RESEARCH ARTICLE
Control of Bacterial Wilt (Ralstonia solanacearum) and Reduction of Ginger Yield Loss through Integrated Management Methods in Southwestern Ethiopia
Eyob Aysanew Benti1, *
Article Information
Identifiers and Pagination:
Year: 2023Volume: 17
E-location ID: e187433152212300
Publisher ID: e187433152212300
DOI: 10.2174/18743315-v17-e230109-2022-39
Article History:
Received Date: 06/09/2022Revision Received Date: 25/11/2022
Acceptance Date: 08/12/2022
Electronic publication date: 10/04/2023
Collection year: 2023

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Bacterial wilt incited by Ralstonia solanacearum is the most important disease affecting ginger production in southwestern Ethiopia. The unavailability of disease-free planting materials, resistant cultivars, and effective chemical compounds are the key constraints in managing the disease.
Objective:
The study was initiated to determine the effect of integrated management methods on bacterial wilt disease and yield loss of ginger through combining hot water, bio-fumigation, soil-solarization and chemical pesticides.
Methods:
A total of seven treatment combinations comprising hot water, bio-fumigation, soil-solarization, Mancozeb, and bleaching powder were tested in a randomized complete block design in three replications. Data on disease incidence, growth, yield, and yield components were recorded from randomly selected plants.
Results:
The use of Mancozeb for seed socking and soil drenching combined with bio-fumigation and soil-solarization reduced the incidence of bacterial wilt by 63.3% and enhanced the rhizome yield by 66.8%. Rhizome and soil treatment using bleaching powder along with soil bio-fumigation also reduced the disease incidence by 38.9% and increased ginger yield by 61.5%. It also provided the highest (6678.7%) marginal rate of return of any treatment combination tested in the experiment. Disease incidence was highly significantly and inversely (r= -0.98**) correlated with rhizome yield. The regression slope estimated that 83.4% of ginger yield loss was associated with bacterial wilt disease.
Conclusion:
A combined application of Mancozeb, bio-fumigation and soil-solarization can be used to control ginger bacterial wilt. Alternatively, bleaching powder for rhizome and soil treatment in conjunction with bio-fumigation can be employed as an integrated management system against the disease.
1. INTRODUCTION
Bacterial wilt caused by Ralstonia solanacearum (R.S.) is one of the most important diseases limiting ginger production in tropical and subtropical regions of the world, particularly in Ethiopia [1]. It causes rapid and fatal vascular wilting to a wide range of host plants and has been ranked as the second most important bacterial pathogen [2]. R.S. is primarily a seed and soil-borne pathogen, frequently spread by latently infected rhizomes to disease-free areas or by planting on infected soils. Wounds created by cultural practices, parasitic insects, or root-knot nematodes are the principal routes of entry for the pathogen [3, 4].
The bacterium colonizes the cortex, has access to the vascular cylinder, and then infects the intercellular spaces of the vascular parenchyma adjacent to xylem vessels. The pathogen then invades and fills the xylem vessels, causing the surrounding parenchymal cells to be severely degraded and destroyed by the hydrolytic enzymes secreted by R.S [5, 6]. Other factors that contribute to wilting include high bacterial densities, byproducts of plant cell wall degradation, and tyloses and gums produced by the plant itself [7].
The direct yield loss caused by bacterial wilt disease varies greatly depending on the host, cultivar, climate, soil type, cropping plan, and strain [8]. In Ethiopia, since the first reports of bacterial wilt on ginger in early 2010, the disease has rapidly spread to major ginger growing areas in the southern and southwestern regions of the country. Disease prevalence ranged between 91.6 and 98.9%, causing yield losses of up to 100%, in areas where small and marginal farmers relied on ginger for a living [9]. A review by Ayana [10] highlighted similar stories from around the world, indicating a 50-100% yield loss of potato in Kenya, 95% loss of tobacco in the United States, 88% loss of tomato in Uganda, and 70% yield loss of potato in India. The propensity of R.S. to develop endophytically, survive in deeper soil layers, migrate with water, and interact with weeds complicates control [11]. Furthermore, no single universally successful approach has been reported. As a result, disease management programs that incorporate cultural, physical, disease resistance, biological, and chemical techniques have received special attention [12].
Since R.S. is a seed and soil-borne pathogen, using disease-free planting material and planting in healthy soils are the principal approaches to avoid the disease [13]. In the absence of these, treating rhizome seeds with hot-water or chemicals may benefit in the elimination of the initial inoculum carried by the seed. Exposing the rhizome to hot-water treatment at 50 oC for 10 minutes effectively disinfected the pathogen on the seed surface [14]. Rhizome soaked in bleaching powder solution, combined with soil bio-fumigation was found to be effective in controlling bacterial wilt and enhancing ginger yield [15]. In a greenhouse trial, soil-drenching with Mancozeb also reduced (96.9%) the severity of tobacco bacterial wilt [16]. Similarly, seed and soil treatment by integrating with other disease management methods was reported to reduce bacterial wilt and improved potato yield [17].
Bio-fumigation is a biological method of controlling soil-borne pathogens using volatile chemicals released from plant residues. When the tissue of plants in the Brassicaceae family is damaged and immediately integrated into the soil, a potent volatile biocidal agent (isothiocyanate) is released, sterilizing the soil environment [18]. The combined use of lemongrass, nitrogen fertilizer, and soil solarization reduced the incidence of ginger bacterial wilt by 42.6% [19]. Essential oils derived from palmarosa, lemongrass, and eucalyptus showed an antibiotic effect on R.S. and reduced bacterial wilt of ginger [20].
Soil-solarization is one of the physical strategies advised for an integrated control strategy of soil-borne diseases. It traps solar energy beneath the plastic sheet and elevates the temperature of the upper layer of the soil surface enough to suppress pathogens of interest [21]. Although the efficacy of this method is temperature dependent, it can be boosted through an integrated measure incorporating plants with allelopathic effects, such as Allium and Brassica species [18]. It has been demonstrated that soil-solarization combined with bio-fumigation with chicken manure improves tomato plant health and yield [22]. Meenu and Jebasingh [23] also advocated integrating soil solarization with other disease management strategies in order to reduce bacterial wilt disease and improve plant vigor.
Currently, bacterial wilt is the most distracting disease threatening ginger production in southwest Ethiopia. Farmers have no control options for this disease; as a result, they discourage producing ginger and other related solanaceous crops. The unavailability of healthy planting materials, resistant cultivars, and effective bactericidal compounds are considered as major constraints for bacterial wilt management. Furthermore, only a few preliminary studies on the integrated management of ginger bacterial wilt have been conducted thus far, with inconclusive results. Thus, the present study was initiated to determine the effect of integrated management methods on bacterial wilt disease and yield loss of ginger through combining hot water, bio-fumigation, soil-solarization and chemical pesticides.
2. MATERIALS AND METHODS
2.1. Study Area
The study was conducted in Southwestern Ethiopia, where bacterial wilt disease on ginger was reported for the first time in the country. The research area is located between the coordinates 6o51’30” North and 35o20’3” East, situated at an altitude range of 900 to 1200 m.a.s.l. The mean maximum and minimum annual temperatures of the area are 30 oC and 16 oC, respectively, with an annual mean rainfall of 1960 mm. The humid climatic conditions and tropical rainforests are typical characteristics of southwestern Ethiopia. Coffee, spices, fruits, oil, and field crops are predominantly cultivated by farmers [24].
2.2. Experimental Treatments and Procedures
The experiment consisted of different treatment combinations, including hot-water, soil bio-fumigation with lemongrass, soil-solarization using polyethylene plastic sheet, Mancozeb® 75% WP, Syngenta India Ltd. Maharashtra, India (MANCOZEB 75% W.P), and Bleaching Powder (Ca(OCl)2), Shriram Vinyl & Chem. Inds., Kota-Rajasthan, India (stable bleaching powder), to control ginger bacterial wilt disease (Table 1). Rhizomes were subjected to hot-water treatment at 50 oC for 10 minutes. MANCOZEB 75% W.P @ 0.1 g/litter for 30 minutes and bleaching powder @ 10% solution for 10 minutes were used to disinfect ginger rhizomes as required per treatment.
Six weeks before planting, young grown lemongrass shoots at a rate of 10 t ha-1 were chopped into pieces and thoroughly incorporated into the soil for bio-fumigation of the experimental plots [19].
| S. No. | Treatment | Treatment Combinations |
|---|---|---|
| 1 | T1 | Hot-water and bio-fumigation |
| 2 | T2 | Hot-water, bio-fumigation, and soil-solarization |
| 3 | T3 | Mancozeb and bio-fumigation |
| 4 | T4 | Mancozeb, bio-fumigation and soil-solarization |
| 5 | T5 | Bleaching powder and bio-fumigation |
| 6 | T6 | Bleaching powder, bio-fumigation, and soil-solarization |
| 7 | T7 | Control |
Experimental plots which received combined applications of soil solarization were moistened with water. Subsequently, the plots were properly leveled and covered with a 25 µm thick polyethylene plastic sheet for six weeks prior to planting.
Soil drenching with MANCOZEB 75% W.P @ 0.3% was done 15 days prior to planting and the second soil drenching was started after the first wilt incidence was observed and continued for 6 times at 15 day intervals [16].
Similarly, bleaching powder was administered at a rate of 25 kg ha-1 and thoroughly mixed with the soil. The plots were then covered with polyethylene plastic and kept undisturbed. After two weeks, the plastic covers were removed and the soil was ploughed to expose remnants of bleaching powder to the air. The rhizomes of the controlled treatment were dipped in distilled water [25].
2.3. Experimental Design
A total of seven treatment combinations were tested against the disease in a randomized complete block design (RCBD) with three replications. Ginger was planted in a net plot size of 3 m x 1.2 m (3.6 m2). The row-to-row and rhizome-to-rhizome distances were 0.3 cm and 0.15 cm, respectively. The spacing between plots and blocks was 0.5 m and 1 m, respectively. Application of fertilizer, weeding, and other agronomic practices were done according to the previous recommendations.
2.4. Data Collection
2.4.1. Disease Assessment
The occurrence of bacterial wilt disease was visually investigated as described by Nelson [25]. The number of infected plants per treatment was counted starting from the first initiation of disease symptoms and proceeded to the final disease assessment date at 15-day intervals. The number of infected plants from a total number of plants per plot was then converted into percentage disease incidence (PDI).
The area under the disease progress curve (AUDPC) in percent-days was also computed from PDI data recorded at each disease assessment date (time) under each treatment of the experiment according to the formula given by Campbell and Madden [26].
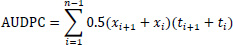 |
where n is the total number of assessments; ti is the time of the ith assessment in days from the first assessment date, xi is the percentage disease incidence at ith assessment date.
2.4.2. Growth and Yield Parameters
Data on plant height (PH), number of tillers per plant (NTPP), rhizome width (RW), rhizome length (RL), and number of fingers per rhizome (NFPR) were recorded based on their standard measurements from ten randomly selected plants per plot, and their means were considered for statistical analysis. Rhizome yield obtained from each treatment was recorded from the two central rows of each plot. Then the yield was measured as kilogram per plot (kg plot-1) and converted into kilogram per hectare (kg ha-1) using the following formula [27].
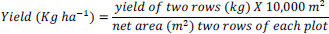 |
2.4.3. Relative Yield Loss and Yield Increase
The relative yield loss of ginger due to bacterial wilt disease was calculated as a percentage yield reduction of controlled plots compared with the most protected plot using the formula of Cerda et al [28].
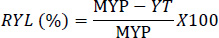 |
where, RYL (%) is the relative yield loss in percentage, MYP is the maximum yield of the protected plot, and YT is the relative yield of other treatments.
The yield increase of ginger due to the integrated application of different disease management methods was calculated as
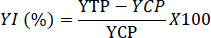 |
where YI (%) is the yield increase in percentage, YTP is the yield of the treated plot, YCP is the yield of the control plot.
2.4.4. Cost-benefit Assessment
A cost-benefit of each treatment was performed using partial budget analysis, and the marginal rate of return was computed by considering the variable cost available in the respective treatment combination [29]. Variable costs include the input and labor costs during the experimental period. The labor cost was 60 ETB man per day. The total gross benefit of the study was calculated from the rhizome yield, considering the local market price (70 ETB kg-1) of the Bench-Sheko Zone. Total input costs were obtained from the summation of the total cost that varied and fixed costs of the products used. The yield and economic data were calculated to compare the advantages of controlling bacterial wilt through the integrated application of different treatment combinations. The marginal rate of return provides the value of the benefits obtained per the amount of additional cost incurred, expressed as a percentage.
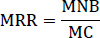 |
where MRR is the marginal rate of returns, MNB is the marginal net benefit compared with the control, and MC is the marginal cost compared with the control.
2.5. Data Analysis
Data on disease incidence, AUDPC, yield, and yield components were subjected to analysis of variance (ANOVA) using R (http://www.R-project.org). Treatment means were compared by the Fisher-pare wise comparison test and the least significant differences (LSD) were inspected at 5% probability. Through correlation analysis, associations between disease parameters and ginger yield and yield components were evaluated. A linear regression model was used to indicate the relationship between rhizome yield loss and disease incidence. The yield loss of ginger was predicted by plotting the rhizome yield data against the final wilt incidence. Regression and correlation were computed using Minitab 14 statistical package (Release 18.0 for Windows®, 2007).
3. RESULTS
3.1. Disease Incidence
The first disease initiation on ginger plants was noted 45 days after planting, and it was confirmed to be bacterial wilt incited by the pathogen R.S. The result from ANOVA showed that disease incidence was highly significantly (P < 0.001) affected by the management methods in all disease assessment dates (DAD). The highest (28.3 and 95.5%) disease incidence was recorded from the control plots at the initial and final DAD, respectively (Table 2). On the contrary, the lowest (1.1 and 35.0%) disease incidence was recorded at the initial and final DAD through the integrated application of T4 (Mancozeb for rhizome soaking and soil drenching, bio-fumigation using lemongrass, along with soil-solarization), respectively. It was also evident that the use of Mancozeb for rhizome socking and soil drenching along with bio-fumigation (T3) gave the second lowest (47.22) PDI in the final DAD. However, the mean PDI was not statistically different in the plots treated with bleaching powder and bio-fumigation with (T6) or without (T5) soil-solarization.
3.2. Area Under Disease Progress Curve
The result of the analysis of variance revealed that the AUDPC was highly significantly (P < 0.001) different among treatments. The highest AUDPC, 1429.17%-days, was recorded in the controlled plots, whereas the lowest (512.5%-days) was from the plots of T4 (seed socking and soil drenching with Mancozeb, bio-fumigation along with soil-solarization), followed by 704.17%-days in T3 (seed socking and soil drenching with Mancozeb along with bio-fumigation using lemongrass), which resulted in a 64.1% and 50.7% reduction of AUDPC over the controlled plots, respectively (Table 2). Similarly, 40.5 and 34.9% of reductions in the AUDPC were recorded through the application of T5 and T6, respectively, over the controlled plots.
3.3. Growth Parameters
The effect of integrating disease management methods was highly significantly (P< 0.001) varied on the plant height (PH), and the number of tillers per plant (NTPP). The highest (71.7 cm) PH was recorded from treatment T6 (rhizome seed and soil treatment with bleaching powder plus bio-fumigation along with soil-solarization) in comparison to the controlled plots (Table 3). Maximum (10.86) NTPP was produced by ginger plants treated with the combined application of Mancozeb for rhizome treatment and soil drenching plus bio-fumigation along with soil-solarization in T4 followed by 10.5 NTPP, from the integrated application of T3 (seed socking and soil drenching with Mancozeb along with bio-fumigation using lemongrass). However, regardless of T1 (hot-water and bio-fumigation) and the controlled plots, the rest of the treatments showed no significant (P < 0.05) difference in the PH. This holds true for the mean number of tillers produced per plant among T4, T3, T5, and T6.
3.4. Yield and Yield Components
Ginger yield and yield components were highly significantly (P < 0.001) affected by the treatment combinations used for managing bacterial wilt disease. The highest rhizome yield (15.09 t ha-1) was harvested from T4 with a maximum (9.7) NRF and a large rhizome size of 9.9 cm in length and 3.74 cm in width (Table 3). On the contrary, the lowest yield of 5.00 t ha-1, and a minimum (4.5) NRF, with a small rhizome size, were obtained from the untreated plots. Seed socking and soil drenching with Mancozeb along with bio-fumigation (T3); and treating rhizomes and the soil with bleaching powder plus bio-fumigation (T5) resulted in 13.0 and 12.18 t ha-1 rhizome yield along with 8.36 and 7.83 rhizome fingers, respectively.
Table 2. Incidence (%) of bacterial wilt disease at the initial and final disease assessment dates as affected by integrated management methods in 2019, southwest Ethiopia.
| Treatment | Disease Incidence (%) | AUDPC (% day-1) |
|
|---|---|---|---|
| IDAD | FDAD | ||
| T1 | 18.33b | 84.44b | 1245.83b |
| T2 | 15.00bc | 79.44b | 1191.67b |
| T3 | 10.56c | 47.22d | 704.17d |
| T4 | 1.11d | 35.00e | 512.50e |
| T5 | 9.44c | 58.33c | 850.00c |
| T6 | 11.67bc | 62.22c | 929.17c |
| T7 | 28.33a | 95.56a | 1429.17a |
| CV (%) | 31.01 | 7.89 | 8.23 |
| LSD (0.05) | 7.44 | 9.27 | 143.61 |
| Treatment | Growth Parameters | Yield Parameters | ||||
|---|---|---|---|---|---|---|
| PH | NTPP | RL | RW | NRF | RY (t ha-1) | |
| T1 | 60.33b | 8.76c | 7.60b | 2.90c | 5.43e | 9.29d |
| T2 | 69.60a | 9.23c | 7.93b | 3.27b | 6.26d | 9.75d |
| T3 | 70.83a | 10.50ab | 9.46a | 3.60a | 7.83bc | 12.18bc |
| T4 | 71.66a | 10.86a | 9.90 a | 3.74a | 9.70a | 15.09a |
| T5 | 70.53a | 10.26ab | 9.23a | 3.54a | 8.36b | 13.01b |
| T6 | 71.73a | 9.93b | 9.66a | 3.67a | 7.43c | 11.56c |
| T7 | 53.53c | 7.03d | 5.97c | 2.72c | 4.50f | 5.00e |
| CV (%) | 2.39 | 3.95 | 4.52 | 5.20 | 6.08 | 5.84 |
| LSD (0.05) | 2.85 | 0.66 | 0.68 | 0.31 | 0.76 | 1.12 |
| Treatment | Relative Yield Loss (%) | Yield Increase (%) |
|---|---|---|
| T1 | 38.39 | 46.18 |
| T2 | 35.40 | 48.72 |
| T3 | 19.24 | 58.95 |
| T4 | 0.00 | 66.87 |
| T5 | 13.75 | 61.57 |
| T6 | 23.37 | 56.75 |
| T7 | 66.86 | 0.00 |
3.5. Relative Yield Loss and Yield Increase
The yield loss assessment of each treatment was calculated relative to the rhizome yield obtained from the maximum protected plots. Percentage rhizome yield increase was also calculated from all treatments in comparison to untreated plots (Table 4). Based on these, the highest (66.86%) relative yield loss was recorded from untreated plots. In addition, a sizeable amount of 38.39 and 35.4% yield loss, was occurred under hot-water treatment of rhizome and bio-fumigation (T1) and hot-water treatment of rhizome plus bio-fumigation and soil-solarization (T2).
It was also evident that rhizome yield response was varied due to the application of diverse treatment combinations. The highest (66.87%) yield increase was obtained by integrating Mancozeb as rhizome treatment and soil drenching plus bio-fumigation along with soil-solarization in T4, followed by a 61.57% of yield increase through the application of bleaching powder for rhizome and soil treatment along with bio-fumigation in T5. In addition, a considerable rhizome yield increase was also recorded through the application of T3 and T6. This indicates that integrating more management methods is indeed essential for the reduction of ginger bacterial wilt and the resultant yield loss.
3.6. Correlation Coefficients of Disease, Yield, and Yield Parameters
Correlation analysis was done to evaluate the association of disease, yield, and yield parameters and to quantify the magnitude of disease influence on the response of the yield and yield components of ginger. The result of correlation analysis showed highly significant (P< 0.001) associations between and within the disease, yield, and yield parameters (Table 5). The correlation coefficient (r = 0.97**) disclosed a strong positive association between the final percentage of disease incidence (PDIf) and AUDPC. The PDIf was highly significantly (P< 0.001) and negatively associated (r = -0.96** and r = -0.98**) with the number of rhizome fingers and rhizome yield obtained ha-1, respectively. Similarly, AUDPC was highly significantly (P< 0.001) and negatively associated (r = -0.94** and r = -0.97**) with the rhizome yield and rhizome fingers, respectively. The yield and yield components of ginger also showed highly significant correlations. Rhizome yield was strongly positively associated with the rhizome finger plant-1 and the rhizome size.
3.7. Regression between Disease Incidence and Rhizome Yield
The yield was predicted from linear regression of disease incidence data. For the estimation of the yield loss due to bacterial wilt disease, the PDIf data was considered as an independent variable and ginger yield as a dependent variable (Fig. 1). The result of the regression model, y =19.92 - 0.1378x, showed that, for every one-unit increase in PDIf, there was a corresponding rhizome yield loss of 0.1378 t ha-1 or 138 kg ha-1 in the experiment. In the study of the association between the disease incidence and yield loss from the model, it was also confirmed that about 83.4% of ginger yield loss was associated with bacterial wilt disease.
| Parameters | PDIf | AUDPC | RL | RW | NRF | RY |
|---|---|---|---|---|---|---|
| PDIf | 1 | - | - | - | - | - |
| AUDPC | 0.97** | 1 | - | - | - | - |
| RL | -0.97** | -0.92** | 1 | - | - | - |
| RW | -0.94** | -0.91** | 0.97** | 1 | - | - |
| NRF | -0.96** | -0.97** | 0.92** | 0.93** | 1 | - |
| RY | -0.98** | -0.94** | 0.95** | 0.91** | 0.96** | 1 |
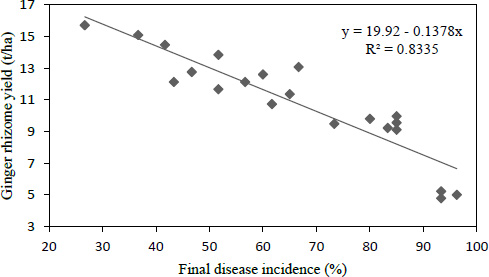 |
Fig. (1). Liner regression indicating the relationship between ginger yield (t ha -1 ) and final disease incidence. |
| Treatment | ARY | GP kg-1 | GB ha-1 | TIC | MC | NB | MNB | MRR |
|---|---|---|---|---|---|---|---|---|
| T1 | 8361 | 70 | 585,270 | 10244 | 6744 | 575025 | 263525 | 3907.3 |
| T2 | 8775 | 70 | 614,250 | 11223 | 7723 | 603026 | 291526 | 3774.5 |
| T3 | 10962 | 70 | 767,340 | 12684 | 9184 | 754655 | 443155 | 4825.1 |
| T4 | 13581 | 70 | 950,670 | 13663 | 10163 | 937006 | 625506 | 6154.4 |
| T5 | 11709 | 70 | 819,630 | 10944 | 7444 | 808685 | 497185 | 6678.7 |
| T6 | 10404 | 70 | 728,280 | 11923 | 8423 | 716356 | 404856 | 4806.2 |
| T7 | 4500 | 70 | 315,000 | 3500 | 0.00 | 311500 | 0.00 | 0.00 |
3.8. Cost-benefit Analysis
The result of the cost-benefit analysis showed an increase in the net benefit and yield of ginger due to the application of different treatment combinations than the controlled plots (Table 6). The lowest (311,500 ETB ha-1) net benefit was calculated from the controlled plots, while the highest (937,006 ETB ha-1) was from the integrated application of Mancozeb for seed and soil treatment plus bio-fumigation, and soil-solarization (T4) followed by 808,685 ETB from (T5). Similarly, the highest marginal net benefit (625,506 ETB ha-1) was obtained from T4, followed by 497,185 ETB ha-1 from the application of bleaching powder as seed and soil treatment, along with bio-fumigation (T5). In terms of the marginal rate of return, which is usually used for comparison of the costs that vary with the net benefits of all treatments, it was evident that the highest (6678.7%) marginal rate of return was obtained from T5, trailed by (6154.4%) from T4.
4. DISCUSSION
The results of the current study indicated that bacterial wilt was highly significantly affected by integrating different management methods. However, the disease was kept to a minimum by the treatments, which comprised Mancozeb or bleaching powder in comparison to the rest of the treatments and control. Application of T4 resulted in a minimum (35.0%) of disease incidence and (512.5 unit-day-1) of AUDPC in comparison to the maximum (95.5% and 1429.1 unit-day-1) of incidence and AUDPC in the controlled plots. This might be attributed to the combined effect of rhizome and soil treatment with Mancozeb, bio-fumigation using lemon grass, and soil-solarization with plastic sheet against the pathogen carried by the seed and soil environment.
Since R.S. is a seed and soil-borne pathogen, chemical treatment of rhizomes and infected soils is the primary method of preventing disease occurrence. Mancozeb has been used for seed and soil treatments to control ginger rhizome rot complex diseases [30]. Despite the lack of evidence for its efficacy in controlling R.S., Bandyopadhyay and Khalko [15] found that the integrated application of Mancozeb for rhizome treatment and cabbage as a soil bio-fumigant reduced the incidence of bacterial wilt by 73.2%. Similarly, R.S. showed a negative reaction in microplates incubated under the pressure of Mancozeb. Furthermore, pre-planting soil drenching with Mancozeb reduced the severity of tobacco bacterial wilt by 94.96% [16].
The combined application of bleaching powder as a seed and soil treatment with (T6) or without soil-solarization (T5) considerably reduced wilt incidence and AUDPC in this experiment. Nelson [25] reported the effectiveness of treating rhizome seed with a bleaching powder solution to sterilize and eradicate R.S. on the surface of ginger rhizome, which is consistent with our findings. Similarly, Singh et al. [31] reported reduction of tomato bacterial wilt by 51.2% when bleaching powder was used in conjunction with other management strategies. Application of bleaching powder to soil raises the pH level of acidic soil, where the growth and multiplication of R.S. promoted. As a result, it inhibits bacterial development and reduces the incidence of wilting disease.
Bio-fumigation is the most frequently used biological method of disinfecting soil using volatile chemicals released from plant residues [32]. Brassicaceae crops are commonly used to control many soil-borne pathogens such as R.S [33]. A powerful biocidal compound (isothiocyanate) is released into the soil environment when the tissue of such plants is damaged and quickly incorporated into the soil prior to planting. Consequently, these volatile substances with varying antimicrobial activity may either directly affect the viability and survival of the pathogen or be involved in inducing systemic resistance [2]. A report by Guji et al. [19] showed that combining lemongrass, fertilizer, and soil-solarization reduced the incidence of ginger bacterial wilt by 42.0%. Paret et al. [20] also demonstrated antibacterial effects and a reduction of ginger bacterial wilt incidence through essential oils extracted from palmarosa and eucalyptus. However, Panth et al. [18] highlighted the limitations of the sole application of bio-fumigation to control soil-borne pathogens. This was in line with our results in treatments comprising hot-water and bio-fumigation, which resulted in the highest mean final PDI and AUDPC next to the controlled treatment.
Soil-solarization is an environmentally friendly physical method of trapping solar energy using a plastic sheet for the control of soil-borne pathogens [21]. In our study, regardless of T4 and T3, the mean final PDI and AUDPC of all treatments were not statistically different. This could be attributed to the fact that the temperature created by solarization was sub-lethal to the pathogen inhabited in the deeper layers of the soil, which hardly reduced the disease [34]. It may, however, have an indirect effect on the pathogen via hindering the leakage of toxic volatiles released from damaged lemongrass tissue during the bio-fumigation process [35]. Soil-solarization, along with bio-fumigation using chicken manure, was reported to improve the plant health index and the yield [22]. The beneficial effect of soil-solarization for 60 days prior to planting together with soil amendment with nitrogen and CaO has been demonstrated by Vinh et al. [14], who reported a 20% bacterial wilt disease incidence reduction in the tomato.
The current findings revealed that ginger growth and yield parameters were notably promoted by the management practices imposed. Plant height, the number of tillers, rhizome length, and width were comparatively increased under integrated management in comparison with control. This indicates the benefits of integrating management tools to control bacterial wilt disease for ginger to grow to the best of its potential. Application of T4 (Mancozeb, bio-fumigation and soil-solarization) and T5 (bleaching powder and bio-fumigation) gave the maximum number of rhizome fingers and increased the rhizome yield by 66.87 and 61.57%, respectively. The increased rhizome fingers and yield advantages might be attributed to the benefits of soil drenching with Mancozeb in 15-day intervals in disinfecting the sphere of soil around the seed and reducing disease occurrence, which was detrimental to the healthy growth and yield of ginger.
In par with our findings, Ghosh and Mandal [17] reported that integrating Mancozeb along with other disease management measures significantly reduced bacterial wilt and resulted in the highest potato yield. Likewise, Mancozeb was suggested as a potential protectant for field management of tobacco bacterial wilt [16]. Furthermore, due to its bactericidal effect on the R.S. population in the soil, the integrated application of bleaching powder may have benefited the healthy growth and yield increase by reducing the disease. In par with our findings, Sharma and Kumar [36] reported a significant amount of disease reduction and yield increase of 123.4% by integrating karanj cake along with bleaching powder for the management of tomato bacterial wilt disease.
Aside from the bio-fumigation benefit of lemongrass, its decomposition in the soil could return a significant amount of organic matter to the soil profile and improve soil properties, both of which can benefit plant development and productivity [37]. Furthermore, applying solarization after soil bio-fumigation with lemongrass could speed up the decomposition process and improve soil properties, which could benefit ginger growth and yield. The benefits of integrating bio-fumigation and soil-solarization to reduce bacterial wilt and improve crop yield have already been demonstrated by [19, 22, 23].
The findings of this study also confirmed the presence of highly and negatively significant (P < 0.001) correlation and association between wilt incidence and growth and yield parameters. This could assert the negative effects of bacterial wilt on the growth, yield, and yield component of ginger during the cropping season. According to the regression model y = 19.92-0.1378x, for every one-unit increase in wilt incidence (percent), there was a corresponding 0.1378 t ha-1 ginger loss during the study period. It was also verified that bacterial wilt disease was responsible for 83.4% of ginger loss in southwest Ethiopia. This complies with the findings of Ghosh et al. [38] who found a strong negative correlation between bacterial wilt disease and the yield of brinjal.
CONCLUSION
The present experimental findings evident that an integrated use of Mancozeb for seed socking and soil-drenching, soil bio-fumigation, along with soil-solarization considerably reduced bacterial wilt disease and associated yield loss of ginger in comparison to the rest of the treatments and control. Thus, it can be used as an integrated management system for ginger bacterial wilt disease. The application of bleaching powder as rhizome seed and soil treatment, along with soil bio-fumigation, was also the second-best treatment combination in terms of disease control and reduction of yield loss. Furthermore, it provided the maximum monetary benefit (MRR) of any treatment combination tested in the experiment. Therefore, it can be utilized as an alternative approach to managing bacterial wilt disease. Nonetheless, since this experiment lasted only one year, more experimental research integrating cultural, physical, host-resistance, biological, and chemical methods should be conducted in different agro-ecologies and years to develop an effective and sustainable management method of ginger bacterial wilt disease.
LIST OF ABBREVIATIONS
| RCBD | = Randomized Complete Block Design |
| PDI | = Percentage Disease Incidence |
| AUDPC | = Area Under The Disease Progress Curve |
RESEARCH INVOLVING PLANTS
The reported experiment was in accordance with the United Nations (UN, 1992) convention on biological diversity.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This experiment did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The author would like to thank Mizan-Tepi University for financing the study. Special thanks also go to farmers and individuals involved in the field preparation and data collection.








