RESEARCH ARTICLE
Structure of Microscopic Fungal Species in Soils at Amber Mining Territories before and during the use of New Technology of Pine Plantation Formation.
Viktoriia Oliferchuk1, *, Dariya Fedorovych2, Leonid Kopiy1, Dmytro Kravtsov3, Nataliia Kendzora4, Hryhoriy Krynytskyy5, Nataliya Hotsii6, Vasyl Suchovich1, Mariya Kopiy1, Mariya Samarska1, Sergiy Kopiy1, Ihor Fizyk1, Anatoliy Novak5, Vasyl Ahiy1
Article Information
Identifiers and Pagination:
Year: 2023Volume: 17
E-location ID: e187433152301190
Publisher ID: e187433152301190
DOI: 10.2174/18743315-v17-e230120-2022-12
Article History:
Received Date: 17/3/2022Revision Received Date: 20/6/2022
Acceptance Date: 21/7/2022
Electronic publication date: 06/03/2023
Collection year: 2023

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction:
Ukraine is one of the European leaders in amber deposits. The main deposits of the mineral are concentrated in the forests of the Rivne, Zhytomyr and Volyn regions. As a result of the extraction process, the integrity of forest’s ecosystems is violated, the fertile soil layer is destroyed, and 3.5 thousand hectares of forests have to be restored.
Aim:
Evaluation of different forest management strategies in degraded soil regeneration.
Objective:
The study aims to explore the response of soil mycobiota to extreme conditions associated with amber mining and to propose biotechnology to restore the fertile soil layer by methods of regenerative land use, namely the use of biomass of various ways of birch cuts, which is the primary succession to the indigenous pine stands in the region.
Materials and Methods:
The study was carried out on the territory of the Klesiv amber deposit in the Ukrainian Polissya. The bioindication method with the help of soil micromycetes was used to assess the quality of the plant development environment in the conditions of ecosystem restoration after amber extraction. To determine the species composition of hyphomycetes, the method of serial dilutions and direct seeding on agar media of soil suspensions was used. The analysis of soil mycobiota was performed using quantitative ecology methods. The method of correlation groups was used to determine the taxonomic diversity of hyphomycetes. To determine the effectiveness of the restoration of the studied forest soils, the express analysis of the content of essential nutrients using NPK-sensor was used. Standard methods for the determination of mobile phosphorus, potassium and nitrogen compounds were used as controls.
Results:
The species composition and taxonomic characteristics of soil micromycetes of forest ecosystems disturbed by amber mining have been studied. It was found that in the areas of amber mining, soil micromycetes form linear connections and three-membered structures, which is characteristic of disturbed biocenoses. In the 60-year-old plantation, soil micromycetes form strong six-membered structures that are characteristic of menopausal ecosystems or intact biocenoses. The biotechnology of restoration of the indigenous plantation characteristic of these conditions - pine with an admixture of hanging birch is offered. The result of the application of this technology will allow to reproduce natural forest ecosystems in large areas.
Conclusion:
For the first time, the structure of fungal complexes in the areas of amber mining has been determined, which indicates that the formation of a stable structure requires time and a systematic approach to the restoration of damaged soils. In the soils disturbed by amber mining, initial linear, three-membered and four-membered structures were formed, the structural genera of which are the “pioneer genera” Penicillium, Mucor, Rhizopus, the species of which were the first to inhabit plant remains.
In the process of reforestation in areas affected by amber mining, biotechnology was used for the first time, which involved the formation of natural pine stands by cutting birch, forming the primary succession in the studied areas.
The comparison of the results of chemical analysis of soils of the studied areas of the Klesiv forestry before and after the application of birch pruning technology for the formation of pine stands proves the effectiveness of the technology, as in all areas nitrogen, phosphorus and potassium were increased in the soil.
1. INTRODUCTION
Anthropogenic transformation of the natural environment, including its main consequence - the change of vegetation - is common in the modern world [1-3]. Historically, the territory of the Ukrainian Polissya has undergone significant anthropogenic impacts caused by agricultural factors [4] and radionuclide contamination [5, 6]. In recent years, another environmental problem has escalated in the Ukrainian Polissya - amber mining, especially illegal, which has long-term serious environmental, social and economic consequences [7-10]. Amber is an organic mineral, a fossilized resin of ancient gymnosperms (including Cupressaceae, Taxodiaceae, and others), and is now highly valued in various industries, including the jewelry, chemical, and pharmaceutical industries. Besides jewelery, amber is used in high-end varnishes, as a flux for soldering and blood transfusion systems. Amber sales are an important source of income for local communities. Amber miners use water pumps specifically made for amber mining out of the old car and van parts, to take advantage of amber’s low density. In popular regions, thousands of men pump the soil full of water up to 30 meters deep, in order to liquefy it, and in the hope that amber will float to the top. Then, clad in swamp boots and old clothes, they wade in with nets to sort through the floating debris and fish out the semi-precious stones [11]. This action washes out most of the organic material from the soil and causes sinkhole formation. The negative consequences of illegal amber mining are related to the disturbance or destruction of natural habitats in large areas and the lack of land rehabilitation after mining. Types of anthropogenic impact associated with amber mining can be divided into two subtypes - direct (breakthrough, water washing) and indirect (felling, changes in water and mineral regime of soils, biological invasions). All these factors significantly slow down the processes of natural regeneration of vegetation, sometimes making them impossible [7, 8, 12]. In recent years, there is a growing scientific interest in identifying new areas affected by illegal amber mining [13-18], the impact of mining on the environment and its influence on the restoration of forest and wetland landscapes of the Polissya, including the territories of the Rivne [12, 19], Zhytomyr [8, 9], and Volyn [7] regions of Ukraine (Fig. 1).
The Klesiv amber deposit is located in the area surrounded by crystalline rocks of the north-western part of the Ukrainian Crystal Shield and sedimentary formations of the north-eastern part of the Volyn-Podilska plate. The purpose of reclamation of used amber deposits or areas of unauthorized mining is not only the partial transformation of disturbed natural territorial complexes, but also the creation of productive and rationally organized anthropogenic landscapes instead. As a result of land reclamation agricultural and forest lands, reservoirs for various purposes, recreational areas, and building areas will be created in the affected areas.
Reclamation involves three stages: the first - the preparatory stage, the second - mining, the third - biological. The preparatory stage of reclamation includes the survey and classification of disturbed lands, the study of its natural conditions (geological structure, rock composition, suitability for biological reclamation and other uses, forecast of dynamics of hydrogeological conditions), and determining the direction of subsequent land use. The mining stage of reclamation involves the preparation of land that was vacated after the mining of deposits for further targeted economical use. The biological stage includes a complex of agrotechnical and phytomeliorative biochemical and other soil properties. The biological stage is performed after the completion of the technical stage and consists of soil preparation, fertilizer application, selection of grass mixtures, crops and crop care. The biological stage is aimed at consolidating the surface layer of the soil by the root system of plants and preventing the development of water and wind erosion of the soil on disturbed lands. We have proposed one of the methods of biological reclamation, namely the biotechnology of soil regeneration through regenerative land use.
Vegetation was studied on the territory of the Klesiv forestry and the ecological analysis of biotopes was carried out [20]. Depending on the method of obtaining amber, the nature of the transformation of edaphotopes in the studied areas differs [21, 22].
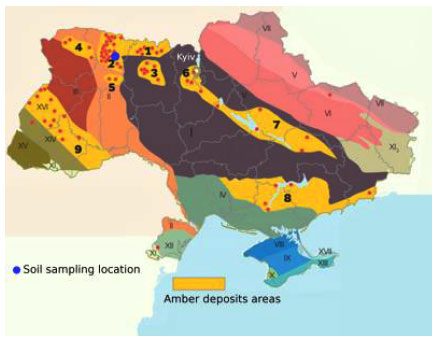 |
Fig. (1). Regions of Ukraine. |
The main forest-forming species is Pinus sylvestris L. with an admixture of Betula pendula Roth. Species such as Betula pubescens Ehrh., Quercus robur L., Populus tremula L., Alnus glutinosa (L.) Gaertn, are usually found in relief depressions.
The authors noted that the ability of vegetation to recover in extreme conditions is reduced (especially when moving north), which worsens the prognosis of the potential regenerability of vegetation as a result of amber mining. However, the factors contributing to the natural course of restoration include the remoteness of large settlements and the relatively high conservation of the surrounding forests, which creates the potential for secondary resettlement of forest species from the surrounding areas. Thus, the measures to restore and monitor changes in succession, in the long run, are appropriate [20].
Anthropogenic impact not only affects vegetation, but also makes changes in environmental conditions, and primarily changes the biota of soils. The use of bioindication with the help of soil micromycetes is relevant in terms of predicting the future trend of vegetation restoration and assessing the potential regenerability of climax ecosystem vegetation. For soil systems, microbial diversity and the functioning of microbial and mycocenosis are crucial for soil health and plant productivity. Thus, it is important to gain increasing knowledge about the microbial structure and functional dynamics of microbial and mycocenosis habitats on soils disturbed by mining. The literature describes the long-term effects of mining such as soil acidification, heavy metals, iron and sulfate pollution, and their effects on soil mycobiota and microbiota, namely, a significant reduction in microbial biomass, richness and diversity, impaired plant growth and productive soil health [23].
Despite these shortcomings, the habitat of mycobiota, which was adapted to extreme conditions, is a unique ecological niche for new microbial phylotypes that can be useful for optimal plant growth and productivity and bioremediation of disturbed soils. Emphasis should be placed on the need to develop new biotechnologies that will affect microbial diversity and useful functions, such as bioremediation of metals, neutralization of acidity, symbiotic assembly, rhizomicrobial biome and plant growth stimulation, sulfates/iron, reduction and biogeochemical processing of nitrogen and carbon.
Globally, large commercial mining companies and other related industries generate diverse wastes, which are one of the main anthropogenic sources of environmental pollution [24-28]. In the mining regions of Australia [29], Brazil [30], Canada [31], England, Wales, Spain, Norway [32, 33], South Africa [34], China [35, 36] and the United States [37, 38] the concerns were reported about various types of pollution that affect terrestrial, aquatic systems, soils, soil and surface water sources [27, 39, 40]. In addition, a large number of rare taxa have been reported that make up the “rare biosphere” in the microbial community, which can play a crucial role in ecosystems [41]. In the aggregate, post-mining environments are unique ecological niches with significantly reduced species richness and diversity, but enriched with microorganisms adapted to sulfates, acids and toxic metals.
In this paper, we researched changes in soil mycobiota in response to extreme conditions associated with amber mining and the application of new technology for the formation of pine stands.
2. MATERIALS AND METHODS
During 2018-2020, in spring, summer and autumn, soil samples were taken from the research station of the Klesiv Forestry (SE Klesiv Forestry of the Rivne region, quarter 27, section 60, area 1.8 hectares, 51.2570 N 26.79917 E). Plantation composition 10 P - forest crops damaged as a result of amber mining. Forest type A2 - fresh birch, distributed within the forest zone – the Polissya. Typical soils are poorly podzolic fluvioglacial or alluvial sands. Soil samples were taken at depths of 0-10 and 20-30 cm. 86 samples were taken, 100 grams of soil per sample, from which 57 species of soil micromycetes belonging to 23 genera were isolated. For microbiological analysis, serial dilutions from 1 gram of soil in 9ml of water were performed for 4 rounds, giving a total dilution of 104, final dilution was plated out on solid nutrient agar media and Chapek medium. Counting of colony forming units (CFU) was done after the 7th day. In spring and autumn, the number of fungal propagules per gram of soil is higher, as usual, and lower in summer. We observed the same phenomenon in the studied ecotopes. The text presents the average values of CFU indicators. The identification of micromycetes was determined by a set of morphological and cultural characteristics of isolates for their cultivation on Chapek's medium using the appropriate determinants of domestic and foreign authors [42, 43]. Morphological features of microscopic fungi were studied using a light ICBM microscope produced by “Lomo”. Analysis of the structure of micromycete groups was performed using generally accepted criteria in ecology [43, 44]. All assays were performed sequentially, each mycological experiment was done in triplicate. Soil nutrient composition analysis was done without repeats.
Indicators of the number of species that are a part of the group were used to determine the capacity of the ecosystem - the ability to create conditions for populations of different species. The more species in the ecosystem, the greater its capacity. Simpson's species wealth index, Berger-Parker dominance index, Shannon diversity index, and Sorensen similarity coefficient were used to characterize species diversity.
The statistical method of correlation groups was used to identify characteristic groups of hyphomycetes in the studied biogeocenoses. Based on the calculations of pair correlations between species, those closely related to each other and forming a certain structure of these relationships in each of the studied biocenoses were identified.
The content of basic nutrients in soils was determined by applying an NPK sensor and using standard methods.
3. RESULTS AND DISCUSSION
3.1. Description of the Proposed Technology of Restoration of the Forest System Damaged due to Amber Mining
Primary succession of birch was formed on the soils where the fertile layer was completely destroyed after amber mining. To restore natural forest plantations of pine in the Klesiv forestry biotechnology of soil restoration was used. After amber mining, the area was planted with pine; birch grew on its own. The age of plantations was 7 years. At this age, birch was cut. One year after pruning, soil samples were taken to explore the species and numerical composition of micromycetes as active transformers of plant residues. The technology involved the step-by-step cutting of birch: ½ of its part on one test area, ⅓ of its part on the second, and cutting to the stump - in the third case. Pruning of birch was carried out in order to allow for the active growth of pine. Thus, the shading of the pine was eliminated. After cutting, the birch remained in the territories and was not taken out. Accordingly, the fungi assimilated the remnants of cut wood. It was important to study the composition of soil deuteromycetes involved in the destruction of wood. Pruning pine on ½ and ⅓ stimulated the development and growth of pine for two reasons: decreased shading of the pine and increased the number of nutrients in the soil. Complete pruning of birch was not effective either in terms of the effective transformation of plant residues or in terms of plantation formation, as productive pines must grow in a mixed culture with other plant species (Table 1).
| Species of Micromycetes |
А2-С (60 years) |
½ Cut Birch | ⅓ Cut Birch | Total Cut of Birch | Control |
|---|---|---|---|---|---|
| Division Zygomycota | |||||
| Mucor hiemalis Wehmer | 10 | 35 | 57 | 45 | 10 |
| M. angulisporus Naumov | - | 43 | 23 | 29 | 12 |
| Mucor sp. | 7 | 17 | 32 | 18 | 5 |
| Rhizopus nigricans Ehrenb. | 12 | 18 | 28 | 24 | 17 |
| R. microsporum v.Tiegh. | - | 12 | 12 | 7 | 11 |
| Mortierella ramanniana (Naumov) | 4 | - | - | 4 | 6 |
| M. isabelina Oudem | 17 | - | 12 | 7 | - |
| Absidia glauca Hagem | 2 | 21 | 24 | 17 | 2 |
| A. coerulea Bain. | 14 | 31 | 42 | 28 | - |
| Zygorhynchus heterogamous (Vuill.) Vuill | 3 | 7 | - | 3 | 4 |
| Division Ascomycota | |||||
| Chaetomium tortile Bain | 5 | - | - | 5 | 7 |
| Chaetomium sp. | 3 | - | - | - | - |
| Class Deuteromycetes | |||||
| Family Moniliaceae | |||||
| Penicillium thomii Maire | 10 | - | - | - | - |
| P. vinaceum Gilman et Abbot | 12 | - | - | - | - |
| P. funiculosum Thom | 15 | 37 | 37 | 35 | 42 |
| P. roseopurpureum Dierck | 12 | 51 | 42 | 43 | 23 |
| P. nigricans (Bainier) Thom | 7 | 17 | 21 | 21 | 15 |
| P. rubrum Stolle | 9 | - | - | - | - |
| P. ochro-chloron Biourge | 5 | - | - | - | 7 |
| P. lividum Westl. | 12 | - | - | - | - |
| P. expansum Link | 14 | - | - | - | - |
| P. tardum Thom | 18 | - | - | - | 3 |
| Penicillium sp. | 7 | - | - | - | - |
| Aspergillus fumigatus Fres. | - | 27 | 21 | 23 | 12 |
| A. awamori Nakazawa | 13 | - | - | - | 2 |
| A. ustus (Bain.) Thom | 2 | - | - | - | 7 |
| A. niger v. Tiegh | 3 | 32 | 24 | 27 | 5 |
| A. oryzae (Ahlburg) Coln. | 10 | - | - | - | 4 |
| Trichoderma viride (Pers. Et S.F. Gray.) | 22 | 32 | 25 | 21 | 12 |
| Family Tuberculariaceae | |||||
| Fusarium oxysporum Schlecht | 5 | - | - | - | 2 |
| F. sambucinum Fuck | 2 | - | - | - | - |
| F. moniliforme Sheld | 10 | - | - | - | - |
| Family Dematiaceae | |||||
| Auerobasidium pullulans (de Bary) Arnaud | 16 | 15 | 27 | 12 | 10 |
| Cladosporium cladosporioides (Fres.) de Vries | 12 | 7 | - | 5 | - |
| Cladosporium macrocarpon Preuss | 7 | 42 | 35 | 32 | 10 |
| Clad. atroseptum Pidopl. et Deniak | 10 | - | - | - | 12 |
| Arthrinium sphaerospermum Fckl. | 18 | - | - | - | 2 |
3.2. General Characteristics of the Mycobiota of Soils Damaged by Amber Mining and after the Application of Birch Pruning Technology
The study of biodegradation processes, and the nature of biodynamic transformation of organic matter is one of the objective indicators of productivity, stability and development of biocenoses. The amount of synthesis and destruction of soil organic matter are factors that determine the level of organization of the biosphere, while the detrital block in terms of complexity of structure and variety of functions plays a key role [45]. We have identified the main genera of micromycetes that inhabit the soils where birch pruning was carried out. These are usually the genera Zigomycota and Ascomycota, whose average CFU (colonies forming organisms) ranged from 156x104 to 178 × 104 in the areas where birch was cut and 87 × 104 in the control area. The number of colonies of fungi in one gram of soil in the territory of A2-C - the territory of the old forest was 198 × 104 CFU.
It is important to emphasize that our results comply with the works of authors who studied the Mycobiota of coniferous, mixed and deciduous forests in the Polissya, as well as Canada and Japan, and found that the predominant soil deuteromycetes were genera Mucor, Mortierella and Penicillium [46]. The nutrient layer is annually replenished with substances that are a source of nutrition for soil destructors. Globally, 95% of its flow is limited due to the soil-microorganisms-vegetation system. Thus, the main role in global biogeochemical transformations in the biosphere belongs to forest biocenoses, which occupy more than 4.1 × 109 ha of land surface [47]. The main role in the destruction of ecosystems belongs to fungi [48]. The biomass of fungi in the soil is 50-70% of the share of all microorganisms, which is 90% of the total number of destructors. The maximum reserves of fungal biomass are found in the soils of the forest zone. Fungi are involved in soil formation by binding mineral particles and ensuring the stability of soil aggregates, and they are the main producers and consumers of the carbon in the soil [49, 50].
The most common type of trophic relationship of soil fungi is associated with the consistent settlement of complex organic and inorganic substrates. A typical example is the mycological degradation and transformation of plant remains, which is relevant in the study of the formation of pine stands, and which became the basis for our research.
The grouping, as a system, is characterized by the interaction between the species that are part of it. In the group of hyphomycetes, the interaction between species is primarily due to trophic relationships. Determining the ratio of micromycete species in the studied soil indicates the content of nutrients in it, their composition and the availability of essential nutrients for the plants. The first degrading agents are types of soil micromycetes that use simple carbohydrates that are formed during the degradation of cellulose. These include most species of the genus Zygomycetes. Mucor sp., Mucor hiemalis, M. angulisporus, Rhizopus nigricans, R. microsporum, and Absidia coerulea were typical and common species (from 30 to 75%) in the studied areas, in ecotopes with ½, ⅓ share of cut birch and fully cut birch. In the final stages of substrate transformation, micromycetes appear that absorb lignin. The trophic relationships of micromycetes of the studied areas occur due to the presence of a complex of specific enzymes that hydrolyze polymers of plant residues and metabolize monomer units. Facultative saprophytes that have settled on dead plant residues have a high level of growth and rapid spread of spores, which is clearly seen in the soils where ½ and ⅓ birches were cut. Here, the assimilation of dead wood was most effective, the number of fungal propagules per 1 gram of soil was 189-192 × 104 CFU.
3.3. Analysis of the Species Composition of Soil Microscopic Fungi in the Studied Ecotopes
Analysis of soil mycobiota of the studied ecotopes revealed the following patterns: a total of 47 species from 15 genera of micromycetes were identified. Table 1 presents 37 species from 12 genera (excluding random species) that were constantly found in the area, and formed biocenoses of the studied areas. For natural pine plantations, the age of which is 60 years, 34 species from 12 genera are characteristic. The species composition of soil micromycetes of the parent stand - the area where amber mining did not take place - differed sharply from the species composition of soil micromycetes of the disturbed areas. The natural stand is characterized by the highest capacity of the ecosystem. Species of the genera Mucor, Rhizopus, Absidia, Aspergillus, Trichoderma, Aureobasidium, Cladosporium, and Penicillium were characteristic of all studied ecotopes. The species P. funiculosum, P. roseopurpureum, Aspergillus fumigatus, A. niger, Aureobasidium pullulans, Cladosporium macrocarpon were constantly found. The frequency of these species in extreme ecotopes is from 24 to 51%. The same species were dominant (frequency of 50% or more) and those that are constantly present (more than 30%) in radionuclide-contaminated soils of the 10-kilometer Chernobyl Exclusion Zone [51]. The authors describe this phenomenon as the formation of radiosensitive strains in the relevant species populations. In the soils of the studied ecotopes, hyphomycetes-bioindicators of radioactive contamination of P. funiculosum, P. roseopurpureum, P. nigricans, Trichoderma viride, Auerobasidium pullulans, Cladosporium macrocarpon were found (Table 1).
The constant occurrence of the described species in the Rivne region (the Klesiv forestry) may indicate an increased level of radiation. Approximately 64 TBq (1b7 Mq) of total 137Cs activity, fell on the territory of Europe in 1986, and 18% fell on Ukraine, including the Rivne region. The migration of radionuclides in forest soils is slow because they have a multilayered vertical structure. Over the course of time, due to the pool of organic acids and oxidative exoenzymes of soil deuteromycetes, hot particles decomposed [51]. Fungi mycelium, numbering from tens of hundreds of kilograms to several tons per hectare, penetrated the upper horizons of the soil and promoted the active transfer of radionuclides in its various layers. According to the authors, Zhdanova N.M. et al., the process of transfer of radionuclides along the vertical profile of soils is slowed down by their fixation on clay minerals. The rate of movement of radionuclides varies depending on the type and physicochemical composition of the soil, but in almost all cases the bulk of radionuclides remains within its rhizosphere layer. Amber mining destroys soil layers, causes harm to vegetation, and radionuclides that still remain in the rhizosphere of plants in some ecotopes migrate chaotically.
As can be seen from Table 1, the frequency of species differs greatly in ecotopes, where pine stands were formed, and in the control area, where pine stands were formed together with birch naturally without the use of technology. The species composition of micromycetes of the mother stand (A2-C) is very diverse and not dominated by species of one division or genus. Areas with cut birch are characterized by the dominance of species of Zygomycota, which indicates an active process of settlement of cut wood by destructive species and its transformation. In the control ecotope, the species composition of micromycetes indicates the gradual formation of plantations, but it is not clear which of the plantations are formed, deciduous or coniferous, as no species were identified that characterize a particular type of plantation. Our data are consistent with the studies of the authors, who noted the difference in fungal flora depending on the type of forest.
3.4. Ecological and Systematic Analysis of Mycocenoses of the Studied Areas where Artificial Restoration of Pine Plantations takes Place after Amber Mining
The analysis of the structure of soil micromycete groups was carried out, where the biotechnology of pine plantations formation was applied by cuts of birch. These data were compared with the indices of ecological diversity of 60-year-old pine plantations. It turned out that in the edaphotopes of pine restoration zones the species composition is depleted compared to the species diversity of microscopic fungi, which is indicated by a decrease of 2.67 (average value for all ecotopes) times the Berger-Parker index, 2.36 times the Shannon index and 2.1 times the Pisl index and a 1.9-times decrease in the Simpson index (Table 2). Regularities are set in spring and summer.
According to the obtained results, birch pruning technologies in the amount of ½ and ⅓ proved to be the most effective for the diversity of micromycetes and their number.
3.5. The Structure of Fungal Complexes of Soils of the Studied Ecotopes of the Klesiv Forestry
The structure and level of complexity of soil fungal complexes were determined using the method of correlation groups according to Terentyev [51-53]. This method involves correlation, in our case, between the frequency of occurrence of a particular genus (or species if the genus was represented by one species) and the soil where the damaged areas are restored after amber mining. The values of the correlation coefficients were determined at the levels of 1.0, 0.95, 0.9 and up to 0.7 and plotted as points within a pie chart, connecting them with lines. The obtained graphic structures (correlation groups) differed in the complexity of the structure. Among them, there were closed and linear (open) groups. Closed groups belong to stable and highly organized ones. Highly organized six-membered complexes of common soil micromycetes (Fig. 2) characteristic of forest ecosystems, where the main forest-forming species is pine, were only in 60-year-old A2-C plantations (60 years).
|
Researched Ecotopes |
Serensen Coefficient | Berger-parker Index | Simpson Index | Shannon Index | Pisl Index |
|---|---|---|---|---|---|
| А2-С (60 years) | 0.57 | 6.8 | 0.22 | 5.08 | 0.61 |
| ½ cut birch | 0.24 | 2.6 | 0.52 | 2.47 | 0.28 |
| ⅓ cut birch | 0.27 | 2.5 | 0.59 | 2.94 | 0.30 |
| Total birch cut | 0.21 | 2.3 | 0.51 | 2.98 | 0.26 |
| Control | 0.29 | 2.8 | 0.47 | 2.31 | 0.29 |
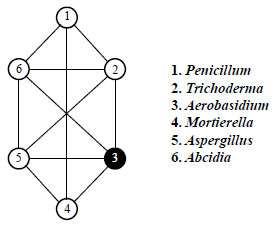 |
Fig. (2). A six-member structure Penicillium, Trichoderma, Aerobasidium, Mortierella, Aspergillus, Absidia. |
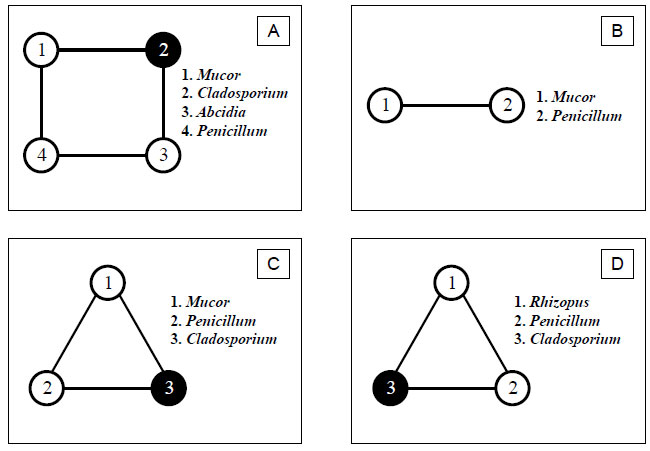 |
Fig. (3). Correlation groups in the restoration of pine plantations by correcting plant biomass: |
|
Researched Ecotopes |
Chemical Indicators, mg / kg | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Nitrogen Easily Hydrolyzed from Organic Compounds |
Nitrogen Potential (Laboratory) |
Phosphorus Available | Potential Phosphorus (Laboratory) | Potassium Exchange | Potential Potassium (Laboratory) | |||||||
| before | after | before | after | before | after | before | after | before | after | before | after | |
| А2-С | 32 | 32 | 62 | 62 | 10 | 10 | 22 | 22 | 120 | 120 | 137 | 137 |
| ½ cut birch | 16 | 25 | 40 | 46 | 8 | 9 | 19 | 21 | 110 | 120 | 130 | 137 |
| ⅓ cut birch | 28 | 30 | 52 | 55 | 8 | 9 | 20 | 21 | 110 | 120 | 132 | 135 |
| Total birch cut | 28 | 30 | 52 | 55 | 8 | 9 | 20 | 22 | 100 | 120 | 130 | 135 |
| Control | 25 | 26 | 51 | 52 | 8 | 8 | 20 | 20 | 120 | 120 | 128 | 129 |
In all research areas, where pine plantations were restored by the correction of the biomass of plants that form the primary succession (in our case, birch), less complex and stable four- and three-membered groups were formed. In areas where birch was completely cut down, only a linear method of communication was established. The structural genera of these groups were mainly species of the genus Zygomycetes - Mucor, Absidia, Rhizopus, as well as Penicillium and Cladosporium (Fig. 3).
- A – Four-member group Mucor, Cladosporium, Absidia, Penicillium;
- B – Linear group Mucor, Penicillium;
- C – Three-member group Mucor, Penicillium, Cladosporium;
- D – Three-member group Rhizopus, Penicillium, Cladosporium.
Observations of the dynamics of fungal complexes, taking into account their location, the degree of soil degradation, and seasons indicate that this structure is quite labile and constantly changing during the restoration of the fertile soil layer. Comparison of structural genera and complexity of the form of correlation groups in soils formed in the areas of pine forest restoration at amber mining sites does not correspond to the stable structure of correlation groups of pine plantations at the age of 60 years. It takes time for forest pine biocenoses to be fully formed. The proposed biotechnology of pine forest restoration is promising but requires further long-term research and constant monitoring of changes in soil mycobiota until the formation of permanent, stable complexes of soil micromycetes.
3.6. Changes in the Chemical Composition of the Soil in the Territory of Amber Mining before and after the Application of the Proposed Method of Birch Cutting
The increase in the number of different groups of micromycetes should lead to changes in the chemical composition of the soil. Therefore, we conducted a comparative analysis of soil samples taken at the studied sites 1 year after pruning. The content of various forms of Nitrogen, Phosphorus and Potassium are given in Table 3. The content of basic nutrients in the 60-year-old forest was the highest and has not changed during our research. Analysis of soils from the studied areas after birch pruning indicated a slight but dynamic increase in basic plant nutrients, indicating active processes of soil enrichment with Nitrogen, Phosphorus and Potassium, which are the result of the active transformation of dead wood remain by soil deuteromycetes (Table 3).
4. CONCLUSION
In the process of reforestation in areas affected by amber mining, biotechnology was used for the first time, which involved the formation of natural pine stands by cutting birch, which formed the primary succession in the studied areas. Bioindication with the use of soil micromycetes is suitable for assessing the effectiveness of the applied biotechnology. The study of the frequency of occurrence of the main species of soil microscopic fungi on the territories revealed an increase in the species composition of micromycetes and the number of fungal propagules in one gram of soil, as well as an increase in the basic elements of plant nutrients: Nitrogen, Phosphorus and Potassium.
ABBREVIATION
| CFU | = Colony Forming Units |
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
All the experimental data are available from the corresponding author [V.O] and shall be provided on request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
| [1] | Alkama R, Cescatti A. Biophysical climate impacts of recent changes in global forest cover. Science 2016; 351(6273): 600-4. |
| [2] | McGrath MJ, Luyssaert S, Meyfroidt P, et al. Reconstructing European forest management from 1600 to 2010. Biogeosciences 2015; 12(14): 4291-316. |
| [3] | Zanon M, Davis BAS, Marquer L, Brewer S, Kaplan JO. European forest cover during the past 12,000 years: A palynological reconstruction based on modern analogs and remote sensing. Front Plant Sci 2018; 253: 1-25. |
| [4] | Khomyak I, Demchuk N, Vasylenko O. Phytoindication anthropogenic transformation of ecosystems on the example of Ukrainian Polissya. Biodivers Conserv 2018; 3(22): 113-8. |
| [5] | Beresford NA, Fesenko S, Konoplev A, Skuterud L, Smith JT, Voigt G. Thirty years after the Chernobyl accident: What lessons have we learnt? J Environ Radioact 2016; 157: 77-89. |
| [6] | Yablokov AV, Nesterenko VB, Nesterenko AV, Sherman-Nevinger JD. Chernobyl: Consequences of the Catastrophe for People and the Environment. New York: John Wiley and Sons 2010. |
| [7] | Kazymyr MM, Bedernichek TY. Reclamation of land violated due to extraction of amber in Polissia: Problems and prospects. Environmental Economics: State, Problems, Prospects 2017; 90-4. |
| [8] | Kovalevsky SB, Marchuk YM, Mayevsky KV, Kurdyuk OM. The scope and consequences of illegal amber extraction on lands of zhytomyr RDFH. Scientific Bulletin of UNFU 2018; 27(10): 69-72. |
| [9] | Kovalevsky SB, Marchuk YМ, Mayevsky КV, Kurdyuk ОМ. Succession processes on the territories disrupted due to illegal amber mining of Zhytomyr Regional Forestry and Hunting Administration. Ukrainian J Forest and Wood Sci 2019; 10(2): 26-40. |
| [10] | Kovalevskyi SB, Kovalevskyi SS. Amber fossils: History of study, methods of getting and effect on forest ecosystems. Sci Bull of UNFU 2019; 29(3): 56-9. |
| [11] | Mining technology. Amber mining: an industry trapped in time and tragedy. Analyis 2017. Available from: https://www.mining-technology.com/analysis/featureamber-mining-an-industry-trapped-in-time-and-tragedy-5773298/ (Accessed 2022-06-18). |
| [12] | Gordeychuk MV. Effect of extraction of the natural landscape of amber Rivne. Phys Geography and Geomorphol 2013; 2(70): 259-62. |
| [13] | Filipovich VE, Shevchuk RM. The methodology and technology assessment of damage caused by ukrainian government as a result of illegal extraction of amber. Ukrainian J Remote Sens 2016; 11: 15-21. |
| [14] | Filipovich VE. Satellite monitoring for the areas of illegal extraction of amber. Ukrainian J Remote Sens 2015; 6: 4-7. |
| [15] | Krasovskyi HYa, Shumeiko VO, Klochko TO, Sementsova NI. Information technologies for monitoring the environmental consequences of amber production in Ukraine. Sci Tech J 2018; 2(18): 107-17. |
| [16] | Masley VM, Mozgovoy DK, Bilousov KG, Horoshilov VS, Bushanska OS, Galich NG. Methods of the impact evaluation of amber mining by multispectral satellite images. Space Sci Tech 2016; 22(6): 26-36. |
| [17] | Shevchuk RM. Verification of results of satellite monitoring of territories of illegal amber mining. Environ Safety and Nat Resour 2017; 3-4(24): 133-7. |
| [18] | Yanchuk R, Prokopchuk A, Trohimets S. Identification and definition of the areas disturbed by mining amber lands based on multispectral satellite imagery Sentinel–2. Suchasni Dosiahnennia Heodezychnoi Nauky Ta Vyrobnytstva 2017; 1(33): 120-4. |
| [19] | Lehkyi VV, Kovalevskii SB. Typological estimation of forest massif of Dubrovytskyi forestry disturbed by unauthorised extraction of amber. Sci Bull UNFU 2018; 28(8): 61-4. |
| [20] | Churilov A, Goncharenko I, Kravchenko O, et al. Phytoindicative assessment and analysis vegetation in disturbed areas after illegal amber mining in the Western Polissya of Ukraine. For Ideas 2020; 1(59): 191-208. |
| [21] | Malanchuk Z, Korniyenko V, Malanchuk Y, Khrystyuk A. Results of experimental studies of amber extraction by hydromechanical method in Ukraine. East-Eur J Enterp Technol 2016; 3(10(81)): 24-8. |
| [22] | Volnenko S, Mel’nichuk G, Kurepa Y, Mamchur S. Extra stocking methods of amber in the Ukrainian Polesie. J Donetsk Min Inst 2017; 1(1): 118-22. |
| [23] | Munyai R, Ogola HJO, Modise DM. Microbial community diversity dynamics in acid mine drainage and acid mine drainage-polluted soils: Implication on mining water irrigation agricultural sustainability. Front Sustain Food Syst 2021; 5: 701870. |
| [24] | Grande JA, Santisteban M, De la Torre ML, Dávila JM, Pérez OE. Map of impact by acid mine drainage in the river network of The Iberian Pyrite Belt (Sw Spain). Chemosphere 2018; 199: 269-77. |
| [25] | Kefeni KK, Msagati TAM, Mamba BB. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J Clean Prod 2017; 151: 475-93. |
| [26] | Rambabu K, Banat F, Pham QM, Ho SH, Ren NQ, Show PL. Biological remediation of acid mine drainage: Review of past trends and current outlook. Environ Sci Ecotechnol 2020; 2: 100024. |
| [27] | Rezaie B, Anderson A. Sustainable resolutions for environmental threat of the acid mine drainage. Sci Total Environ 2020; 717: 137211. |
| [28] | Steyn M, Oberholster PJ, Botha AM, Genthe B, Van Den Heever KPE, Weyers C. Treated acid mine drainage and stream recovery: Downstream impacts on benthic macroinvertebrate communities in relation to multispecies toxicity bioassays. J Environ Manage 2019; 235: 377-88. |
| [29] | Lei L, Song C, Xie X, Li Y, Wang F. Acid mine drainage and heavy metal contamination in groundwater of metal sulfide mine at arid territory (BS mine, Western Australia). Trans Nonferrous Met Soc China 2010; 20(8): 1488-93. |
| [30] | Galhardi JA, Bonotto DM. Hydrogeochemical features of surface water and groundwater contaminated with Acid Mine Drainage (AMD) in coal mining areas: A case study in southern Brazil. Environ Sci Pollut Res Int 2016; 23(18): 18911-27. |
| [31] | Sracek O, Choquette M, Gélinas P, Lefebvre R, Nicholson RV. Geochemical characterization of acid mine drainage from a waste rock pile, Mine Doyon, Québec, Canada. J Contam Hydrol 2004; 69(1-2): 45-71. |
| [32] | Hallberg KB. New perspectives in acid mine drainage microbiology. Hydrometallurgy 2010; 104(3-4): 448-53. |
| [33] | Romero A, González I, Galán E. Stream water geochemistry from mine wastes in Peña de Hierro, Riotinto area, SW Spain: A case of extreme acid mine drainage. Environ Earth Sci 2011; 62(3): 645-56. |
| [34] | Ochieng GM, Seanego ES, Nkwonta OI. Impacts of mining on water resources in South Africa: A review. Sci Res Essays 2010; 5: 3351-7. |
| [35] | Wang Z, Xu Y, Zhang Z, Zhang Y. Acid Mine Drainage (AMD) in abandoned coal mines of Shanxi, China. Water 2020; 13(1): 8. |
| [36] | Wu P, Tang C, Liu C, Zhu L, Pei T, Feng L. Geochemical distribution and removal of As, Fe, Mn and Al in a surface water system affected by acid mine drainage at a coalfield in Southwestern China. Environ Geol (Berl) 2009; 57(7): 1457-67. |
| [37] | Acharya BS, Kharel G. Acid mine drainage from coal mining in the United States – An overview. J Hydrol 2020; 588: 125061. |
| [38] | Blowes D, Ptacek C, Jambor J, Weisener C. The geochemistry of acid mine. Environ Geochem 2005; 9: 149-204. |
| [39] | Kumar A, Kumar A, Cabral MMSP, et al. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int J Environ Res Public Health 2020; 17(7): 2179. |
| [40] | Massányi P, Massányi M, Madeddu R, Stawarz R, Lukáč N. Effects of cadmium, lead, and mercury on the structure and function of reproductive organs. Toxics 2020; 8(4): 94. |
| [41] | Luo ZH, Li Q, Lai Y, Chen H, Liao B, Huang L. Diversity and genomic characterization of a novel parvarchaeota family in acid mine drainage sediments. Front Microbiol 2020; 11: 612257. |
| [42] | Domsch KH, Gams W, Anderson TH. Compendium of soil fungi. London Ect.: Academic Press 1980. |
| [43] | Ellis MB. Dematiaceous Hyphomycetes. England: Commonwealth Mycol. Inst. Kev 1993. |
| [44] | Kurakov AV. Mushrooms in the nitrogen cycle in soils: Author's abstract of the dissertation of a doctor of biological sciences. Moscow 2003. |
| [45] | Methods of experimental mycology: Handbook. Kiev: Naukova Dumka 1982. |
| [46] | Bylyi VI, Ellanskaya IA, Kyrylenko TS. Soil Micromycets. Kiev: Naukova dumka 1984. |
| [47] | Chervonny AYe, Voloshchuk NM. Micromycetes isolated from ramial chipped wood with leaves of soft deciduous trees. Forestry and Forest Melioration 2009; 116: 140-5. |
| [48] | Bunio L, Hudyk O, Oliferchuk V, Tsvilynyuk O, Terek O. Mycological characteristic of root area of plants Carex hirta L. in oil-polluted sod-podzolic soil. Visnyk of the Lviv University. Series Biology 2014; 64: 124-36. |
| [49] | De Godoy FPH, De Souza ALT, Tanaka MO, Sebastiani R. Decomposition and stabilization of organic matter in an old-growth tropical riparian forest: Effects of soil properties and vegetation structure. For Ecosyst 2021; 8(1): 13. |
| [50] | Guo X, Luo Z, Sun OJ. Long-term litter type treatments alter soil carbon composition but not microbial carbon utilization in a mixed pine-oak forest. Biogeochemistry 2021; 152(2-3): 327-43. |
| [51] | Zhdanova NN, Zakharchenko VA, Vasilevskaya AI, Shkolny AT, et al. Mycobiota of the Ukrainian Polesie: The consequences of the Chernobyl catastrophe. Kiev: Naukova dumka 2013. |
| [52] | Borisova VN. Micromycetes of forest litter in various ecosystems. Kiev: Naukova Dumka 1988. |
| [53] | Terentyev PV. The method of correlational Pleiades. Bulletin of the Leningrad University 1979; 9: 137-42. |








