All published articles of this journal are available on ScienceDirect.
Microbiological Quality of Feed
Abstract
Background:
In most countries, the microbiological quality of complete feed mixtures, grains, silages and hay is sadly a much-underrated aspect of official feed control. Monitoring the microbiological quality of feeds downgrades any of the poor quality feeds and stimulates feed mills to produce better quality feeds, therefore, enforcing the prominence of high-quality feeds on the market.
Objectives:
To collect the results of the microbiological feed quality, done over a period of one year, all of which originate from Croatia, Slovenia, and Switzerland. Furthermore, identify the presence of feedingstuffs with poor microbiological quality in some parts of the European market and therefore have the most impact on animal health and welfare.
Methods:
Feed quality was assessed through a single Verband deutscher landwirtschaftlicher Untersuchungs - und Forschungsanstalten (VDLUFA) method based on a systematic approach, which categorizes feed into categories from 1 to 4 according to bacteria, moulds, and yeasts content, and their ecology and hazard significance.
Results:
Although the most analyzed samples of feed belong to the most desirable quality level 1, it should be emphasized that almost all feed types can be found samples of quality level 4, unacceptable for feed.
Conclusion:
The obtained results give an overview of the advantages of monitoring the microbiological quality of feeds for governmental authorities, producers, and consumers alike and potentially provide more information concerning the new aspects of the risk assessment of certain types of feeds or their raw materials.
1. INTRODUCTION
Modern animal systems, which aspire to be successful and sustainable must rely on the quality of feed, nutrition, and welfare of the animals [1,2], as without these elements it is not possible to elevate animal productivity to meet the nowadays demands of animal production [3]. It is well known that the content of bacteria and moulds affects the quality of feed and consequently animal health and production. Even low-feed contamination with pathogenic bacteria, such as Salmonella, Clostridium, Listeria genera and E.coli can cause serious health and epidemiological problems [4, 5]. Saprophytic bacteria, moulds, and yeasts in excessive numbers cause undesirable changes in the colour, taste, and smell of the feed as a result of their metabolic activity. It reflects the loss in the feed of dry matter because microorganisms use carbohydrates as an energy source, they degrade the fats and proteins and consequently reduce the feed's nutritive values. Additionally, by producing volatile metabolites, a microorganism changes the smell and taste of a feed and as a result means a decrease in feed intake, which can cause digestive disorders, and consequently decreases the animals’ productivity and is a violation of the animals’ immune system and consequently its health and welfare [6, 7]. The microbiological quality of feed implies a level of feed contamination with bacteria, moulds, and yeast. Spoilage microorganisms can be introduced to the feedstuffs, during a crop’s growth in the field, during harvesting and postharvest handling, or during storage and distribution [8]. In addition, as a result of contamination of feedstuffs with moulds, the feedstuffs can contain mycotoxins, secondary moulds metabolites which can harm the animals’ health [9, 10]. The microbiological analysis of feed quality allows one to identify any dangerous species of moulds (mycotoxins producers), that are often not covered by the traditional feed safety spectrum. These moulds could originate either from disease-stricken plant material (e.g. Fusarium genera) or from inadequate storage conditions (e.g. Aspergillus and Penicillium genera) [11, 12]. The same is true for the aerobic mesophilic bacterial flora of these feeds, which can be either associated with epiphytic bacteria from the open-air fields or from the flora that develops during storage [7]. Apart from these bacteria and moulds, yeast can also cause spoilage to feed. While yeast can be added to feed as probiotics (e.g.Saccharomyces spp), an increased amount/quantity of the yeast of other species is considered spoilage [13].
The microbiological quality of feeds is unfortunately a much-underrated aspect of official feed control. The reason can lie in the fact that although the European Union has one of the most strict regulations, it has only prescribed the desirable qualitative conditions for feed. EU Regulation 178/2002 states [14]:" Feed shall be deemed to be unsafe for its intended use if it is considered to: — have an adverse effect on human or animal health"; and EU Regulation 767/2002 states [15]: “Feed business operators placing feed on the market shall ensure that the feed: is sound, genuine, unadulterated, fit for purpose and of merchantable quality”. It should be emphasized that it is left to the Member States how to regulate what is perfectly fit with regards to the microbiological quality.
Indeed, monitoring the microbiological quality of feeds downgrades the poor quality feeds, stimulates mills to produce better quality feeds, and enforces the prominence of high-quality feeds on the market. Those high-quality feeds, when widely available on the markets, will then improve the performances of farm animals and thus increase the number of more successful farmers. Moreover, monitoring microbial feed quality also reveals those mills with systemic bad hygiene, an unsatisfactory storage environment, and working with low-grade raw materials.
The aim of the presented study was to gather the results of microbiological feed quality of three European countries: Croatia, Slovenia and Switzerland and to produce a survey of the monitoring over a period of one year.
2. MATERIALS AND METHODS
In this study, 155 complete feed mixtures, grains, silages and hay samples from Croatia, 47 samples from Slovenia, and 125 samples from Switzerland were sampled in 2019 (Table 1).
Sampling was provided according to the Commission regulation EU Regulation 152/2009 laying down the methods of sampling and analysis for the official control of feed [16]. In Croatia and Switzerland, most of the samples were sampled in the frame of official monitoring of feed and some of them were at the customer’s request. In Slovenia, all the samples were analyzed at the customer’s request. Samples were analyzed by the Association of German Agricultural Analytic and Research Institutes (Verband deutscher landwirtschaftlicher Untersuchungs - und Forschungsanstalten) -VD LUFA method, which is based on a systematic approach, categorizes feed into categories from 1 to 4 according to bacteria, moulds, and yeasts content, and their ecology and hazard significance. VD LUFA method [17] merges into four standard operating procedures, which combine as the usual classical microbiological methods ISO 21527-1:2008 [18], ISO 21527-2:2008 (moulds counting) [19], and ISO 4833:2003 (mesophilic bacteria counting) [20] with some modification. For bacteria number counting tryptose agar with trifeniltetrazolium chloride (TTC) is used. Under incubation conditions (30°C/3 days), dependent on the bacteria's metabolic activities; TTC is reduced to colour formazin. In that way, uncoloured bacteria become yellow, orange (saprophytic bacteria), and red or pink (spoilage indicators) and can be classified.
| - | Croatia (n) |
Slovenia (n) |
Switzerland (n) |
|---|---|---|---|
| Ruminant feed | 37 | 1 | 66 |
| Poultry feed | 40 | 4 | 11 |
| Swine feed | 22 | 1 | 14 |
| Rabbit feed | 1 | 2 | 1 |
| Horse feed | - | - | 11 |
| Mixed grains | - | - | 5 |
| Maize | 7 | - | 6 |
| Oat | 1 | 26 | 1 |
| Barley | 4 | - | - |
| Soy meal | 25 | - | - |
| Silage | 18 | 5 | 8 |
| Hay | - | 8 | 2 |
| TOTAL | 155 | 45 | 125 |
The method is used in three stages:
(i) Counting the bacteria, moulds, and yeasts present in the samples [21, 22]
(ii) Identifying and classifying them into 7 functional categories [23].
According to the VD LUFA method bacteria, moulds and yeasts are divided into micro-organisms groups (MG) considering their ecology and hazard signification. Therefore Product-typical bacteria (yellow-pigmented representatives of the genera (Pantoea, Enterobacter, Stenotropho-monas, etc.Pseudomonas spp./Enterobacteriaceae) or moulds and Dematiacea (Dematiaceae, Verticillium spp., Acremonium spp., Fusarium spp., Aureobasidium spp.) are allocated into a micro-organism group (MG 1 and MG 4) each. Spoilage-indicating bacteria (Bacillus spp., Staphylococci/Micrococci) or moulds(Aspergillus spp, Penicillium spp, Scopulariopsis spp, and Walemia spp) are allocated into two micro-organism groups each (MG 2 and 3; MG 5 and 6). Yeasts of all species are to be assessed as spoilage-indicating micro-organisms; they are allocated to micro-organism group 7 (MG 7). In contrast to the spoilage indicating bacteria of MG 2 “Streptomycetes” are characteristic only for certain processes of spoilage (advanced storage spoiling). Furthermore, they occur in unspoiled feeds in distinctly lower germ concentrations than the other spoilage indicating bacteria of MG 2. Therefore a separate micro-organism group (MG 3) was formed for the Streptomycetes. In the case of spoilage indicating moulds the Mucorales (Zygomy-cetes) were separated as MG 6 from the other indicator moulds (MG 5) because Mucorales produce a higher share of mycelium and thus of biomass than that of other spoilage indicating moulds.
iii) Comparing the obtained concentrations of microorganisms categories to elaborate the orientation values for each type of matrices and taking into account the types of animals feeding [17]. In that way feed and feedstuffs are classified into 4 quality levels; Q1-normal, desirable; Q2- slightly or moderately reduced; Q3- reduced or distinctly reduced; Q4- unsound, unacceptable for feeding
3. RESULTS
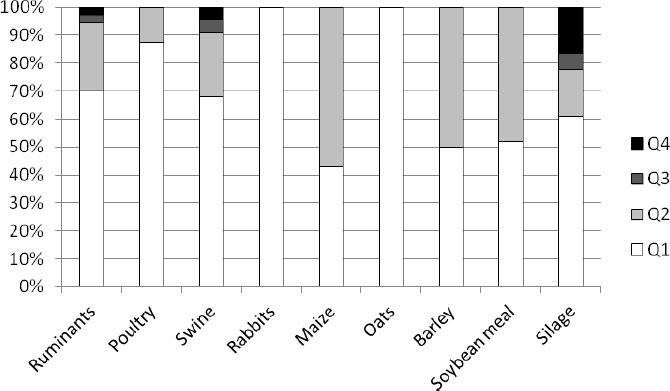
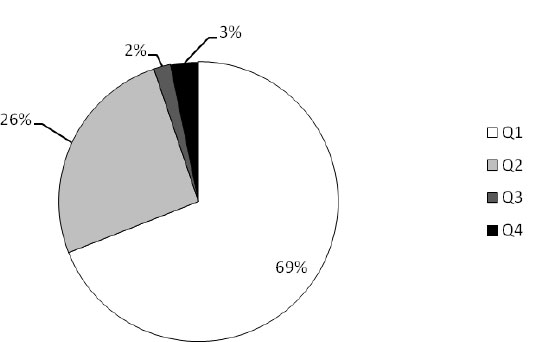
The results of the microbiological feed quality assessment of the analyzed samples from three European countries are shown in Figs. (1-6). In Croatia, in the frame of official control, 125 feed and feeding stuff samples were assessed. In the desirable Q1 level (Fig. 1) all the analyzed samples of rabbits’ feed and oats were classified. Around 40% to 60% of samples of maize, barley, and soybean meal were classified as Q2, in which microbiology quality was slightly reduced, as well as 20% of feeds for ruminants, swine, and silage. Also, about 5% of the analyzed samples of ruminant and swine feed and silage as well were classified as distinctly reduced (Q3). The highest percent (15%) of unsound samples (Q4) belongs to silage, followed by swine (7%) and ruminants’ (4%) feeds. Taking all into account, 69% of all the assessed feeds belong to Q1, 26% to Q2, 2% to Q3, and 3% to Q4 (Fig. 2).
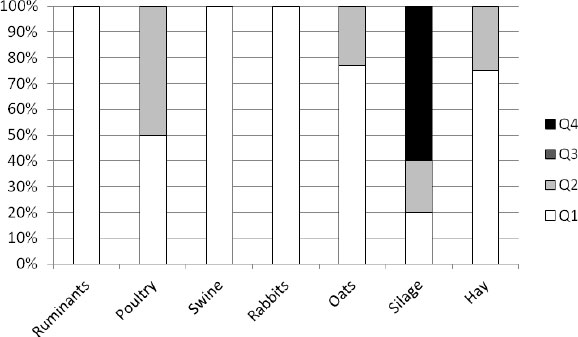
In Slovenia 46 feed and feedingstuff samples were analyzed at the customers' request (Fig. 3). All the analyzed samples for ruminants, swine, and rabbits were classified at the Q1 level. In restricted microbiological quality, the Q2 level was classified at 50% of poultry feed, 25% of oats and hay, and 20% of silage samples. As much as 60% of silage samples were classified as unsound at the Q4 level.
In general, 70% of the assessed feeds in Slovenia were classified in Q1, and 24% in Q2 while none of the assessed samples were classified as Q3. As unsound (Q4) 6% of samples were assessed (Fig. 4).

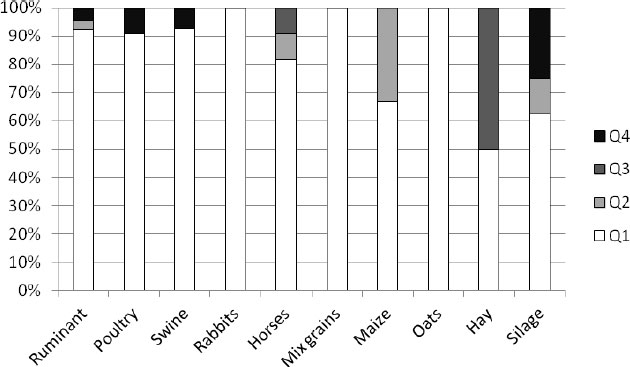
In the frame of official control, 130 samples of feed and feedingstuffs were analyzed in Switzerland (Fig. 5). All the analyzed samples of rabbits, mixed grains, and oats were classified at the desirable Q1 level. At the Q2 level 35% of maize, about 10% of silage and horses’ feed, and 3% of ruminants’ feed were classified. 50% of hay samples and 10% of horses’ feed were classified as feed distinctly reduced in Q3. As unsound (Q4) 5% of ruminant feed, almost 10% of poultry feed, 5% of swine feed, and a high 25% of silage.
In total, 88% of the analyzed feeds in Switzerland were assessed as of a desirable quality (Q1). In Q2 were classified as 5% of the analyzed samples, in Q3 2%, and in Q4 as unsound 5% of samples (Fig. 6).
The data supporting the findings of the article is available in the PUH at [https://puh.srce.hr/s/3ayFz2TtsBqedFZ] reference number [24].

4. DISCUSSION
A survey of the microbiological quality level of feeds and feeding stuff was done on samples from three European countries: Croatia, Slovenia, and Switzerland. In this study about 70-80% of the analyzed feed was assessed as at a desirable Q1 level, and about 20% of the analyzed samples from Croatia and Slovenia were classified as Q2 as was previously described by Bucher and Thalman [25]. Simultaneously, in Switzerland, just 5% of the analyzed samples were assessed as slightly reduced quality Q2. While Bucher and Thalman [25] in their study obtained about 4% of samples as reduced quality Q3 and about 2% of unsound feed Q4, in this study 2% were assessed as Q2 and 3-6% as Q4. It can be explained that Bucher and Thalman [25] analyzed random samples for the pilot study, and this study used random samples too, however, samples from customers who had a problem with feeding were also used (targeted sampling).
The worst assessed samples, unsound Q4, in this study were silage. As much as 60% of the analyzed silage samples in Slovenia, 25% of silage samples in Switzerland, and 15% in Croatia were assessed as undesirable for feeding. According to Wyss and Pradervand [26] whose study shows that silage immediately after ensiling cannot be recommended for feeding (Q4), some of the bad results can be explained, for example, bad technology of ensiling. The ensiling process is defined as involving the following steps: harvesting the crop at the optimal stage of maturity, chopping, loading into a silo, compacting and sealing to exclude air, storing, and finally unloading for animal feeding. If any of those steps are not performed in the proper way, there can be dramatic changes for the worst [27, 28]. Considering that Slovenian samples targeted those samples at the customer’s request it is not surprising that there is such a high percentage (60%) of samples of undesirable quality.
Plant feeding stuffs are the primary and most important source of fungi in feed (field fungi, MG 4) and additionally, contaminants which occur during storage (storage fungi, MG 5) [29, 30]. In this study, significant percentages of grains and hays were assessed as a feed with a slightly reduced or reduced quality because of the increased count number of moulds. Poor quality hay is a big problem for farmers and horse breeders, causing digestible and breathing complications [31]. In Croatia, as much as 55% of maize and 50% of barley and soya bean meal samples were classified in Q2. In Slovenia, 25% of the analyzed barley samples and in Switzerland 35% of the maize samples were classified as Q2. Therefore, it was expected that some percentage of the compound feeds were assessed as reduced quality. In Croatian and Switzerland in almost all the compound feed (excluding the feed for rabbits) all four microbiological feed categories are present and in Slovenia, more than 50% of poultry feed is classified as Q2. Moreover, an increased number of moulds could be an indicator of mycotoxins present [32]. Using the VD LUFA method one can easily decide what kinds of mycotoxins to analyze in order to distinguish the moulds according to their species. Microorganisms, especially moulds in hay can cause problems, especially to horses’ respiratory and digestion systems which are very sensitive [33]. Therefore, the high percentage of reducing the microbiological quality of hay in Switzerland (50% of samples were classified in Q3) and 25% in Slovenia (classified in Q2) is a good reason to consider that before starting feeding with hay, silage and feed they should be checked for their microbiological quality. By checking the microbiological quality of feed and feeding stuffs before use, a lot of potential issues in animal and feed production could be avoided, because bad material can then be excluded from the feed production. Also, by checking the final products the feed mill will have an insight into the hygiene of the production line and storage conditions. Furthermore, it is vital to state that only quality and nutritionally and microbiologically sound feed will be able to raise the level of animal production and satisfy the health and welfare needs of modern farm animals [34].
It is a great pity that no European regulation proscribes the specified numbers/values for the microbiological quality of feed, while for food for human consumption, there exist guidelines for certain types of food with proscribed orientation values for pathogens and for saprophytes microorganisms (EU 178/2002) [14]. In the European Union, the Member States proscribe for themselves the values for microbiological quality (EU 183/2005) [35].
CONCLUSION
The obtained results of the presented study give an overview of the advantages of monitoring the microbiological quality of feeds for governmental authorities, producers, and consumers alike and potentially provide information regarding the new aspects of the risk assessment of certain types of feeds or their raw materials.
LIST OF ABBREVIATIONS
| TTC | = Trifeniltetrazolium Chloride |
| MG | = Micro-organisms Groups |
| VDLUFA | = Verband deutscher landwirtschaftlicher Untersuchungs - und Forschungsanstalten |
CONSENT FOR PUBLICATION
Not applicable
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the PUH at https://puh.srce.hr/s/3ayFz2TtsBqedFZ reference number [24].
FUNDING
None.
CONFLICTS OF INTEREST
Authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thank the Croatian Veterinary Institute; Faculty of Veterinary Medicine the University of Zagreb; Agroscope; and the Institute of Food Safety, Feed and Environment, the Veterinary Faculty, Ljubljana for helping to provide equipment for the research.


