All published articles of this journal are available on ScienceDirect.
Growth Promoting Potential of Phosphate Solubilizing Enterobacter Cloaca and Enterobacter Hormaechei on Maize and Cowpea Seedlings
Abstract
Introduction:
Phosphorus plays a range of functions in the proper growth and development of plants. Numerous microbial species, including bacteria, fungi, actinomycetes, and even algae have been found to play a crucial role in the solubilization of phosphate. This study was therefore aimed at exploring the growth-promoting potential of phosphate solubilizing Enterobacter species on maize and cowpea seedlings.
Methods:
Five strains that showed remarkable phosphate solubilization potential were used for the study. The bacterial isolates consist of three strains of Enterobacter cloaca and two strains of Enterobacter hormaechei. Growth promotion studies were carried out under laboratory conditions (in blotters) and green house (soil environment). In the blotter study, percent germination, germination index, germination time, germination rate, and vigor index were estimated, while shoot and root lengths, number of leaves, and wet weight were estimated in the greenhouse study.
Results:
In both seedlings, percent germination, germination index, and vigor index showed significantly higher values in seeds primed with the isolates than in the water-treated seeds (p≤ 0.05). Throughout the period of planting, the shoot and root lengths of the isolate-treated seedlings showed significantly higher values than the untreated control setups. This observation was irrespective of the maize and cowpea seedlings. In both seedlings, shoot and root lengths were directly proportional to days of growth. The bacterial strains showed significantly higher growth promoting potential on the seedlings.
Conclusion:
Therefore, the availability of these phosphate solubilizing microorganisms in the soil could enhance the growth of the seeds.
1. INTRODUCTION
Phosphorus is a major limiting nutrient for plant growth, it plays a number of different roles in plant nutrition and encourages the growth of deeper roots [1]. However, only a little amount of phosphorus present in the soil is accessible to plants. The rhizosphere is known to support complex microbial populations comprising pathogens, saprophytes, epiphytes, endophytes, and many beneficial microbes, both qualitatively and quantitatively [2, 3]. It is estimated that the microbial load of the rhizosphere is typically less than 108 in bulk soil, although it can range from 1010 to 1012 per gram of soil [4, 5]. Bacterial species are the most prevalent microbes in the rhizosphere, and they are inevitably going to have a big impact on the plant. Several bacterial strains are indicated to cover up to 15% of plant root surface [6].
Most biological functions require phosphorus, including respiration, photosynthesis, energy transfer and storage, signal transduction, cell division and elongation, production of roots and seeds, nitrogen fixation, and other processes [7, 8]. Crop health and yield are both impacted when plants are unable to obtain phosphorus from the soil. It has been reported that about 40% of the world's arable land has production issues because of phosphorus deficiency [9]. Phosphate solubilizing bacteria interact with vesicular arbuscular mycorrhizae through the release of phosphate ions into the soil, which could result in a synergistic interaction that improves the exploitation of sources of weakly soluble phosphorus [10].
Numerous microbial species, including bacteria, fungi, actinomycetes, and even algae, play a crucial role in the solubilization of phosphate. They mostly consist of bacteria, which outperform the fungus in solubilizing phosphorus [11]. Some bacterial strains that have been implicated as phosphate solubilizers are Pseudomonas and Bacillus, Arthrobacter, Serratia, Chryseobacterium, Phyllobacterium, Xanthomonas, Enterobacter, Pantoea, Klebsiella, and Kushneria sinocarni [12-14].
Due to the chemical fixation of phosphorus in the soil and interactions with other metallic elements present in the rhizosphere, the majority of the phosphorus (95–99%) contained in the soil is insoluble and hence unable to be utilized by plants [15, 16]. Large quantities of fertilizer are frequently applied in order to increase the availability of phosphorus for plants. When fertilizers are applied in soils, there is the possibility of being converted to insoluble forms, thus necessitating the need for phosphate solubilizers that make the phosphorus available to plants [17, 18]. This study was therefore aimed at exploring the growth-promoting potential of phosphate solubilizing Enterobacter species on maize and cowpea seedlings.
2. MATERIALS AND METHODS
2.1. Test Bacterial
The bacterial strains were obtained from the rhizosphere of the plant, a total of 52 bacterial strains were isolated from rhizospheres of leguminous and non-leguminous plants, using the standard pour plating procedure. All the test strains were screened for phosphate solubilization ability. The five strains that showed remarkable phosphate solubilization potential were used for the study. The bacterial isolates consist of three strains of Enterobacter cloaca [E. cloacae OP023807, E. cloacae OP023808, E. cloacae OP023809] and two strains of E. hormaechei [E. hormaechei OP023806 and E. hormaechei OP0238010].
2.2. Viability Testing of Seeds
Two crops (cowpea and maize seedlings) were used, cowpea variety white sokoto which is indigenous to the study area and maize variety SUWAN 2 were used for the trial. Before planting, the crops were surface sterilized in 5% sodium hypochlorite solution for 5 min and assessed for viability. Preliminary viability testing was carried out by soaking approximately 100 seeds of the respective test crops in 200 mL of distilled water at a 250 mL capacity.
Seeds that floated were discarded, while the ones that settled were deemed to be viable. Further viability of the seeds that passed the preliminary viability was carried out by planting seven presoaked seeds (in distilled water for 2 h). The seeds were then planted in transparent plastic cups (40 cm in depth and 80 cm in diameter) that contained 3.5 g of adsorbent cotton wool and allowed to grow for 7 d. Seeds were deemed viable when a minimum of 50% germination was obtained.
2.3. Growth Promotion in Blotters
Growth promotion in blotters was carried out by steeping surface sterilized seeds from already established viable seeds in 18 h old broth cultures of the respective bacterial strains for 2 h. A setup that contained seeds steeped only in water served as a control.
At the expiration of steeping, seven seeds were withdrawn and planted in plastic cups, as described earlier. Each of the seed lots were observed daily for germination and estimation of percent germination, germination index, germination time, germination rate and vigor index as follows:
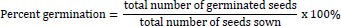 |
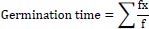 |
Where f is the number of seeds germinated on day x
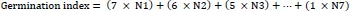 |
Where N1, N2, N3....N7 represent the number of seeds that germinated on the first, second, third till the 7th day.
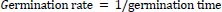 |
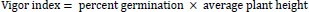 |
2.4. Growth Promotion in Soil
For the study in soil, the respective steeped seeds with different inoculum treatments and water control were planted in polythene bags containing 600 g of sterile soil. Following planting, the setups were incubated in a greenhouse for 21 d.
To maintain the soil's moisture, plants were irrigated with an equivalent volume of distilled water. Three replicates of each treatment were used in the experimental unit. Every 7 days, for a 21 d duration, shoot and root lengths, number of leaves, and wet weight (g).
2.5. Statistical Analysis
All values were presented as means and standard deviations of triplicate samples. Comparison of means was carried out using the One-Way Analysis of Variance (ANOVA) test, while multiple comparisons were carried out using the Turkey multiple range test. All statistical analyses were carried out using the SPSS Statistical Software (version 23.0).
3. RESULTS
3.1. Growth Promotion in Blotters
In both cowpea and maize seedlings, percent germination, germination index, and vigor index showed significantly higher values in seeds primed with the isolates than in the water-treated seeds (p≤ 0.05). For the cowpea seeds primed with the respective isolates, the percent germination, germination index, and vigor index ranged from 76.19-90.48%, from 59.00 to 70.67 and from 379.25 to 915.25, respectively. In control water-treated seeds, values of 47.61 (percent germination), 48.67 (vigor index), and 336.73 (vigor index) were recorded. The highest percent germination and vigor index values were recorded in cowpea seeds treated with E. cloacae OP023809 (Table 1).
In the case of maize seedlings, percent germination that ranged from 76.19 to 90.47% was observed for the isolate-treated seeds, while 52.38% germination was observed in the control setup. Moreover, a germination index and vigor index that ranged from 47.00 to 71.33 and from 861.16 to 1012.00 was observed for the isolate-treated seeds, while values of 42.00 (germination index) and 183.66 (vigor index) were recorded. The highest germination and vigor values were recorded in seedlings treated with E. cloacae OP023807 and E. cloacae OP023809, respectively (Table 1).
3.2. Growth Promotion in Soil
Throughout the period of planting, the shoot and root lengths of the isolate-treated seedlings showed significantly higher values than the untreated control setups. This observation was irrespective of the maize and cowpea seedlings. In both seedlings, shoot and root lengths were directly proportional to days of growth. At the expiration of the 21 d growth period, the highest shoot lengths of 20.40 cm and 29.70 cm, 25.00 cm and 25.80 cm, 25.10 cm and 23.40 cm, 26.3 cm and 26.90 cm, 25.6 cm and 29.70 cm, 13.7 cm and 16.00 cm were observed for seeds treated with E. cloacae OP023807, E. cloacae OP023808, E. hormaechei OP023810, E. cloacae OP023809, E. hormaechei OP023806 and untreated control setups, respectively (Table 2).
Generally, significantly higher leaves were observed in the isolated treated seeds than the control seedlings. Moreover, the number of leaves was directly proportional to the day of planting. This observation was irrespective of the maize and cowpea seedlings. At the end of the planting period, the average number of leaves in the maize seedlings were 5, 4.6, 4, 4, 4.67, and 3.3, while in the cowpea seedlings, it was observed to be 7.67, 6, 6, 7.67, 7.67 and 5 for the E. cloacae OP023807, E. cloacae OP023808, E. hormaechei OP023810, E. cloacae OP023809, E. hormaechei OP023806 and untreated control setups, respectively (Table 3).
| - | Final % Germination | Germination Time | Germination Index |
Vigor Index |
Germination Rate |
|---|---|---|---|---|---|
| Cowpea | |||||
| E. hormaechei OP023806 | 85.71 (± 14.29) |
5.37 (± 0.07) |
59.60 (± 9.23) |
379.25 (± 253.78) |
0.19 (± 0.00) |
| E. cloacae OP023807 | 76.19 (± 21.82) |
5.13 (± 0.16) |
62.66 (± 21.83) |
471.43 (± 483.21) |
0.19 (± 0.00) |
| E. cloacae OP023808 | 80.95 (± 8.24) |
5.13 (± 0.23) |
70.67 (± 21.73) |
402.381 (± 696.94) |
0.19 (± 0.00) |
| E. cloacae OP023809 | 90.48 (± 16.50) |
5.28 (± 0.12) |
62.67 (± 16.01) |
915.51 (± 431.36) |
0.19 (± 0.00) |
| E. hormaechei OP023810 | 76.19 (± 21.82) |
5.11 (± 0.06) |
59.00 (± 13.45) |
523.50 (± 676.04) |
0.19 (± 0.00) |
| Water | 47.61 (± 43.64) |
5.22 (± 0.31 |
48.67 (± 8.08) |
336.73 (± 583.24) |
0.19 (± 0.01) |
| Maize | |||||
| E. hormaechei OP023806 | 76.19 (± 8.24) |
5.17 (± 0.08) |
66 (± 4.58) |
1012 (± 176.2) |
0.19 (± 0.00) |
| E. cloacae OP023807 | 90.47 (± 8.25) |
5.23 (± 8.25) |
71.33 (± 23.46) |
998.57 (± 712.14) |
0.19 (± 0.00) |
| E. cloacae OP023808 | 80.95 (± 8.24) |
5.28 (± 0.11) |
70.06 (± 11.60) |
753.80 (± 114.48) |
0.18 (± 0.00) |
| E. cloacae OP023809 | 76.19 (± 16.50) |
5.51 (± 0.15) |
47.00 (± 9.16) |
1006.67 (± 199.34) |
0.18 (± 0.00) |
| E. hormaechei OP023810 | 80.95 (± 21.82) |
5.30 (± 0.02) |
63.67 (± 17.50 |
861.16 (± 624.71) |
0.19 (± 0.00) |
| Water | 52.38 (± 21.82) |
5.22 (± 0.21) |
42.00 (± 13.74) |
183.66 (± 131.69) |
0.19 (± 0.00) |
Table 2.
| Treatments | Lengths (cm) | |||||
|---|---|---|---|---|---|---|
| Maize Seedlings | Cowpea Seedlings | |||||
| Shoot length | Day7 | Day 14 | Day 21 | Day 7 | Day 14 | Day 21 |
| E. cloacae OP023807 | 13.1 (± 1.22) |
16.57 (± 1.01) |
20.40 (± 1.9) |
14.9 (± 0.81) |
20.00 (± 3.28) |
29.70 (± 2.52) |
| E. cloacae OP023808 | 11.2 (± 1.39) |
20.17 (± 2.57) |
25.00 (± 1.0) |
18.2 (± 2.76) |
23.5 (± 0.5) |
25.80 (± 1.06) |
| E. hormaechei OP023810 | 9.50 (± 1.13) |
11.70 (± 1.14) |
25.10 (± 1.94) |
14.1 (± 0.66) |
16.67 (± 0.58) |
23.40 (± 3.15) |
| E. cloacae OP023809 | 7.17 (± 1.03) |
13.7 (± 0.83) |
26.3 (± 1.25) |
16.7 (± 1.53) |
21.87 (± 1.52) |
26.90 (± 1.51) |
| E. hormaechei OP023806 | 8.23 (± 2.46) |
12.6 (± 1.83) |
25.6 (± 0.91) |
14.9 (± 0.81) |
20.00 (± 3.28) |
29.70 (± 2.52) |
| Untreated control | 3.33 (± 0.58) |
5.37 (± 0.71) |
13.7 (± 15.3) |
8.93 (± 2.35) |
13.13 (± 1.21) |
16.00 (± 1.00) |
| Root length | ||||||
| E. cloacae OP023807 | 6.73 (± 2.36) |
10.50 (± 0.87) |
17.70 (± 1.48) |
7.93 (± 1.37) |
14.80 (± 2.15) |
16.7 (± 2.34) |
| E. cloacae OP023808 | 6.07 (± 0.60) |
11.1 (± 0.85) |
14.00 (± 2.65) |
12.40 (± 2.48) |
17.00 (± 2.0) |
19.80 (± 0.28) |
| E. hormaechei OP023810 | 5.17 (± 0.76) |
8.11 (± 0.53) |
11.70 (± 0.58) |
14.00 (± 3.61) |
18.21 (± 2.06) |
22.06 (± 2.27) |
| E. cloacae OP023809 | 6.20 (± 0.59) |
11.70 (± 12.5) |
13.30 (± 0.04) |
12.70 (± 1.26) |
15.00 (± 1.00) |
17.00 (± 1.25) |
| E. hormaechei OP023806 | 11.00 (± 1.00) |
13.7 (± 0.58) |
16.00 ± 1.00 |
7.93 (± 1.37) |
14.80 (± 2.15) |
16.70 (± 2.34) |
| Untreated control | 4.00 (± 0.00) |
7.00 (± 0.00) |
13.00 (± 0.00) |
3.07 (± 1.10) |
8.37 (± 0.81) |
11.30 (± 0.93) |
| Treatment Type | Number of Leaves | |||||
|---|---|---|---|---|---|---|
| Maize Seedlings | Cowpea Seedlings | |||||
| Day 7 | Day 14 | Day 21 | Day 7 | Day 14 | Day 21 | |
| E. cloacae OP023807 | 3.0 (± 0.00) |
4.00 (± 0.00) |
5.00 (± 0.00) |
2.00 (± 0.00) |
5.00 (± 0.00) |
7.67 (± 0.58) |
| E. cloacae OP023808 | 3.0 (± 0.00) |
3.67 (± 0.58) |
4.60 (± 0.58) |
2.00 (± 0.00) |
4.33 (± 1.56) |
6.00 (± 1.73) |
| E. hormaechei OP023810 | 3.0 (± 0.00) |
3.67 (± 0.58) |
4.00 (± 0.00) |
2.00 (± 0.00) |
5.00 (± 0.00) |
6.00 (± 1.73) |
| E. cloacae OP023809 | 2.33 (± 0.47) |
4.00 (± 0.00) |
4.00 (± 0.00) |
2.00 (± 0.00) |
5.00 (± 0.00) |
7.67 (± 0.58) |
| E. hormaechei OP023806 | 2.33 (± 0.58) |
4.00 (± 0.00) |
4.67 (± 0.58) |
2.00 (± 0.00) |
5.00 (± 0.00) |
7.67 (± 0.58) |
| Untreated control | 1.00 (± 0.00) |
3.00 (± 0.00) |
3.3 (± 0.58) |
2.00 (± 0.00) |
3.00 (± 1.73) |
5.00 (± 0.00) |
| Treatment Type | Weight (g) | |||||
|---|---|---|---|---|---|---|
| Maize Seedlings | Cowpea Seedlings | |||||
| Day 7 | Day 14 | Day 21 | Day 7 | Day 14 | Day 21 | |
| E. cloacae OP023807 | 1.60 (± 0.38) |
1.84 (± 0.35) |
2.07 (± 0.33) |
1.29 (± 0.09) |
1.29 (± 0.09) |
2.06 (± 0.49) |
| E. cloacae OP023808 | 1.44 (± 0.11) |
1.67 (± 0.14) |
2.57 (± 0.09) |
1.22 (± 0.38) |
1.80 (± 0.51) |
2.37 (± 0.32) |
| E. hormaechei OP023810 | 1.47 (± 0.27) |
1.73 (± 0.29) |
1.94 (± 0.10) |
1.47 (± 0.14) |
1.78 (± 0.23) |
2.34 (± 0.15) |
| E. cloacae OP023809 | 1.34 (± 0.50) |
1.44 (± 0.05) |
2.22 (± 0.07) |
1.20 (± 0.36) |
1.92 (± 0.26) |
2.37 (± 0.33) |
| E. hormaechei OP023806 | 0.9 (± 0.15) |
1.28 (± 0.22) |
2.08 (± 0.07) |
1.29 (± 0.09) |
2.06 (± 0.49) |
2.06 (± 0.49) |
| Untreated control | 0.8 (± 0.10) |
1.16 (± 0.04) |
1.24 (± 0.06) |
1.10 (± 0.29) |
1.15 (± 0.13) |
2.3 (± 0.04) |
Moreover, the weight of the seedlings increased with increase in the planting days. For the maize seedlings, significantly higher weight was observed in the isolated treated seedlings, when compared with the control setup. For the cowpea seedlings, weight weights were only observed to be significantly higher in seedlings treated with E. cloacae OP023808 and E. cloacae OP023809 when compared with the control setup (Table 4).
4. DISCUSSION
The results from this study revealed that seedlings treated with the isolates had significantly higher germinability values for cowpea and maize seedlings when compared with the control setup. The direct improvement in vigor index suggests that phosphate solubilizing bacteria had a favorable impact on seed quality and germination [19]. There are further reports of the plant growth-promoting bacterium Enterobacter cloacae stimulating the growth of tomato, pepper, and mung bean plants [20]. When maize and wheat were inoculated with Pantoea cypripedii and Pseudomonas plecoglossicida, Kaur and Sudhakara [21] reported a significant improvement in growth parameters, grain yield, and total phosphate uptake. Additionally, according to Panhwar et al. [22], the addition of organic acids to phosphate-solubilizing bacteria increased the amount of soluble phosphate in the soil solution, promoted root development, and boosted plant biomass in aerobic rice seedlings.
The study also revealed significantly higher shoot and root lengths for maize and cowpea in the isolate treated seedlings under soil conditions. In a similar study, Rodríguez and Fraga [23], Enterobacter sp. was observed to show potential for improvement of growth of okra seedlings. In addition, Walpola and Yoon [24] also studied the effect of two phosphate solubilizing bacterial strains (Pantoea agglomerans and Burkholderia anthina) on mung bean plants under greenhouse conditions. The study revealed enhanced shoot and root length, dry matter, and overall phosphate uptake.
Additionally, Kolekar et al. [25] observed that inoculating Vigna radiata plants with a mixture of yeast, bacteria, and fungi improved their growth and yield. Similarly, under semi-arid conditions, maize seeds treated with phosphate solubilizing bacteria showed a considerable increase in yield and yield components [26]. In addition, Egamberdieva et al. [27] observed that the presence of Enterobacter hormaechei promoted tomato growth in both saline and non-saline soil, which could be attributed to the fact that phosphorus can help improve the tolerance of the plant to salinity [28].
The presence of phosphate solubilizing bacteria in the rhizosphere has been attributed to a significant increase in plant height and seed weight [29]. Phosphate solubilizing bacteria have also been implicated in the improvement of wheat and rice performance [22]. Panhwar et al. [22] reported that treatment with phosphate solubilizing bacteria boosted wheat production and encouraged rice growth. However, different bacterial species have different solubilization capacities for phosphate. According to reports, phosphate dissolvers' long-term stability and capacity pose major obstacles to their widespread use in boosting agricultural output [30]. There is evidence that Enterobacter sp. can create large amounts of indole acetic acid, one of the primary direct ways for promoting plant growth, in addition to solubilizing phosphate. In plants, indole acetic acid influences responses to light and gravity as well as cell division, extension, and differentiation and stimulates seed germination [30, 31].
CONCLUSION
The study revealed the growth promoting potential of the bacterial strains on cowpea and maize seedlings. The influence of these selected strains is connected to their ability to make phosphorus available to the plant by increasing its solubility in the soil. Therefore, the availability of these phosphate solubilizing microorganisms in the soil could enhance the growth of the test seeds. Application of E. cloacae and E. hormaechei strains used in this study could increase the productivity and yield of the test crops.
LIST OF ABBREVIATIONS
| SPSS | = Statistical Package for the Social Sciences |
| ANOVA | = Analysis of Variance |
CONSENT FOR PUBLICATION:
Not applicable.
FUNDING
None.
AVAILABILITY OF DATA AND MATERIALS:
The data and supportive information is available within the article.
CONFLICT OF INTEREST
The authors declare no conflict of interest financial or otherwise.
ACKNOWLEDGEMENTS
The authors are grateful to Afe Babalola University for the provision of facilities to carry out the study.


