and biochemical processes to occur, but actual visible germination, that is, radicle emergence, is prevented [11, 12].
Commonly used priming techniques include hydropriming (steeping seeds in water), osmo-priming (the use of osmotic solutions), halopriming (the use of salt solutions), and the use of plant growth hormones (e.g., auxins and gibberellins). Chemical seed priming has been shown to enhance the agronomic parameters of a variety of crops [13-18].
Biopriming entails the steeping of seeds in bacterial suspensions for a predetermined duration in order to allow for bacterial colonization of the spermosphere and initiation of physiological processes of germination [19-21]. Improved germination and seedling development are beneficial effects of bacterial biopriming [22]. Moreover, they can enhance plant growth and root development, improve germination speed, and reduce the susceptibility of plants to diseases and environmental stress [23]. The use of plant growth-promoting bacteria (PGPB) as bio-primers holds great potential in agricultural production [24, 25]. Due to the critical importance of seeds in agriculture, it is necessary to explore the optimization of the application of priming techniques used in order to reduce wastage and ensure the best results. This study was therefore aimed at assessing the effects of steeping duration and inoculum concentration on the germination and seedling growth of five seed crops during priming by growth-promoting rhizobacteria.
2. MATERIALS AND METHODS
2.1. Preliminary Viability Testing of the Seeds
The seeds of the five crops used for the study were cowpea (Vigna unguiculata), soya bean (Glycine max), sorghum (Sorghum bicolor), sesame (Sesamum indicum), and okra (Abelmoschus esculentus). The seeds were obtained from local markets in Ado-Ekiti, Ekiti State, Nigeria. Before use, the seeds were surface sterilized in 5% (v/v) sodium hypochlorite solute (v/v) for 5 min. After surface sterilization, the seeds were rinsed three times in sterile distilled water.
All tested seeds were subjected to viability testing before use in the study. Preliminary viability testing was carried out by putting 500 surface-sterilized seeds in a 500 mL beaker containing around 500 mL of distilled water. Seeds that floated were regarded as not viable and discarded while the rest were subjected to further viability testing. Seeds that passed this preliminary test were further tested for viability by planting a known number of seeds on three point five (3.5) grams of absorbent cotton wool (serving as blotters) that were placed in transparent plastic containers (80 cm in diameter and 40 cm depth) and incubated for five days. Seeds were considered viable when a percent germination of 70 was obtained.
2.2. Rhizosphere Bacteria
Five bacterial species were used for the study. The bacteria comprised two species of Providencia vermicola, two species of P. rettgeri, and one species of Bacillus cereus. The test bacterial species were isolated from rhizospheres within Afe Babalola University environs using the standard pour plating procedure and identified using the 16S rRNA polymerase chain reaction (PCR) procedure. Following identification, the sequences were deposited on the NCBI database and the following ascension numbers were obtained: Providencia vermicola (OP830490), P. rettgeri (OP830497), P. rettgeri (OP830496), P. vermicola (OP830492) and Bacillus cereus (OP830499) and were referred to as isolates F, G, H, I and J, respectively.
2.3. Germination and Seedling Growth Studies
Two parameters (effects of steeping duration and initial inoculum concentration) were investigated in this study. To determine the effects of steeping duration, previously ascertained viable seeds of the respective crops were primed in broth cultures of the respective isolates and allowed to stand for a known duration. Every 1 h, for a five-hour duration, 21 seeds of the respective crops were withdrawn from the solution and planted in triplicates (7 seeds per lot) in plastic cups containing blotters and incubated under laboratory light for eight days. Daily germination was recorded throughout incubation, while final germination, germination index, mean germination time and vigor index were estimated.
Where f is the number of seeds germinated on day x
- Germination index (GIX) = (8 × N1) + (7 × N2) + (6 × N3) + … + (1 × N8) [28]
Where N1, N2, N3 … N8 represent the number of seeds that germinated on the first, second, third until the 8th day, and 8, 9, 7, …1 are the weights given to the number of germinated seeds on the first, second, third day up to the 8th day.
- Vigor index (VIX) = FGP × average plant height [29]
In the case of determination of the effect of initial inoculum concentration on germination and seedling growth of the seeds, varying dilution concentrations of the bacterial broth cultures were used for steep priming. Inoculum dilutions were prepared using water/broth culture ratios of 4:1, 3:2, 2:3, 1:4, and 0:5. The concentrations for 0:5 dilutions of the respective inoculums were 360,274 (F), 2,980,000 (E), 2,468,000 (G), 653,464 (H), and 246,379 (I) CFU/mL. Seeds were steeped at the respective dilutions for the optimal steeping duration before planting and incubated as described earlier. The steeping duration used for each isolate was the respective optimal steeping duration for the vigor index in the steeping duration experiment, representing the lowest steeping duration that produced the highest significant vigor index. Where there was no data for the steeping duration experiment, the seeds were steeped at the various inoculum concentration for 2 hours. Following planting and incubation, germination and seedling growth parameters were measured, as described earlier.
3. RESULTS
3.1. Effect of Steeping Duration
3.1.1. Cowpea
At different steeping durations, the final germination of the cowpea seeds did not follow any particular trend at the different steeping durations. Generally, the significantly highest germination was observed in seeds that were steeped for 1-3 h, 3 and 5 h, 2 and 3 h, 1-4 h, and 2-4 h in the presence of inoculums F, G, H, I and J, respectively. With respect to germination time, significantly lower values were observed in seeds steeped for 1 and 3 h (inoculum F), 1-3, and 5 h (inoculum G), 1-3 h (inoculum H), 4 and 5 h (inoculum I), and 1 h (inoculum J). Nonetheless, the trend in mean germination time of the seeds did not follow any pattern with steeping duration. In the case of germination and vigor indices, although no consistent pattern of increase or decrease with steeping duration was observed, the significantly highest germination index values were recorded in seeds that were steeped for 1-3 h (inoculum F), 2, 3, and 5 h (inoculum G), 2 and 3 h (inoculum H), 1-4 h (inoculum I) and 2-4 h (inoculum J). Significantly highest vigor index values were observed in seeds that were steeped for 1 and 3 h (inoculum F), 3 and 5 h (inoculum G), 1-3 h (inoculum H), 1-4 (inoculum I), and 1-3 h for inoculum J (Table 1).
3.1.2. Soybean
The final germination of the soybean seeds did not differ significantly across the different steeping periods for seeds treated with inoculum F. For seeds treated with inoculums G, H, I, and J, significantly higher final germinations were observed at 1 and 4 h, 1, 2, and 4 h, 2 h, and 1-2 h, respectively. In the case of the mean germination time of the soybean seeds, no consistent trend with steeping duration was observed in the presence of the respective inoculums. However, significantly lower mean germination times of 2-5 h (inoculum F), 2 h (inoculum G), 3 and 5 h (inoculum H), 4 h (inoculum I), and 1, 2, 4, and 5 h (inoculum J). For the germination index, although, no consistent trend of increase or decrease was observed for the seeds at the different steeping durations, seeds showed significantly higher values at 1, 2, and 4 h (inoculums G and H), 2 h (inoculum I), and 1 and 2 h (inoculum J). However, there was a significant difference in germination index values for seeds treated with inoculum F at different steeping durations. The vigor index of the soybean seeds showed significantly higher values at steeping durations of 1 and 3 h, 1 and 4 h, 1, 2 and 4 h, 2 h, and 1, 2, 4, and 5 h for seeds treated with inoculums F, G, H, I and J, respectively (Table 2).
| - | Time | F | G | H | I | J |
|---|---|---|---|---|---|---|
| Final germination | 1 h | 85.71a (± 0.00) |
42.86a (± 0.00) |
57.14ac (± 15.65) |
64.29a (± 23.47) |
50.00ac (± 23.47) |
| 2 h | 78.57a (± 7.82) |
42.86a (± 0.00) |
78.57b (± 23.47) |
71.43a (± 15.65) |
78.57b (± 7.82) |
|
| 3 h | 85.71a (± 0.00) |
57.14b (± 15.65) |
78.57b (± 7.82) |
78.57a (± 7.82) |
64.29bc (± 7.82) |
|
| 4 h | 57.14b (± 15.65) |
35.71a (± 7.82) |
35.71a (± 23.47) |
64.29a (± 7.82) |
64.29bc (± 7.82) |
|
| 5 h | 35.71c (± 23.47) |
57.14b (± 15.65) |
57.14c (± 0.00) |
35.71b (± 7.82) |
50.00c (± 23.47) |
|
| Mean germination time | 1 h | 5.23a (± 0.00) |
5.81ab (± 0.01) |
5.50a (± 0.21) |
5.57ac (± 0.07) |
5.22a (± 0.24) |
| 2 h | 5.38b (± 0.10) |
5.57a (± 0.08) |
5.43a (± 0.10) |
5.61a (± 0.12) |
5.48b (± 0.06) |
|
| 3 h | 5.33b (± 0.02) |
5.51a (± 0.05) |
5.54a (± 0.28) |
5.66a (± 0.15) |
5.41b (± 0.02) |
|
| 4 h | 5.31ab (± 0.03) |
6.02b (± 0.57) |
5.84b (± 0.17) |
5.45bc (± 0.07) |
5.47b (± 0.12) |
|
| 5 h | 5.37b (± 0.15) |
5.68a (± 0.20) |
5.87b (± 0.21) |
5.41b (± 0.10) |
5.50b (± 0.00) |
|
| Germination index | 1 h | 147.00a (± 0.00) |
49.50ac (± 1.64) |
78.00ad (± 8.76) |
87.00a (± 26.29) |
80.00ac (± 17.53) |
| 2 h | 123.00a (± 4.38) |
60.00ab (± 3.29) |
117.00b (± 48.20) |
94.50a (± 11.50) |
111.00b (± 15.34) |
|
| 3 h | 132.00a (± 8.76) |
80.50b (± 27.93) |
107.50ab (± 12.60) |
95.00a (± 1.10) |
102.50ab (± 10.41) |
|
| 4 h | 92.50b (± 29.03) |
34.50ac (± 8.22) |
44.00c (± 31.77) |
96.00a (± 17.53) |
95.00abc (± 4.38) |
|
| 5 h | 59.50c (± 42.17) |
76.00b (± 31.77) |
60.00cd (± 14.24) |
56.00b (± 15.34) |
73.50c (± 34.51) |
|
| Vigor index | 1 h | 1777.96a (± 119.38 |
229.59ac (± 2.68) |
502.24ab (± 242.34) |
514.59a (± 372.56) |
579.69ac (± 483.90) |
| 2 h | 879.49b (± 416.83 |
125.82a (± 30.52) |
890.31a (± 625.63) |
580.82a (± 460.98) |
784.18a (± 6.15) |
|
| 3 h | 1484.08a (± 189.13 |
563.27b (± 412.47) |
849.49a (± 383.96) |
585.41a (± 99.15) |
650.20a (± 97.03) |
|
| 4 h | 486.22c (± 96.69) |
106.43a (± 8.83) |
235.82b (± 232.61) |
635.31a (± 151.35) |
444.59bc (± 171.36) |
|
| 5 h | 320.82c (± 349.65 |
453.88bc (± 214.84) |
215.51b (± 21.46) |
129.18b (± 113.35) |
244.29b (± 197.40) |
| - | Time | F | G | H | I | J |
|---|---|---|---|---|---|---|
| Final germination | 1 h | 57.14a (± 15.65) |
78.57a (± 7.82) |
85.71a (± 0.00) |
50.00a (± 7.82) |
57.14ab (± 15.65) |
| 2 h | 50.00a (± 39.12) |
57.14bc (± 0.00) |
85.71a (± 15.65) |
85.71b (± 0.00) |
64.29b (± 7.82) |
|
| 3 h | 64.29a (± 7.82) |
50.00b (± 23.47) |
42.86b (± 15.65) |
50.00a (± 7.82) |
42.86c (± 0.00) |
|
| 4 h | 50.00a (± 7.82) |
71.43ac (± 15.65) |
78.57a (± 7.82) |
50.00a (± 7.82) |
50.00ac (± 7.82) |
|
| 5 h | 42.86a (± 15.65) |
42.86b (± 15.65) |
57.14c (± 0.00) |
71.43c (± 15.65) |
50.00ac (± 7.82) |
|
| Mean germination time | 1 h | 5.70ac (± 0.12) |
5.77a (± 0.29) |
5.61ab (± 0.15) |
5.61a (± 0.04) |
5.63a (± 0.14) |
| 2 h | 5.54b (± 0.04) |
5.28b (± 0.09) |
5.60ad (± 0.04) |
5.59a (± 0.06) |
5.62a (± 0.13) |
|
| 3 h | 5.65ab (± 0.17) |
5.54c (± 0.14) |
5.55ac (± 0.06) |
5.66ac (± 0.17) |
6.09b (± 0.17) |
|
| 4 h | 5.55b (± 0.06) |
5.66ac (± 0.21) |
5.65bd (± 0.03) |
5.42b (± 0.09) |
5.59a (± 0.10) |
|
| 5 h | 5.59bc (± 0.10) |
5.55c (± 0.06) |
5.50c (± 0.00) |
5.79c (± 0.17) |
5.73ab (± 0.09) |
|
| Germination index | 1 h | 75.00a (± 26.29) |
95.50a (± 10.41) |
115.50a (± 12.60) |
68.00a (± 12.05) |
78.50ad (± 29.03) |
| 2 h | 70.50a (± 54.22) |
95.00a (± 4.38) |
117.50a (± 25.74) |
116.00b (± 2.19) |
86.00a (± 2.19) |
|
| 3 h | 86.00a (± 20.81) |
66.50b (± 25.74) |
60.00b (± 19.72) |
67.50a (± 18.07) |
39.50b (± 7.12) |
|
| 4 h | 70.50a (± 8.22) |
95.00a (± 35.05) |
104.00a (± 12.05) |
70.00a (± 7.67) |
68.00cd (± 5.48) |
|
| 5 h | 57.50a (± 16.98) |
60.00b (± 19.72) |
84.00c (± 0.00) |
86.00a (± 27.39) |
62.00c (± 5.48) |
|
| Vigor index | 1 h | 379.80ab (± 145.09 |
763.47a (± 129.44) |
1135.10a (± 197.18) |
195.92a (± 26.83) |
610.61a (± 438.85) |
| 2 h | 280.51ad (± 277.55 |
390.61b (± 139.95) |
971.02a (± 521.57) |
880.41b (± 20.12) |
619.18a (± 166.78) |
|
| 3 h | 524.90b (± 243.23 |
355.10b (± 216.85) |
276.73b (± 160.07) |
479.69c (± 161.30) |
273.67b (± 1.34) |
|
| 4 h | 263.27ad (± 17.44) |
875.31a (± 593.11) |
888.16a (± 105.52) |
505.10cd (± 149.56) |
511.63ab (± 175.49) |
|
| 5 h | 160.20cd (± 28.39) |
355.71b (± 225.57) |
466.94b (± 169.91) |
623.67d (± 86.74) |
358.16ab (± 54.33) |
| - | Time | F | G | H | I | J |
|---|---|---|---|---|---|---|
| Final germination | 1 h | 100.00a (± 0.00) |
85.71a (± 0.00) |
92.86a (± 7.82) |
ND | ND |
| 2 h | 100.00a (± 0.00) |
85.71a (± 15.65) |
100.00b (± 0.00) |
ND | ND | |
| 3 h | 92.86a (± 7.82) |
100.00b (± 0.00) |
78.57c (± 7.82) |
ND | ND | |
| 4 h | 92.86a (± 7.82) |
85.71a (± 15.65) |
100.00b (± 0.00) |
ND | ND | |
| 5 h | 92.86a (± 7.82) |
92.86ab (± 7.82) |
100.00b (± 0.00) |
ND | ND | |
| Mean germination time | 1 h | 5.21ab (± 0.12) |
5.33a (± 0.29) |
5.17a (± 0.03) |
ND | ND |
| 2 h | 5.30a (± 0.10) |
5.30a (± 0.23) |
5.19a (± 0.14) |
ND | ND | |
| 3 h | 5.13bc (± 0.07) |
5.27a (± 0.08) |
5.18a (± 0.19) |
ND | ND | |
| 4 h | 5.21a (± 0.15) |
5.31a (± 0.20) |
5.61b (± 0.18) |
ND | ND | |
| 5 h | 5.21ac (± 0.15) |
5.23a (± 0.18) |
5.06a (± 0.07) |
ND | ND | |
| Germination index | 1 h | 172.50a (± 11.50) |
135.00a (± 28.48) |
164.50a (± 11.50) |
ND | ND |
| 2 h | 161.00b (± 6.57) |
138.00a (± 5.48) |
175.50ac (± 14.79) |
ND | ND | |
| 3 h | 168.00ab (± 7.67) |
168.00b (± 7.67) |
140.50b (± 30.12) |
ND | ND | |
| 4 h | 161.00b (± 0.00) |
145.50ab (± 39.98) |
124.50b (± 26.84) |
ND | ND | |
| 5 h | 161.00b (± 0.00) |
158.00ab (± 3.29) |
189.00c (± 7.67) |
ND | ND | |
| Vigor index | 1 h | 1154.29a (± 62.60) |
652.04a (± 2.01) |
1291.12ab (± 443.21) |
ND | ND |
| 2 h | 951.43b (± 164.32 |
758.67ab (± 334.78) |
1445.71b (± 53.21) |
ND | ND | |
| 3 h | 900.61b (± 158.95 |
1012.14b (± 235.52) |
957.96a (± 463.66) |
ND | ND | |
| 4 h | 793.16b (± 215.62 |
623.06a (± 268.94) |
1042.86a (± 28.17) |
ND | ND | |
| 5 h | 888.06b (± 76.12) |
699.49a (± 102.28) |
1127.14ab (± 28.17) |
ND | ND |
3.1.3. Sorghum
Generally, no consistent patterns in final germination, mean germination time, germination index, and vigor index were observed in the sorghum seeds at different steeping durations. This observation was irrespective of the isolates used for treatment. Final germination of the seeds treated with inoculum F showed no significant difference between the different steeping durations. However, seeds steeped for 3 and 5 h (inoculum G) and 2, 4, and 5 h (inoculum H) showed significantly higher final germination. Seeds treated in inoculums F and H were observed to show significantly lower mean germination time at steeping times of 1, 2, 4, and 5 h and 1, 2, 3, and 5 h, respectively, but there was no significant difference at the different steeping durations for seed steeped in inoculum G. In addition, seeds treated with inoculums F and G showed significantly higher germination index and vigor index at 1 and 3 h and 3, 4, and 5 h, respectively, while seeds treated with inoculum H showed significantly higher germination and vigor indices at 2 h and 5 h, respectively (Table 3). Significantly higher vigor index values were observed at 1 h (inoculum F), 2 and 3 h (inoculum G), and 1, 2, and 5 h (inoculum H).
3.1.4. Sesame
Final germination values of the sesame seeds were observed to be remarkable at different steeping durations in the presence of the respective inoculums. The final germination of sesame seeds did not follow any consistent trend with steeping duration. Similarly, the mean germination time, germination index, and vigor index of the seeds did not also follow any consistent trend with steeping duration. Significantly lower mean germination times were observed for seeds steeped for 3 and 4 h (inoculum F), 1, 2, and 4 h (inoculum G), 2 h (inoculum H), 1 and 2 h (inoculum I), and 1-4 h (inoculum J). In the case of germination index, significantly higher values were observed for seeds steeped for 3 and 4 h (inoculum F), 1 and 2 h (inoculum G), 2 h (inoculum H), 1, 2, 3, and 5 h (inoculum I), and 3 and 4 h (inoculum J). Although the vigor index of seeds that were treated with inoculum F did not differ significantly between the different steeping durations, a significantly higher vigor index was recorded at 1 and 2 h, 2 and 4 h, 1, 3, and 4 h, and 3 h steeping time for seeds treated with inoculums G, H, I and J, respectively (Table 4).
3.1.5. Okra
As was observed for the other crops, no consistent pattern of decrease or increase in final germination, mean germination time, germination index, and vigor index was observed in the presence of the different inoculums at the respective steeping durations. This observation was constant irrespective of the inoculum used for treatment. For seeds treated with inoculum F, the lowest final germination, mean germination time, germination index values, and vigor index values were generally recorded for seeds steeped for 1 and 2 h. For the inoculum G-treated seeds, the lowest final germination, mean germination time, germination index, and vigor index were recorded in seeds steeped for 3 h. The lowest final germination and vigor index values were observed for seeds treated with inoculums I and J at 1 h steeping duration, while 1 h and 4 h showed the lowest germination index values for both inoculums (Table 5).
| - | Time | F | G | H | I | J |
|---|---|---|---|---|---|---|
| Final germination | 1 h | 71.43a (± 0.00) |
100.00a (± 0.00) |
92.86a (± 7.82) |
85.71ab (± 15.65) |
78.57a (± 7.82) |
| 2 h | 85.71a (± 0.00) |
100.00a (± 0.00) |
92.86a (± 7.82) |
78.57bc (± 7.82) |
85.71b (± 0.00) |
|
| 3 h | 100.00a (± 0.00) |
100.00a (± 0.00) |
78.57b (± 7.82) |
92.86a (± 7.82) |
100.00c (± 0.00) |
|
| 4 h | 100.00a (± 0.00) |
92.86b (± 7.82) |
100.00a (± 0.00) |
85.71ac (± 0.00) |
100.00c (± 0.00) |
|
| 5 h | 100.00a (± 0.00) |
92.86b (± 7.82) |
92.86a (± 7.82) |
92.86a (± 7.82) |
92.86d (± 7.82) |
|
| Mean germination time | 1 h | 5.33a (± 0.06) |
5.23a (± 0.04) |
5.46a (± 0.04) |
5.14a (± 0.06) |
5.21a (± 0.03) |
| 2 h | 5.41a (± 0.10) |
5.23a (± 0.04) |
5.16b (± 0.01) |
5.16a (± 0.08) |
5.27a (± 0.05) |
|
| 3 h | 5.23b (± 0.04) |
5.33b (± 0.07) |
5.37c (± 0.15) |
5.26b (± 0.07) |
5.23a (± 0.04) |
|
| 4 h | 5.27b (± 0.00) |
5.25a (± 0.02) |
5.51a (± 0.03) |
5.49c (± 0.09) |
5.27a (± 0.23) |
|
| 5 h | 5.59c (± 0.10) |
5.54c (± 0.04) |
5.46a (± 0.04) |
5.46c (± 0.04) |
5.50b (± 0.00) |
|
| Germination index | 1 h | 115.50a (± 3.83) |
171.50a (± 3.83) |
140.00ad (± 15.34) |
154.00a (± 23.00) |
136.50a (± 11.50) |
| 2 h | 133.00b (± 7.67) |
171.50a (± 3.83) |
165.00b (± 12.05) |
140.00ac (± 7.67) |
143.50a (± 3.83) |
|
| 3 h | 171.50c (± 3.83) |
161.50bc (± 7.12) |
126.00c (± 23.00) |
157.50a (± 19.17) |
171.50b (± 3.83) |
|
| 4 h | 168.00c (± 0.00) |
157.50cd (± 11.50) |
145.00ad (± 3.29) |
126.50bc (± 7.12) |
168.50b (± 22.46) |
|
| 5 h | 136.50b (± 11.50) |
133.50d (± 14.79) |
140.00ac (± 15.34) |
140.00ab (± 15.34) |
136.50a (± 11.50) |
|
| Vigor index | 1 h | 433.67a (± 162.08 |
629.29a (± 3.91) |
555.71a (± 111.11) |
711.02a (± 346.29) |
510.31ad (± 39.23) |
| 2 h | 443.88a (± 40.91) |
588.57ac (± 12.52) |
732.55b (± 197.52) |
370.31b (± 89.31) |
627.55a (± 141.51) |
|
| 3 h | 517.86a (± 47.73) |
696.43b (± 47.73) |
471.53ac (± 101.83) |
586.73ac (± 139.73) |
752.86b (± 82.94) |
|
| 4 h | 451.43a (± 6.26) |
526.53c (± 99.26) |
704.29b (± 15.65) |
521.02abc (± 44.94) |
1020.71c (± 63.38) |
|
| 5 h | 434.29a (± 59.47) |
306.02d (± 38.79) |
357.96c (± 64.83) |
399.69bc (± 105.19) |
432.76cd (± 137.60) |
| - | Time | F | G | H | I | J |
|---|---|---|---|---|---|---|
| Final germination | 1 h | 42.86a (± 0.00) |
35.71a (± 23.47) |
21.43a (± 23.47) |
28.57a (± 0.00) |
14.29a (± 0.00) |
| 2 h | 28.57b (± 15.65) |
28.57a (± 0.00) |
28.57a (± 0.00) |
35.71a (± 7.82) |
42.86bd (± 0.00) |
|
| 3 h | 42.86a (± 15.65) |
7.14b (± 7.82) |
50.00b (± 23.47) |
35.71a (± 7.82) |
50.00bc (± 7.82) |
|
| 4 h | 50.00a (± 7.82) |
42.86a (± 15.65) |
50.00b (± 7.82) |
28.57a (± 0.00) |
21.43a (± 7.82) |
|
| 5 h | 42.86a (± 0.00) |
28.57a (± 0.00) |
28.57a (± 0.00) |
42.86a (± 46.95) |
35.71d (± 7.82) |
|
| Mean germination time | 1 h | 5.26a (± 0.29) |
5.41a (± 0.10) |
2.75a (± 3.01) |
5.88a (± 0.37) |
5.75a (± 0.27) |
| 2 h | 5.22a (± 0.24) |
5.45a (± 0.49) |
5.21b (± 0.23) |
5.00a (± 0.00) |
5.08b (± 0.08) |
|
| 3 h | 5.75b (± 0.27) |
3.00b (± 3.29) |
5.34b (± 0.17) |
5.18a (± 0.19) |
5.30b (± 0.17) |
|
| 4 h | 5.55bc (± 0.01) |
5.10a (± 0.11) |
5.70b (± 0.06) |
5.82a (± 0.44) |
5.25b (± 0.27) |
|
| 5 h | 5.48ac (± 0.18) |
5.12a (± 0.13) |
6.36b (± 0.70) |
2.79b (± 3.05) |
5.70a (± 0.22) |
|
| Germination index | 1 h | 71.50a (± 13.69) |
56.50ab (± 38.89) |
31.50ac (± 34.51) |
31.50a (± 7.12) |
18.00a (± 3.29) |
| 2 h | 46.00b (± 19.72) |
43.50b (± 13.69) |
49.50ac (± 7.12) |
70.00b (± 15.34) |
80.50b (± 3.83) |
|
| 3 h | 48.00b (± 6.57) |
7.50c (± 8.22) |
84.00b (± 46.01) |
59.00ab (± 3.29) |
81.00b (± 4.38) |
|
| 4 h | 65.50a (± 11.50) |
77.50a (± 23.55) |
61.00bc (± 4.38) |
34.00ab (± 9.86) |
35.00c (± 7.67) |
|
| 5 h | 63.50a (± 7.12) |
52.50a (± 3.83) |
24.00a (± 13.15) |
60.00ab (± 65.73) |
47.00d (± 17.53) |
|
| Vigor index | 1 h | 154.59ab (± 67.40) |
128.57a (± 117.59) |
109.90a (± 120.39) |
73.67a (± 23.92) |
22.04a (± 2.24) |
| 2 h | 99.80a (± 79.14) |
105.71a (± 4.47) |
87.55a (± 11.40) |
114.08a (± 18.56) |
219.80b (± 44.26) |
|
| 3 h | 111.22a (± 42.70) |
16.02b (± 17.55) |
338.78b (± 281.69) |
134.59a (± 16.88) |
302.55c (± 101.38) |
|
| 4 h | 202.35b (± 92.22) |
157.55a (± 118.04) |
147.14a (± 57.90) |
60.41a (± 12.97) |
65.92ad (± 43.59) |
|
| 5 h | 112.96a (± 13.75) |
83.67ab (± 8.05) |
61.63a (± 23.25) |
330.00b (± 361.50) |
120.51d (± 33.65) |
| - | - | Isolate F | Isolate G | Isolate H | Isolate I | Isolate J |
|---|---|---|---|---|---|---|
| Final germination | C1 | 35.71ab (± 7.82) |
78.57a (± 7.82) |
64.29ab (± 7.82) |
50.00a (± 7.82) |
100.00a (± 0.00) |
| C2 | 35.71ab (± 7.82) |
78.57a (± 7.82) |
71.43a (± 0.00) |
50.00a (± 7.82) |
57.14b (± 0.00) |
|
| C3 | 21.43a (± 7.82) |
50.00b (± 7.82) |
57.14b (± 15.65) |
28.57b (± 0.00) |
42.86c (± 15.65) |
|
| C4 | 50.00b (± 7.82) |
78.57a (± 23.47) |
85.71a (± 0.00) |
64.29c (± 23.47) |
57.14b (± 0.00) |
|
| C5 | 21.43a (± 23.47) |
71.43a (± 15.65) |
71.40a (± 15.66) |
71.43c (± 0.00) |
35.71c (± 7.82) |
|
| Mean germination time | C1 | 5.16a (± 0.17) |
5.19a (± 0.11) |
5.21a (± 0.03) |
5.34a (± 0.02) |
5.26a (± 0.07) |
| C2 | 5.08a (± 0.08) |
5.43b (± 0.01) |
5.22a (± 0.15) |
5.34a (± 0.02) |
5.23ac (± 0.00) |
|
| C3 | 5.00a (± 0.00) |
5.26ac (± 0.12) |
5.22a (± 0.07) |
5.61bc (± 0.12) |
5.30b (± 0.07) |
|
| C4 | 5.34a (± 0.20) |
5.39bc (± 0.07) |
5.23a (± 0.00) |
5.52b (± 0.08) |
5.32b (± 0.00) |
|
| C5 | 2.72b (± 2.98) |
5.33bc (± 0.15) |
5.44b (± 0.08) |
5.73c (± 0.29) |
5.19c (± 0.04) |
|
| Germination index | C1 | 63.00ab (± 7.67) |
137.00a (± 4.38) |
112.00ab (± 15.34) |
80.50a (± 11.50) |
168.50a (± 7.12) |
| C2 | 66.50a (± 11.50) |
118.00ac (± 12.05) |
123.00b (± 10.95) |
80.50a (± 11.50) |
98.00b (± 0.00) |
|
| C3 | 42.00bc (± 15.34) |
82.00b (± 5.48) |
98.00a (± 23.00) |
39.00b (± 3.29) |
70.00c (± 23.00) |
|
| C4 | 78.50a (± 1.64) |
120.50ac (± 31.22) |
145.00c (± 2.19) |
90.00a (± 28.48) |
92.00b (± 0.00) |
|
| C5 | 32.00c (± 35.05) |
113.00c (± 15.34) |
106.50ab (± 16.98) |
89.00a (± 18.62) |
63.00c (± 15.34) |
|
| Vigor index | C1 | 160.92ac (± 91.32) |
787.04ac (± 133.80) |
575.00a (± 146.99) |
456.43ac (± 127.76) |
730.71a (± 0.78) |
| C2 | 118.57ab (± 32.42) |
858.47ac (± 284.26) |
909.18b (± 128.55) |
348.27ab (± 183.65) |
368.98bc (± 17.88) |
|
| C3 | 44.59b (± 28.50) |
310.51bd (± 95.35) |
416.33a (± 328.63) |
100.82b (± 3.58) |
358.78bd (± 283.92) |
|
| C4 | 246.02c (± 126.65) |
1060.51a (± 589.42) |
1099.59b (± 37.56) |
768.67c (± 570.41) |
480.00b (± 26.83) |
|
| C5 | 88.16ab (± 96.58) |
506.33cd (± 197.85) |
632.24a (± 190.03) |
524.49ac (± 223.56) |
208.16cd (± 111.33) |
3.2. Effect of Initial Inoculum Concentration
3.2.1. Cowpea
Generally, no consistent trend in the germination and seedling growth parameters was observed at the different initial inoculum concentrations. This observation was constant irrespective of the inoculum used for treatment. In the presence of the respective initial concentrations of inoculum J, the final germination of cowpea seeds was significantly highest in seeds treated at dilution 4:1. For inoculums H, I, and F, the significantly highest final germination values were observed in seeds treated in dilutions 1:4, 0:5, and 1:4, respectively. In the case of inoculum G-treated seeds, the significantly lowest final germination was observed in 2:3 dilution. In the case of mean germination time, the significantly lowest values were recorded at 3:2 and 0:5 dilutions, at 1-4 dilutions, 4:1 and 3:2 dilutions, and at 4:1 and 2:3, for inoculums J, H, I, F and G, respectively. In the case of the germination index, the significantly highest values were recorded in seeds treated in dilutions 4:1 (inoculum J), 4:1, 3:2, and 0:5 (inoculum H), 4:1, 3:2, and 1:4 (inoculum G). However, the significantly highest lowest germination index values were recorded in dilutions 2:3 and 0:5 for inoculums I and F treated seeds, respectively. With respect to the vigor index, the significantly highest values were recorded at 1:4 dilution, 4:1, 1:4, and 0:5 dilutions, 4:1 and 1:4 dilutions, and 4:1, 3:2, and 1:4 dilutions, for seeds that were treated with inoculums H, I, F, G, respectively, and 4:1 dilution for inoculum J-treated seeds (Table 6).
3.2.2. Soybean
For the soybean seeds, none of the parameters investigated showed any specific pattern of increase or decrease at the respective initial inoculum concentrations. Significantly higher final germination values were observed at dilutions of 4:1 for inoculums H, I, and G -treated seeds, 4:1, 3:2, and 2:3 for inoculum J-treated seeds and 3:2, 2:3, and 0:5 for inoculum F-treated seeds. In the case of mean germination time, significantly lower values were recorded at dilutions 0:5 (inoculum J), 2:3 (inoculum H), 4:1, 3:2, 2:3, and 0:5 (inoculum I), 3:2 (inoculum F), and 4:1 (inoculum G). Significantly highest germination index values were observed at dilutions 4:1, 3:2, and 2:3 (inoculum J), 4:1 (inoculum H), 4:1 and 1:4 (inoculum I), 3:2, 2:3, and 0:5 (inoculum F), and 3:2, 2:3, and 1:4, and 4:1 (inoculum G), respectively. Significantly highest vigor index values were observed at dilutions 3:2 (inoculum J), 4:1 (inoculum H), 4:1 (inoculum I), 3:2 and 2:3 (inoculum F), and 4:1 and 2:3 (inoculum G), respectively (Table 7).
3.2.3. Sorghum
In the presence of the respective inoculums, the final germination percentages of the sorghum seeds did not differ significantly between the respective concentrations used for treatment, except for inoculums G and H. In the case of mean germination time, the lowest values were recorded at dilutions of 4:1 and 3:2 for inoculum H and 4:1, 3:2, 2:3, and 1:4 for inoculums J and F. However, no significant difference in mean germination time for inoculums I and G at the respective steeping durations. For the germination index, although the values were not observed to follow any consistent trend with the concentration of inoculum, the significantly highest values were recorded at dilutions of 4:1, 3:2, 2:3, and 1:4 (inoculums J, H, and G) and 3:2 and 1:4 (inoculum F). However, the germination index values did not differ significantly at the respective dilutions used for treatment in inoculum I (Table 8). For the vigor index, significantly higher values were recorded at 3:2 dilution (inoculums J and H) and 4:1, 3:2, 2:3, and 1:4 (inoculum G). However, the vigor index did not differ significantly at the respective dilutions used for treatment with inoculums I and F (Table 8).
| - | - | Isolate F | Isolate G | Isolate H | Isolate I | Isolate J |
|---|---|---|---|---|---|---|
| Final germination | C1 | 57.14a (± 15.65) |
100.00a (± 0.00) |
85.71a (± 0.00) |
100.00a (± 0.00) |
57.14a (± 31.30) |
| C2 | 78.57b (± 7.82) |
71.43b (± 0.00) |
57.14b (± 0.00) |
64.29b (± 23.47) |
78.57a (± 7.82) |
|
| C3 | 85.71b (± 0.00) |
78.57b (± 7.82) |
42.86c (± 15.65) |
64.29b (± 7.82) |
57.14a (± 15.65) |
|
| C4 | 50.00a (± 7.82) |
64.29b (± 7.82) |
50.00b (± 7.82) |
78.57bc (± 7.82) |
28.57b (± 15.65) |
|
| C5 | 85.71b (± 0.00) |
71.43b (± 31.30) |
50.00b (± 7.78) |
92.86ac (± 7.82) |
14.29b (± 15.65) |
|
| Mean germination time | C1 | 5.30a (± 0.02) |
5.28a (± 0.02) |
5.75a (± 0.00) |
5.33ab (± 0.07) |
5.64a (± 0.15) |
| C2 | 5.15b (± 0.00) |
5.49b (± 0.06) |
5.71ac (± 0.38) |
5.27ab (± 0.05) |
5.72a (± 0.20) |
|
| C3 | 5.27a (± 0.05) |
5.56c (± 0.06) |
5.16b (± 0.18) |
5.21a (± 0.03) |
5.52a (± 0.14) |
|
| C4 | 5.46c (± 0.16) |
5.49b (± 0.03) |
5.49c (± 0.04) |
5.36b (± 0.20) |
5.16a (± 0.17) |
|
| C5 | 5.30a (± 0.01) |
5.56c (± 0.07) |
5.55ac (± 0.06) |
5.33ab (± 0.16) |
2.62b (± 2.87) |
|
| Germination index | C1 | 94.50a (± 26.84) |
165.50a (± 2.74) |
104.00a (± 0.00) |
161.50a (± 7.12) |
72.50ac (± 33.41) |
| C2 | 140.50b (± 14.79) |
103.50b (± 3.83) |
73.50b (± 19.17) |
108.50b (± 42.17) |
93.50a (± 3.83) |
|
| C3 | 143.50b (± 3.83) |
110.00b (± 5.48) |
74.00b (± 19.72) |
112.00b (± 15.34) |
84.50a (± 30.12) |
|
| C4 | 74.00c (± 4.38) |
93.00b (± 8.76) |
72.00b (± 14.24) |
123.50b (± 2.74) |
49.00bc (± 23.00) |
|
| C5 | 140.50b (± 0.55) |
99.00b (± 39.44) |
70.50b (± 8.22) |
148.50a (± 0.55) |
24.50b (± 26.84) |
|
| Vigor index | C1 | 489.39a (± 149.11) |
605.71ab (± 65.73) |
740.20a (± 0.67) |
2012.14a (± 149.45) |
486.53a (± 466.35) |
| C2 | 965.61b (± 347.97) |
489.80ac (± 16.77) |
239.18b (± 25.04) |
816.73b (± 668.00) |
760.20b (± 72.66) |
|
| C3 | 1046.33b (± 266.26) |
834.80b (± 45.94) |
282.04b (± 182.87) |
856.02b (± 225.91) |
233.88ac (± 38.90) |
|
| C4 | 225.51c (± 41.81) |
279.69c (± 47.95) |
201.02b (± 108.87) |
1061.33b (± 139.84) |
117.96c (± 96.80) |
|
| C5 | 1375.10d (± 14.75) |
546.94a (± 455.62) |
322.76b (± 143.64) |
1527.55c (± 148.22) |
78.98c (± 86.52) |
| - | - | Isolate F | Isolate G | Isolate H | Isolate I | Isolate J |
|---|---|---|---|---|---|---|
| Final germination | C1 | 92.86a (± 7.82) |
100.00a (± 0.00) |
100.00a (± 0.00) |
92.86a (± 7.82) |
92.86a (± 7.82) |
| C2 | 100.00a (± 0.00) |
100.00a (± 0.00) |
92.86b (± 7.82) |
92.86a (± 7.82) |
100.00a (± 0.00) |
|
| C3 | 92.86a (± 7.82) |
100.00a (± 0.00) |
100.00a (± 0.00) |
92.86a (± 7.82) |
92.86a (± 7.82) |
|
| C4 | 100.00a (± 0.00) |
100.00a (± 0.00) |
100.00a (± 0.00) |
92.86a (± 7.82) |
100.00a (± 0.00) |
|
| C5 | 92.86a (± 7.82) |
92.86b (± 7.82) |
100.00a (± 0.00) |
100.00a (± 0.00) |
92.86a (± 7.82) |
|
| Mean germination time | C1 | 5.04a (± 0.04) |
5.03a (± 0.03) |
5.03a (± 0.03) |
5.04a (± 0.04) |
5.12a (± 0.13) |
| C2 | 5.06a (± 0.00) |
5.08a (± 0.02) |
5.00a (± 0.00) |
5.00b (± 0.00) |
5.13a (± 0.00) |
|
| C3 | 5.03a (± 0.03) |
5.06a (± 0.07) |
5.12b (± 0.06) |
5.06a (± 0.07) |
5.07a (± 0.01) |
|
| C4 | 5.03a (± 0.03) |
5.08a (± 0.02) |
5.13b (± 0.07) |
5.06a (± 0.07) |
5.12a (± 0.06) |
|
| C5 | 5.10b (± 0.03) |
5.06a (± 0.07) |
5.23c (± 0.11) |
5.08a (± 0.02) |
5.21b (± 0.02) |
|
| Germination index | C1 | 178.50ac (± 19.17) |
192.50a (± 3.83) |
192.50a (± 3.83) |
178.50a (± 19.17) |
171.50ab (± 26.84) |
| C2 | 189.00ab (± 0.00) |
186.00ab (± 3.29) |
182.00ab (± 15.34) |
182.00a (± 15.34) |
182.00a (± 0.00) |
|
| C3 | 178.50ac (± 11.50) |
189.00a (± 7.67) |
182.50a (± 7.12) |
175.00a (± 7.67) |
175.00ab (± 15.34) |
|
| C4 | 192.50b (± 3.83) |
186.00ab (± 3.29) |
182.00ab (± 7.67) |
175.00a (± 7.67) |
182.50a (± 7.12) |
|
| C5 | 171.50c (± 11.50) |
175.50b (± 22.46) |
170.00b (± 13.15) |
186.00a (± 3.29) |
161.00b (± 15.34) |
|
| Vigor index | C1 | 781.84a (± 260.89) |
742.86ab (± 29.73) |
876.43a (± 121.28) |
770.61a (± 44.71) |
740.71a (± 99.37) |
| C2 | 847.86a (± 71.20) |
922.86a (± 18.78) |
988.37b (± 47.17) |
735.71a (± 35.99) |
1274.29b (± 92.33) |
|
| C3 | 791.12a (± 160.85) |
789.29ab (± 313.77) |
901.43a (± 15.65) |
719.39a (± 93.22) |
780.20a (± 21.69) |
|
| C4 | 781.43a (± 1.56) |
942.14a (± 280.90) |
686.43c (± 19.56) |
823.16a (± 248.49) |
853.57a (± 215.18) |
|
| C5 | 796.12a (± 98.14) |
640.92b (± 150.79) |
774.29d (± 82.94) |
675.71a (± 123.63) |
755.61a (± 103.40) |
| - | - | Isolate F | Isolate G | Isolate H | Isolate I | Isolate J |
|---|---|---|---|---|---|---|
| Final germination | C1 | 92.86a (± 7.82) |
100.00a (± 0.00) |
100.00a (± 0.00) |
100.00a (± 0.00) |
100.00a (± 0.00) |
| C2 | 100.00b (± 0.00) |
100.00a (± 0.00) |
100.00a (± 0.00) |
100.00a (± 0.00) |
100.00a (± 0.00) |
|
| C3 | 100.00b (± 0.00) |
92.86a (± 7.82) |
100.00a (± 0.00) |
92.86b (± 7.82) |
92.86b (± 7.82) |
|
| C4 | 100.00b (± 0.00) |
92.86a (± 7.82) |
100.00a (± 0.00) |
100.00a (± 0.00) |
100.00a (± 0.00) |
|
| C5 | 100.00b (± 0.00) |
78.57b (± 23.47) |
100.00a (± 0.00) |
100.00a (± 0.00) |
78.57c (± 7.82) |
|
| Mean germination time | C1 | 5.32ab (± 0.10) |
5.27a (± 0.00) |
5.51a (± 0.27) |
5.27a (± 0.00) |
5.24a (± 0.12) |
| C2 | 5.27a (± 0.08) |
5.27a (± 0.08) |
5.35b (± 0.09) |
5.33ab (± 0.07) |
5.23a (± 0.04) |
|
| C3 | 5.39b (± 0.05) |
5.29a (± 0.06) |
5.30b (± 0.04) |
5.40b (± 0.14) |
5.42b (± 0.10) |
|
| C4 | 5.34ab (± 0.08) |
5.37b (± 0.04) |
5.36ab (± 0.10) |
5.30a (± 0.04) |
5.37b (± 0.03) |
|
| C5 | 5.30ab (± 0.04) |
5.25a (± 0.02) |
5.34b (± 0.00) |
5.34ab (± 0.00) |
5.37b (± 0.02) |
|
| Germination index | C1 | 150.50a (± 3.83) |
168.00a (± 0.00) |
144.00ac (± 26.29) |
168.00a (± 0.00) |
169.50a (± 13.69) |
| C2 | 168.00b (± 7.67) |
168.00a (± 7.67) |
159.00ab (± 9.86) |
161.50a (± 7.12) |
171.50a (± 3.83) |
|
| C3 | 153.50ac (± 8.22) |
154.00ab (± 7.67) |
164.50b (± 3.83) |
143.00b (± 27.39) |
137.50b (± 6.02) |
|
| C4 | 161.00bc (± 7.67) |
147.00ab (± 15.34) |
158.50bc (± 10.41) |
164.50a (± 3.83) |
158.00c (± 3.29) |
|
| C5 | 164.50b (± 3.83) |
133.00b (± 38.34) |
161.00b (± 0.00) |
161.00a (± 0.00) |
121.50d (± 9.31) |
|
| Vigor index | C1 | 521.12a (± 59.80) |
603.57a (± 69.64) |
581.43a (± 48.51) |
485.00ab (± 16.43) |
562.14a (± 8.61) |
| C2 | 603.57b (± 35.21) |
635.00a (± 27.39) |
580.00a (± 1.56) |
452.14ab (± 72.77) |
473.57b (± 63.38) |
|
| C3 | 510.71a (± 0.78) |
605.71a (± 100.15) |
589.29a (± 85.29) |
423.98b (± 66.06) |
402.04c (± 44.71) |
|
| C4 | 587.86b (± 52.42) |
547.86a (± 111.89) |
581.43a (± 12.52) |
501.43a (± 7.82) |
550.71a (± 13.30) |
|
| C5 | 582.86b (± 28.17) |
402.65b (± 213.05) |
503.57b (± 93.11) |
490.71a (± 71.20) |
350.82c (± 75.79) |
3.2.4. Sesame
For the sesame seeds, apart from seeds treated with dilution 0:5 of inoculums J and G that showed significantly lower final germination than the other dilutions, seeds treated at the respective dilutions of the other inoculums did not differ significantly between the dilutions used for treatment. Similarly, the mean germination time of the sesame seeds did not differ significantly between most of the respective dilutions of the inoculums used for treatment. Significantly lowest germination index values were recorded at dilutions 0:5 (inoculum J), 4:1 and 1:4 (inoculum H), 4:1 and 2:3 (inoculum F), while significantly lowest vigor index values were recorded at dilutions 4:1 and 2:3 (inoculum F), 0:5 (inoculums G and J), and 4:1, 3:2, and 2:3 (inoculum I) (Table 9).
3.2.5. Okra
As was observed for the other crops, none of the germination and seedling growth parameters showed any consistent trend with inoculum concentration. This was observed irrespective of the inoculum used for treatment. However, significantly higher final germination percentages were observed at dilutions 3:2 (inoculum J), 4:1, 3:2, 2:3, and 0:5 (inoculum H), 3:2, 2:3, 1:4, and 0:5 (inoculum I), 3:2, 1:4, and 0:5 (inoculum F), and 4:1 and 2:3 (inoculum G). Significantly highest mean germination time was however observed at dilutions of 3:2 and 2:3 (inoculum J), 1:4 (inoculum H), 2:3 (inoculum I), and 4:1 (inoculum F), and 4:1 and 0:5 (inoculum G). In the case of germination index, the significantly lowest values were recorded at dilution 2:3 and 1:4 for inoculum J, 1:4 for inoculum H, 4:1, 2:3, and 1:4 (inoculums I and F), and 1:4 and 0:5 for inoculum G. For the vigor index, significantly lower values were obtained at 2:3 and 1:4 dilutions (inoculum J), 4:1, 2:3, and 1:4 dilutions (inoculum I), 4:1, 3:2, 2:3, and 1:4 (inoculum F), and 3:2,1:4, and 0:5 for inoculum G (Table 10).
| - | - | Isolate F | Isolate G | Isolate H | Isolate I | Isolate J |
|---|---|---|---|---|---|---|
| Final germination | C1 | 42.86a (± 31.30) |
78.57a (± 7.82) |
71.43ab (± 15.65) |
42.86a (± 0.00) |
71.43a (± 0.00) |
| C2 | 78.57b (± 7.82) |
50.00b (± 23.47) |
78.57a (± 7.82) |
57.14ab (± 15.65) |
85.71b (± 15.65) |
|
| C3 | 42.86a (± 31.30) |
78.57a (± 7.82) |
71.43ab (± 15.65) |
50.00ab (± 7.82) |
64.29a (± 7.82) |
|
| C4 | 57.14ab (± 0.00) |
57.14b (± 15.65) |
64.29b (± 7.82) |
50.00ab (± 7.82) |
50.00c (± 7.82) |
|
| C5 | 71.43ab (± 31.30) |
57.14b (± 0.00) |
71.40ab (± 0.00) |
64.29b (± 23.47) |
71.43a (± 0.00) |
|
| Mean germination time | C1 | 5.48a (± 0.02) |
5.53a (± 0.01) |
5.16ab (± 0.04) |
5.30a (± 0.16) |
5.18a (± 0.11) |
| C2 | 5.39b (± 0.04) |
5.26b (± 0.28) |
5.25b (± 0.00) |
5.43b (± 0.08) |
5.26b (± 0.10) |
|
| C3 | 5.39b (± 0.12) |
5.31bc (± 0.07) |
5.00a (± 0.00) |
5.77c (± 0.05) |
5.22b (± 0.03) |
|
| C4 | 5.35b (± 0.03) |
5.18b (± 0.19) |
5.43c (± 0.26) |
5.45b (± 0.01) |
5.00c (± 0.00) |
|
| C5 | 5.34b (± 0.03) |
5.49ac (± 0.08) |
5.16a (± 0.18) |
5.47b (± 0.02) |
5.00c (± 0.00) |
|
| Germination index | C1 | 63.50a (± 46.56) |
111.00a (± 10.95) |
127.00a (± 30.67) |
70.50ab (± 7.12) |
126.00ac (± 7.67) |
| C2 | 121.00b (± 15.34) |
78.50b (± 24.65) |
129.50a (± 10.41) |
88.00a (± 27.39) |
144.50a (± 34.51) |
|
| C3 | 70.00ac (± 53.68) |
127.00a (± 7.67) |
140.00a (± 30.67) |
61.50b (± 11.50) |
109.50bc (± 11.50) |
|
| C4 | 89.50ab (± 2.74) |
98.50c (± 15.88) |
94.50b (± 4.93) |
74.50ab (± 11.50) |
98.00b (± 15.34) |
|
| C5 | 113.00bc (± 47.10) |
82.50bc (± 3.83) |
127.00a (± 14.24) |
93.50a (± 32.32) |
140.00a (± 0.00) |
|
| Vigor index | C1 | 247.55a (± 266.48) |
547.76a (± 141.74) |
604.29a (± 262.24) |
144.49a (± 34.88) |
416.33ad (± 53.65) |
| C2 | 503.88ab (± 230.04) |
282.45b (± 211.49) |
506.63a (± 142.52) |
333.98bc (± 210.93) |
756.33b (± 338.92) |
|
| C3 | 259.18a (± 276.10) |
591.94a (± 130.89) |
607.76a (± 308.07) |
168.16ab (± 47.39) |
256.43ac (± 37.67) |
|
| C4 | 244.90a (± 43.82) |
355.92b (± 147.77) |
398.06a (± 16.66) |
213.88abd (± 41.14) |
225.20c (± 95.80) |
|
| C5 | 628.37b (± 526.04) |
317.96b (± 18.33) |
441.33a (± 54.21) |
377.14cd (± 246.81) |
534.18d (± 37.45) |
4. DISCUSSION
The final germination percentage denotes the number of germinated seeds in a lot, in terms of percentage, and higher the value, the better. For cowpea, generally, it peaked at 2 or 3 h and dipped afterward and these drops were most significant. This implies that the lower steeping durations were sufficient for adequate imbibition of desirable biomolecules and adherence of a sufficient number of microorganisms present in the medium, and that longer steeping durations can inhibit germination. Longer steeping duration can result in the imbibition of excess liquid [30, 31], leading to the development of over-bloated seeds which may not germinate. A prime hydropriming duration of 4 h for “Oloyin” beans was observed by Fabunmi et al. [32], using the conventional priming technique that involved drying the seed to their original moisture content.
While there was no consistent pattern between steeping duration and final germination for the isolates in the case of soybean, the longest steeping duration (5 h) recorded the lowest final germination for all isolates, except for isolate I. Using the conventional priming technique, Aminu et al. [33] reported that 8 hours was required for optimal hydropriming of different soybean varieties at 2 different locations. In a study by Kujur and Lal [34], polyethylene glycol 6000 (5%) was used for osmo-priming at 8, 12, 24, and 48 hours, the highest germination percent was obtained at 48 hrs. Priming in ZnSO4 for 10 h was required for maximal final germination percentage, germination index, and seedling vigor index. These studies highlighted here used the conventional priming technique that involves drying the seeds before planting, hence the longer optimal steeping duration recorded.
Final germination was not affected by steeping duration for sorghum and sesame. This could be related to the unique seed structures of sesame and sorghum, as both do not have large endosperms that can soak up large volumes of liquid and sorghum has a hardy pericarp that perhaps limits imbibition. In a study on sesame seeds by Shim et al. [19], priming was shown to be dependent on duration and there was a steady increase in final germination up to a priming period of 4 days. This result shows the lengthy amount of time required for the effective priming of sesame.
The germination pattern was erratic in the case of okra. Low germination values obtained for okra can be linked to the hard seed coat which restricts imbibition and, therefore, interferes with seed germination [35]. Kaur et al. [36] observed that germination percentage significantly increased in treated okra seeds. Their results indicated that the highest seed germination was recorded for seeds soaked for a very long period—24 hours in all treatments, likely as a result of the high level of impermeability provided by its special seed coat. Therefore, a longer steeping duration is likely needed for effective biopriming for the isolates used in this study, in order to allow for adequate imbibition of microbial metabolites.
Mean germination time represents the weighted mean of the germination time. Mean germination time was stable across crops for the isolates and no observable patterns with steeping duration were observed, although there were significant results. This shows that the isolates have a rather limited impact on the crops with regard to improving the germination speed. A result that is not in tandem with the reduced mean germination time for osmo- and hydro-primed sorghum seeds of different genotypes [37].
The germination index appears to be the most comprehensive measurement parameter combining both germination percentage and speed [38]. For cowpea, generally, the germination index peaked at 1-3 h and dipped till the last steeping duration, except for isolate G. In the case of sorghum, the germination index did not follow a consistent pattern for most of the isolates. Although the pattern shown by the germination index with respect to steeping duration was not found to be stable; however, lower values tended to occur at longer steeping durations. For both cowpea and soybean, the low germination index values observed can be related to the low final germination and mean germination time values recorded; therefore, a low value in either of these parameters can produce a low germination index. For sorghum, consistently high values were obtained at different steeping durations for the isolates for germination index. However, no consistent relationships with steeping duration were obtained for the isolates in the case of sesame and okra. For the last three crops (i.e., sorghum, sesame, and okra), the inconsistency in germination index values relates to the time-independent nature of the germination pattern for them.
The vigor index is an important indicator of plant biomass. Height is highly correlated with plant biomass [39]. Moreover, the higher in height a plant is, the closer is it to the maturity and reproduction stages [40]. For the vigor index, the larger values were largely confined to the shorter steeping duration periods of 1-3 h. This highlights the deleterious effects of over-imbibition that can occur in a liquid medium at longer steeping durations. The over-imbibition resulted in soggy seeds which limited the germination percentage, an important computational component of the vigor index. However, no observable pattern with steeping duration was obtained for soybean, sorghum, and sesame. The pattern for okra was haphazard.
The parameters for these crops were observed to be independent of the initial concentration Higher concentrations of the isolates did not appear to significantly hamper or promote final germination, mean germination time, germination index, and seedling vigor index. This implies that the use of higher concentrations may be inefficient. However, blotters have low levels of competing microbial species, which likely eliminates the use of higher concentrations. Under soil conditions where competition for food, resources, and space could be intense, the use of a higher inoculum concentration may become necessary.
Nonetheless, the use of a high concentration seems to be a necessity for chemical priming. Arif et al. [41] noted that a higher osmotic potential of -1.1 MPa of Polyethylene glycol (PEG) was effective for faster germination of soybean in a study that involved the use of different osmotic potentials (0.2, -0.5, -1.1 and -1.8 MPa). Moreover, a conc-dependent result was observed in a sorghum chemo priming experiment by Arief et al. [42]. The study involved the use of 5 different concentrations of KNO3 (0.5, 1%, 1.5, and 2%), 1.5% KNO3 was found to give the best results in terms of germination percentage, germination rate, and seedling dry weight. Conversely, in another study by Mereddy et al. [35], the lowest concentration of KNO3 (0.25%) gave the highest germination level, while the highest concentration (3%) generated the lowest germination percentage in okra seeds. Moreover, in this same study, the lowest concentration (0.25%) produced the best results with regard to the number of days to reach 50% germination. The same chemical was used in both studies; however, different results were obtained, pointing to the role of the nature of the seed (that is, its anatomy and physiology) in priming.
CONCLUSION
This study shows that steeping duration is a more important factor than the initial inoculum concentration in the area of biopriming. The benign effect of the use of a heavy inoculum for biopriming in contrast to the potentially deleterious effect of the use of higher concentrations of chemical priming lends further credence to the use of bio-primers in place of chemical primers. However, further research still needs to be carried out to better understand the usability of these isolates under field conditions.
LIST OF ABBREVIATIONS
| PEG | = Polyethylene Glycol |
| NCBI | = National Center for Biotechnology Information |
| PGPB | = plant growth-promoting bacteria |
FUNDING
None.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the Zenodo Repository at https://openpublichealthjournal.com/availability-of-data-materials.php.
CONFLICT OF INTEREST
The authors declare no conflict of interest financial or otherwise.
ACKNOWLEDGEMENTS
The authors are grateful to Afe Babalola University for providing facilities for conducting the study.



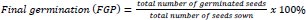 [
[ [
[




