All published articles of this journal are available on ScienceDirect.
Apoptosis and Remodeling in Ovary of Water Deer and Sika Deer at Pregnant and Non-pregnant Stages
Abstract
Background:
Many studies have been conducted on the sika deer, an extinct species in Korea, to analyze the physiological characteristics of restoration and reproductive physiological characteristics. The reproductive physiological mechanisms of water deer and sika deer, especially the function, morphological changes, and ovarian characteristics, are unknown.
Objective:
We aimed to study the differences in the reproductive physiology of water deer and sika deer and determine the difference in function through ovarian morphological analysis and cell remodeling.
Methods:
Water deer and sika deer ovaries were collected during the estrus and pregnancy seasons from the Korean Peninsula and Russia–Korean Peninsula border, respectively, and analyzed. Morphological analysis and in situ zymography were conducted to confirm the activity of matrix metalloproteinases (MMPs), analyze the immunofluorescence of Casp-3 protein, and assess the morphological changes in the ovaries.
Results:
The results of the analysis confirmed the ovaries of water deer and sika deer to be morphologically different. The corpus luteum of sika deer showed large differences in size and morphology compared to water deer, and many changes were also observed in the corpus luteum cells. However, the activity of MMPs and apoptosis in the follicles of sika deer were higher than those of water deer. Water deer and sika deer showed similar corpus luteum patterns during estrus and pregnancy.
Conclusion:
In our study, morphological differences were found to be present between water deer and sika deer ovaries; however, cell remodeling demonstrated a similar pattern. Therefore, the endocrine function and reproductive efficiency for reproduction would be similar.
1. INTRODUCTION
Successful development of follicles is essential for offspring production and pregnancy maintenance. In particular, the body is remodeled through the production of estrogen and progesterone, and the feedback mechanism can be controlled [1-3]. As a result, the ovary remodels numerous cells as it undergoes estrus and pregnancy, and the three important cells in remodeling the ovary are activated. The ovarian cells are composed of germ cells and somatic cells, such as granulosa and theca cells. After ovulation, these cells are remodeled to form the corpus luteum [4, 5]. Ovarian function involves the mechanism of apoptosis, activation of extracellular matrix (ECM) by matrix metalloproteinases (MMPs), and apoptosis by Casp-3 [6, 7]. This provides the functional normality of the ovary, induces estrus and pregnancy at the correct time, and allows it to perform normal physiological functions. In other words, all mammals generally produce offspring for action, and if a problem arises through a sophisticated mechanism, it may lead to various diseases, leading to infertility. However, it is a seasonal breeding animal, and wild animals have some differences; the development of follicles and time of corpus luteum formation is considered to be different from Artiodactyla (e.g., domesticated cattle), which remodels cells by correct control of hormones, especially in the case of water deer and sika deer [8-11].
Water deer have a similar habit to sika deer during estrus and pregnancy. In sika deer, the development of follicles in the estrus goes through at least 5 to 8 cycles, where ‘silent ovulations’ occur, similar to those experienced in monovulatory species [12-14]. In particular, the ovaries of water deer and sika deer have a flat surface, and it is challenging to observe protruding follicles compared to the ovaries of sheep [15, 16] or goats [17], which have irregularities on the surface due to the protrusion of the follicle and corpus luteum [8, 18]. In addition, the estrus cycle patterns of water deer and sika deer are very similar, and it is known that ovulation occurs in about five to eight cycles in estrus, which can be seen as having a rapid change in the state of the ovary [19, 20]. According to a study by Kim et al. [4], the action of MMPs in remodeling ovarian cells in estrus and pregnancy is different from that of domesticated Artiodactyla, suggesting that this pattern is similar to breeding vulnerable species by increasing apoptosis in pregnancy. In other words, when deer and water deer exist as a herd during the breeding season, 'silent ovulations' occur for about 8 to 10 days, indicating that ovulation occurs without showing any clear signs of estrus similar to those observed in monovulatory species. The reorganization of the corpus luteum is different from that of general Artiodactyla animals, and discontinuous follicular growth and regression occur during estrus, resulting in the formation of two to four dominant follicles [5, 21, 22]. However, the association between cell remodeling and functional performance of the corpus luteum and follicles of water deer and sika deer, as well as reproductive physiology data for the restoration of endangered (water deer) and extinct (sika deer) animals are unknown. Therefore, this study aimed to determine whether there is a difference in the development of irregular follicles and corpus luteum on ovarian tissue remodeling in estrus and pregnancy, under the assumption that the functional composition of the ovaries of water deer and sika deer is similar.
2. MATERIALS AND METHODS
2.1. Ovary of Water Deer and Sika Deer
To examine the ovaries of the endangered water deer and sika deer, an extinct species in the Korean peninsula, we captured water deer from Ansan, Gyeonggi-do, Korea, in accordance with Article 19, paragraph 1 of the Wildlife Protection and Management Act and under the guidance of the Institutional Animal Management and Use Committee of the Wildlife Conservation and Research Center (Capture Permit No. 2013-1) from November to January. Water deer with completed placenta formation were captured from March to May. The captured sika deer individuals were presumed to be Korean Peninsula deer living in Primorskaya State Agricultural Academy and the border of Primorskaya and North Korea, with ovaries evolved at a similar time to water deer. In addition, estrus and pregnancy were divided (in the previous study, Primorskaya State Agricultural Academy signed an MOU (dated 11.25.2014)). All ovarian tissues were collected from 5 animals, each in water deer and sika deer groups.
2.2. Hematoxylin and Eosin
The ovaries of water and sika deer were stored at -80 °C, thawed for use in the experiment, and fixed in 70% ethanol alcohol with 0.2% diethyl pyrocarbonate for 24 h. After that, dehydration was conducted for 15 min twice in the order of 70%, 95%, and 100% ethanol alcohol, and xylene, and then placed in molten paraffin at 60 °C to induce paraffin penetration twice for 2 h, and then hardened for 24 h at room temperature and stored at 4 °C until used in the experiment. In the hematoxylin and eosin (H&E) staining for morphological analysis, paraffin blocks were cut into 10 μm to make a slide, and a representative section of each ovary was randomly selected and routinely stained with H&E for histological examination under an optical microscope (×40).
2.3. In situ Zymography
To perform in situ zymography experiments, the tissues were first deparaffinized and hydrated twice for 10 min in xylene, 100% ethanol, and 95% ethanol, washed in ddW for 5 min, and boiled in 10 mM sodium citrate for 10 min. After that, they were rubbed on ice for 20 times and then washed in ddW 3 times for 5 minutes, and emulsion (ddW, 10% SDS, 2% glycerol) and zymography reaction buffer (1 M Tris-HCL, 5 M NaCl, 1 M CaCl2, 0.2 mM ZnCl2, 0.2 mM ZnCl2, 0.2% Triton X-100, 0.02% NaN3 in 1×PBS; pH 7.5) in a 1:2 ratio, mounted on slides, and stored in a slide box filled with 1M Tris for 48 hours at 37 °C. After completion of the reaction, routine H&E staining was performed for histological examination using an optical microscope [23].
2.4. Immunofluorescence
Paraffin blocks of ovaries tissues from each group were sectioned to 5 μm thickness. Tissue slides were prepared following deparaffinization and hydration using xylene and ethanol and permeabilization at -20 °C with 0.1% Triton X-100 in 1× PBS (PBS-T). The samples were blocked at room temperature (RT) for 30 min in TPBS (1× PBS with 0.01% Tween-20) containing 5% normal horse serum (NHS) and 1% normal goat serum (NGS). All tissue slides were incubated in a dark room at RT for 1 h, with primary antibody Casp-3 (ab4051; Abcam, Cambridge, UK) diluted to 1:200 in blocking solution. The cells were incubated in the dark with a blocking solution for 30 min to induce secondary antibody conjugation. Subsequently, all slides were incubated with Alexa 594-conjugated anti-rabbit secondary antibody (ab150080; Abcam) diluted to 1:300 in blocking solution in a dark room at RT for 1 h. The secondary antibody solution was decanted and washed thrice with PBS for 5 min each in the dark. The Image J program (Ver. 1.53t; National Institutes of Health, USA) was used to compare and analyze the detection level of the target protein by immunofluorescence analysis.
3. RESULTS
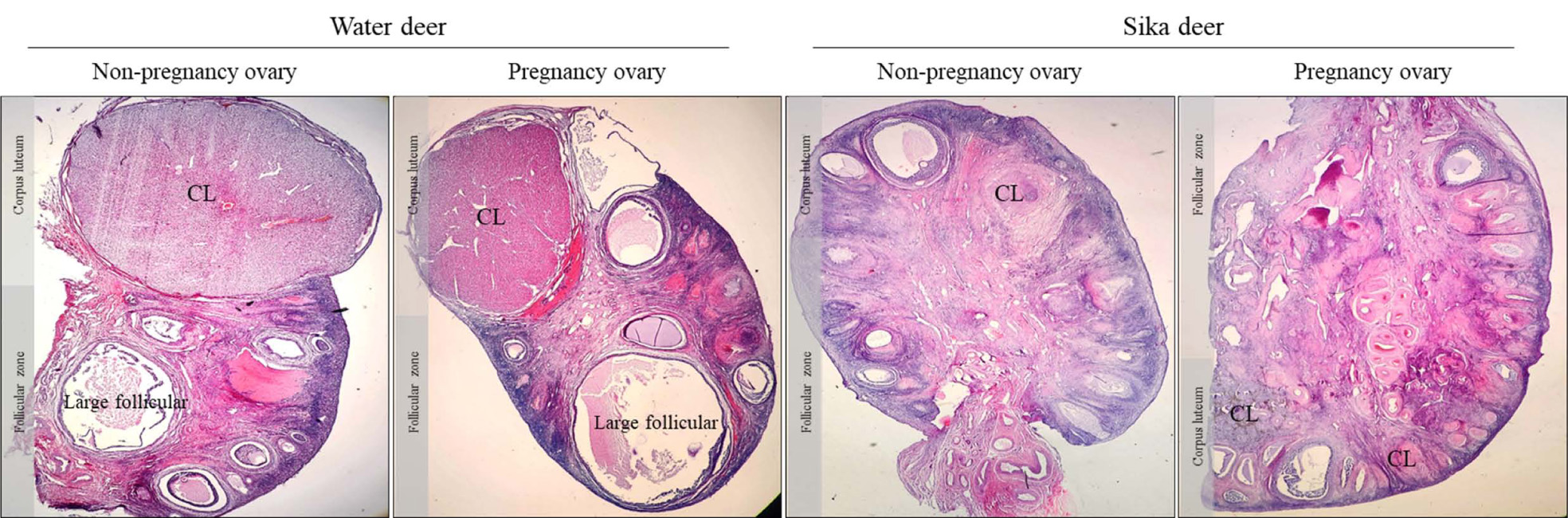
3.1. Analysis of Morphological differences between Water Deer and Sika Deer Ovary
Morphological differences in the ovaries of water deer and sika deer during estrus and pregnancy are shown in Fig. (1). Both water and sika deer were found to have morphologically similar estrus and pregnancy, and observing changes over time was challenging. However, various other differences between water deer and sika deer were documented. The water deer had larger follicular and corpus luteum, and developmental follicles were present. The distribution of stroma cells in the ovary was lower than that of sika deer, and the development of theca cells around the follicle was high. However, it was difficult to observe the correct corpus luteum; we observed that it formed as a resistant corpus luteum rather than an externally protruding corpus luteum in sika deer. In addition, the follicles of sika deer did not exhibit large follicles but had early follicular development. The distribution of stromal cells constituting the entire ovary was very wide, and the development of granulosa cells and theca cells in the follicle was high. In addition, in terms of morphology and cell composition, the corpus luteum of water deer showed normal large or small lutein cells, but that of sika deer had a slightly modified corpus luteum and differences in cell shape.
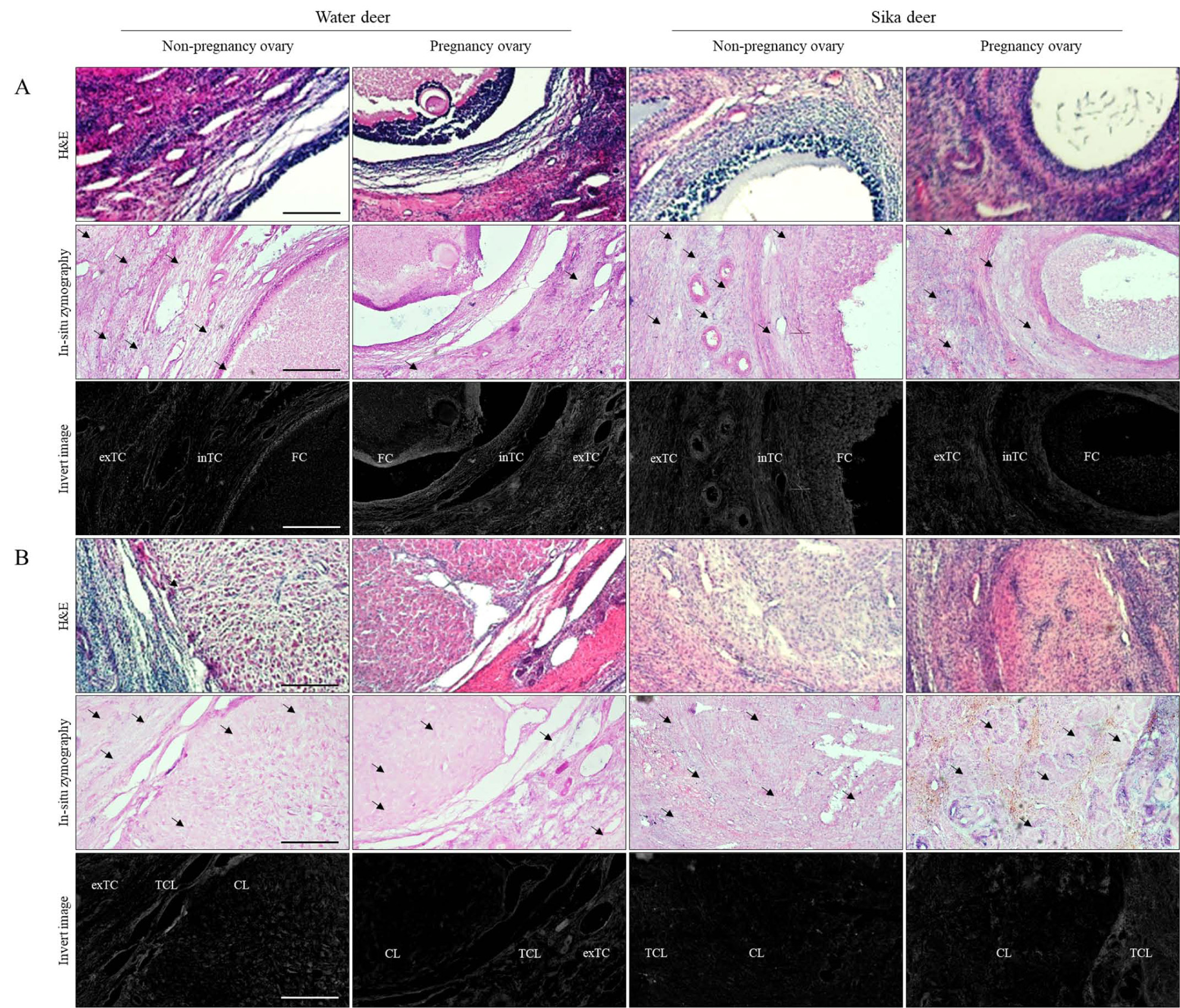
3.2. Cytoplasmic Remodeling in Follicles and Corpus Luteum in Water Deer and Sika Deer
Zymography results, which determine morphological changes in the activity of MMPs in follicles and the corpus luteum during estrus and pregnancy, showed differences between each cycle and individual. MMPs activity in follicular development was activated in the external and internal theca cells in non-pregnant water deer, whereas MMP activity during pregnancy was low. Unlike water deer, sika deer’s MMPs showed low activity in non-pregnancy follicles and very high activity in intrathecal cells during pregnancy, and some extra theca cell sections were found to be slightly lower than water deer (Fig. 2). Overall, the activity of MMPs in sika deer was much higher than in water deer in the corpus luteum, especially in corpus luteum derived from granulosa cells. In water deer, MMPs activity was low in the entire theca cells and corpus luteum in estrus, but they were highly active in the entire corpus luteum section during pregnancy. MMPs activity of sika deer was similar to the difference in water deer follicles, with high activation in the corpus luteum on estrus and low activation in the corpus luteum during pregnancy.
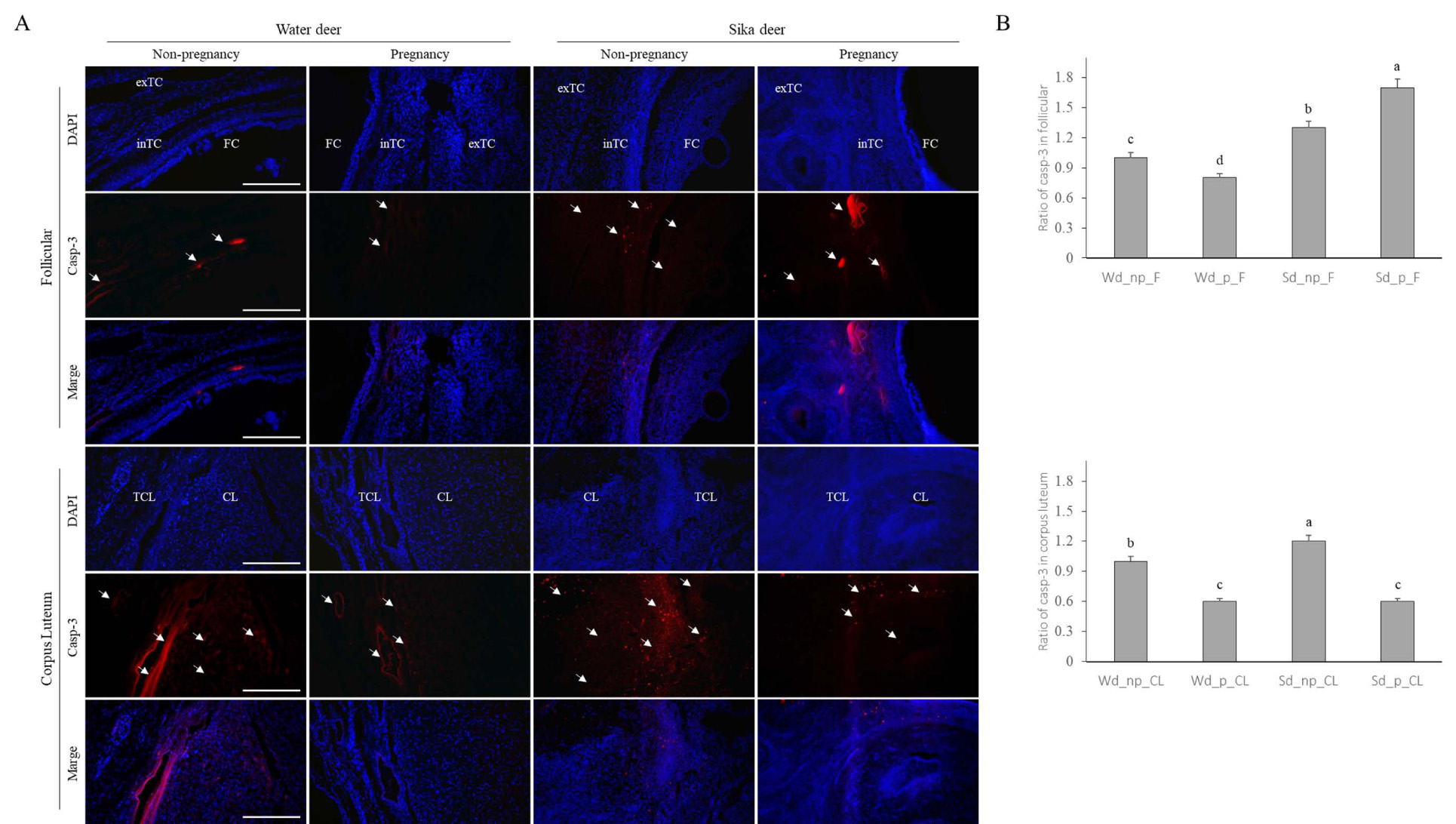
3.3. Detection of Casp-3, Apoptosis Factor in the Follicles, and Corpus Luteum of Water Deer and Sika Deer
The results of immunofluorescence analysis to determine the effect of tissue remodeling and cell death on the detection of Casp-3 protein and apoptosis factor in the ovary are shown in Fig. (3). First, the detection of Casp-3 in follicular development was higher in sika deer than in water deer and was generally detected in all theca cells, especially in the intrathecal cells of the follicular cells during pregnancy. Analysis of MMPs in the corpus luteum revealed that the water deer differed from the zymography pattern. The detection of Casp-3 was higher in the estrous corpus luteum of water deer than in the pregnancy corpus luteum, especially in the theca corpus luteum. However, the overall detection rate was low during pregnancy. Similar to water deer, the detection of Casp-3 in sika deer on estrus corpus luteum was very high, and the detection in corpus luteum, including theca corpus luteum, was much higher than that during pregnancy (Fig. 3A).
Overall, comparing water deer and sika deer, the detection of Casp-3 in follicles was generally higher in sika deer than in water deer, and it increased significantly during pregnancy. The detection of Casp-3 in the corpus luteum showed similar patterns, but the detection in the corpus luteum of sika deer was higher than that in water deer (Fig. 3B).
4. DISCUSSION
Morphological changes in the ovary affect reproductive physiological functions and are important in determining the normal estrous cycle through endocrine action [4, 24]. In particular, the formation of the corpus luteum after ovulation is an important organ that produces progesterone for pregnancy maintenance and tissue remodeling during estrus relapse. It also affects the physiology of individual reproduction and provides successful hormonal feedback [25, 26]. However, in some seasonal animals, the development and regression of follicles occur rapidly during estrus and pregnancy, and silent ovulation is sometimes formed, which leads to unstable reproductive physiology [14]. That is, the morphological changes of the ovaries during estrus and pregnancy show a different pattern from that of domesticated cattle; in particular, it is different from the function of the cattle corpus luteum during estrus and pregnancy [5, 8, 9].
In this study, the morphological changes in the ovaries of the endangered or huntable water deer in Korea and the Korean Peninsula-type sika deer near Russia were analyzed for their differences to determine the developmental changes occurring through the cloning of the sika deer in the future. Further, there were many differences observed in the morphological changes in the ovaries of water and sika deer. These two species have a similar reproductive cycle and are known to undergo estrus at the same time in the herd, and the sexual cycle, according to the development of follicles, is known to be five to eight times during the breeding season [16, 17]. However, in a comparative analysis of ovaries, the formation of at least one giant follicle and the development of several developmental follicles increased despite the formation of the corpus luteum [9]. Unlike in water deer, giant follicles in sika deer did not exist, but developmental follicles appeared to have developed. As shown in the study by Pérez et al. [8], the estrus ovary forms a smaller follicle and corpus luteum than those observed in other small ruminants, and it seems that it undergoes a different morphological change from that of the water deer in our study. The formation and regression of follicles depend on the presence of the corpus luteum, and follicle development is inhibited by sufficiently non-affecting follicle-stimulating hormone (FSH) because the secretion of progesterone causes the functional decline of FSH and maintains the corpus luteum by maintaining high levels of luteinizing hormone (LH) [27, 28]. Therefore, it is thought that the activity of MMPs remodeling the ovary is very low in the theca cell zone, in which the cells surround the follicle [29]. However, despite the development of the estrus corpus luteum, it was confirmed that MMPs were active in the extra- and intra-theca cells, and apoptosis was rarely observed. However, the activity of MMPs around follicles during pregnancy is reduced, and the morphological changes in the follicle appear to decrease.
Unlike water deer, MMPs were activated in the theca cells of follicles during pregnancy rather than during estrus, and apoptosis was activated in follicles during estrus and pregnancy. This seems to have formed a functional change in the ovary to rapidly recover the return of estrus function according to the possibility of unstable pregnancy in wild water deer and sika deer. These results are thought to affect the inconsistent steroid hormone secretion in wild sika deer species during pregnancy [30]. In particular, compared to water deer, pregnancy follicles of sika deer have a very high level of apoptosis in the intrathecal cell section, similar to cattle, and tissue remodeling also seems to act rapidly [4, 9]. In the results of corpus luteum remodeling, water deer and sika deer showed similar results, unlike the development of ovaries, which is different from the corpus luteum composition of domesticated cattle. The corpus luteum secretes very important hormones for the possibility and maintenance of pregnancy, and the action of major hormones changes according to the formation of the corpus luteum, leading to morphological changes in the uterus and ovaries. Although the secretion of hormones is important in maintaining the corpus luteum shape, in the direct development and disassembly of the corpus luteum, the corpus luteum is reconstituted according to the degree of activity of MMPs and apoptosis, and pregnancy can be determined [31]. However, apoptosis in theca lutein cells and corpus lutein cells in the corpus luteum during estrus is rapidly increased compared to that in pregnancy, whereas MMP activity appears to be predominant in the large luteum cell zone of the corpus luteum during pregnancy. The action of MMPs does not increase apoptosis at a certain level, unlike the results of Kim et al. [5]. These results showed a similar pattern for sika deer. The corpus luteum of sika deer was found to be much smaller than that of water deer, and it was difficult to observe the general cell shape of the corpus luteum. Contrary to our expectation, cell remodeling in sika deer was confirmed to be similar to that in water deer despite abnormal luteal cells, and the results showed ovary function to be the same in water deer and sika deer. In studies on ovarian changes associated with water deer and sika deer, follicle and corpus luteum development showed to be different from those of other animals [5, 9, 11]. It is believed that the development of non-specific corpora lutein and follicles in water deer and sika deer may lead to incomplete estrus. In addition, we believe that the findings of our study can be used as very important basic data related to the implantation and development of embryos of different species through morphological changes of the ovary during the estrous and gestational stages of endangered species.
CONCLUSION
Our study was performed to assess reproductive physiological differences through tissue remodeling of follicles and corpus luteum in the ovaries of wild water deer and sika deer. In the study results, the ovarian tissue of water deer and sika deer showed very different morphological differences, but the growth of follicles continued to increase even during pregnancy, and similar patterns were observed in the changing corpus luteum. In particular, unlike water deer, the morphology of the sika deer corpus luteum was very small, and the shape of the luteum cells was also different, but cell remodeling was similar. Therefore, the functional activity of the ovaries of water deer and sika deer was similar. This knowledge can be used as baseline data on interspecies cloning and reproductive physiological conditions required to restore the sika deer, an extinct Korean peninsula species.
LIST OF ABBREVIATIONS
| MMPs | = Matrix metalloproteinases |
| RT | = Room Temperature |
| NHS | = Normal Horse Serum |
| NGS | = Normal Goat Serum |
| FSH | = Follicle-Stimulating Hormone |
| LH | = Luteinizing Hormone |
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


