All published articles of this journal are available on ScienceDirect.
The Use of Unripe Banana Flour as a Functional Feed Ingredient on Blood Profile, Serum Biochemical and Intestinal Indices of Broilers
Abstract
Background:
The study evaluated the effect of unripe banana flour (UBF) given individually or in combination with probiotics or multienzymes on blood profile, serum biochemical parameters and intestinal indices of broilers.
Methods:
About 392 broiler chicks were assigned to four groups (each with seven replicates) including CONT (birds provided with control feed), UBF (birds provided with 5% UBF in feed), UBFPRO (5% UBF plus 0.05% probiotics) and UBFZYM (5% UBF plus 0.05% multienzyme). Blood and intestinal segment and digesta were collected on days 21 and 38.
Results:
On day 21, the mean corpuscular volume (MCV) value in UBF was lower (p < 0.05) than UBFPRO. At day 38, haemoglobin levels in UBFZYM were higher (p < 0.05) than in CONT and UBF. Mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC) were higher (p < 0.05) in UBFZYM than in the other groups. UBFZYM had higher (p < 0.05) leukocyte counts than CONT. Uric acid levels in UBFZYM were lower (p < 0.05) than in CONT on day 21. On day 38, UBFPRO chicks had lower (p = 0.05) pH values in the duodenum than other groups. UBFZYM had higher (p = 0.05) pH values in the ileum, while UBFPRO had higher (p = 0.05) pH values in the caecum than the CONT groups. Lactic acid bacteria were found in higher (p < 0.05) concentrations in the ileum of UBFPRO and UBFZYM than in the CONT at day 38. The lactic acid bacteria to coliform ratio in UBFPRO and UBFZYM ileums was also higher (p = 0.07) than in CONT. On day 38, the crypt depth of the duodenum in UBFZYM was lower (p < 0.05) than in CONT. The duodenum villus height to crypt depth ratio was higher (p < 0.05) in UBFZYM than in the other groups. The villus height to crypt depth ratio in the UBF ileum was higher (p < 0.05) than in the CONT. When compared to CONT broilers, UBF, UBFPRO, and UBFZYM broilers had higher (p < 0.05) daily weight gain and feed efficiency.
Conclusion:
Broiler growth performance was improved when UBF was given alone or in combination with probiotics or multienzymes. The use of probiotics or multienzymes in combination with UBF improved the intestinal bacterial population, while multienzymes improved broiler physiology and intestinal morphology.
1. INTRODUCTION
The ban on the use of antibiotic growth promoters (AGP) has posed a negative impact on the growth and health of broilers, thus threatening the sustainability of the broiler production. To support the sustainability of the broiler industry, the presence of an alternative to AGP is very much needed. Various AGP alternatives have been explored, one of which is functional feed ingredients. Functional feed is generally defined as a feed ingredient that have potential health effects other than basic nutrition [1]. Several active components in the functional feed are believed to have critical roles in promoting health and well-being of the host, including prebiotics and antioxidant compounds. Aside from the high energy content, unripe banana flour (UBF) has been reported to be rich in soluble fibre and resistant starch that can act as a prebiotic agent [2, 3]. This property is highly beneficial for the intestinal microbial balance of broiler chickens. The UBF also showed high antioxidant activity [3, 4], which may be beneficial in improving the physiological condition of broilers reared under intensive production system. Taking all these above facts into consideration, UBF has great potential to be developed into functional feed ingredients for broiler chickens during the post-antibiotic era.
UBF is generally prepared from green bananas that have been abandoned or discarded during the fruit selection and processing. Discarded green bananas are so plentiful in some banana-producing areas that if not utilized, they can pose environmental problems [5]. Unripe banana flour can also be obtained from various underconsumed banana cultivars that have low economic value [4]. As a tropical country, Indonesia is home to various cultivars of bananas [6]. Some banana cultivars have a high economic worth, whilst others possess a low economic value due to a lack of customer demand (underconsumed banana). In the previous study, we evaluated some banana cultivars in Indonesia, including Klutuk, Norowito, Raja Bandung, and Pisang Hijau [4]. It was shown that all unripe banana cultivars are high in energy content and can support the growth of Lactobacillus casei (lactic acid bacteria-based probiotics), suggesting that all banana cultivars possess prebiotic potential. Based on the 2,2-diphenylpicrylhydrazyl (DPPH) assay, it was further shown that the underconsumed banana cultivars of Klutuk and Norowito have high antioxidant activity [4]. Owing to these conditions, the unripe banana cultivars, particularly Klutuk and Norowito, may be employed as local and cheap functional feed ingredients for broilers.
Currently, the use of UBF for broiler chicken diets is still limited. To the best of our knowledge, only Dumorné et al. [7] used UBF as an energy source for broiler chickens. They confirmed that the inclusion of banana flour into broiler feeds of up to 20% did not adversely affect the growth performance of broilers [7]. However, the use of UBF as a functional feed ingredient and its impact on the physiological condition of broiler chickens have not been studied. Besides being applied individually, active components such as prebiotics are often combined with other active ingredients to obtain a synergistic effect so as to further increase their effectiveness in improving the physiological condition and health of broiler chickens. In this study, UBF as a functional feed ingredient was combined with probiotics or enzymes, and further evaluated its impact on physiological conditions in broilers. Overall, the purpose of this study was to determine the effect of UBF given individually or in combination with probiotics or enzymes on blood profile, serum biochemical parameters and intestinal indices of broiler chickens.
2. MATERIALS AND METHODS
2.1. Production of Unripe Banana Flour
The green banana “Norowito” cultivar was harvested from the banana garden in Pati district, Central Java province, Indonesia. After being peeled and washed, the banana was sliced and then sun-dried. The dried unripe banana was ground and sifted (2 mm) to yield UBF. The sample was obtained for the measurement of proximate contents [8], total phenols (Folin-Ciocalteu method [9]), antioxidant activity (2,2-diphenylpicrylhydrazyl [DPPH] method [10]) and pH values (Portable pH Meter OHAUS ST300). The proximate analysis documented that UBF contained crude protein of 3.89 ± 0.01, crude fibre 3.51 ± 0.30, ash 3.82 ± 0.06 and crude fat 0.32 ± 0.06% dry matter. Unripe banana flour also contained total phenols of 1.51 ± 0.02 mg/100 g, showed the DPPH scavenging activity of 94.4 ± 0.21% and had pH values of 6.04 ± 0.02.
2.2. Broiler Chicken Trial
The trial was set up in a completely randomized manner. During the first week of rearing, 392 Cobb broilers (unsex) were grown together using a commercial starter feed (according to the feed label; containing 22-24 percent crude protein, 5% maximum crude fiber, 5% crude fat, and 7% ash). The chicks were then individually weighed (mean body weight 180 ± 0.97 g) and randomly assigned to one of four treatment groups, each with seven replicates/pen and 14 chickens per pen (1×1 m2). The experimental groups included CONT (birds provided with control feed), UBF (birds provided with 5% UBF in feed), UBFPRO (birds provided with 5% UBF in feed plus 0.05% probiotics) and UBFZYM (birds provided with 5% UBF plus 0.05% multienzyme). Commercial probiotic (Vetafarm, Wagga Wagga, NSW, Australia) used in this study contained 1.8 × 108 cfu/g of Lactobacillus acidophilus, L. rhamnosus, L. plantarum, L. delbrueckii ssp. bulgaricus, Bifidobacterium bifidum, Enterococcus faecium and Streptococcus salivarius ssp. thermophilus. The multienzyme (Natuzyme, Bioproton Europe Oy, Kaarina, Finland) used was composed of cellulose (6,000,000 u/kg), xylanase (10,000,000 u/kg), β-glucanase (700,000 u/kg), α-amylase (700,000 u/kg), pectinase (70,000 u/kg), protease (3,000,000 u/kg), lipase (5,000 u/kg) and phytase (1,300,000 u/kg).
| Items (%, Unless Otherwise Noted) | Grower Feeds (Day 8-21) | Finisher Feeds (Day 22-38) | ||
|---|---|---|---|---|
| CONT | UBF, UBFPRO, UBFZYM | CONT | UBF, UBFPRO, UBFZYM | |
| Yellow maize | 56.7 | 51.1 | 65.0 | 59.3 |
| Palm oil | 1.15 | 1.25 | 1.25 | 1.35 |
| Soybean meal | 39.6 | 40.1 | 31.2 | 31.8 |
| DL-methionine, 990 g | 0.20 | 0.20 | 0.20 | 0.20 |
| Bentonite | 0.50 | 0.50 | 0.50 | 0.50 |
| Limestone | 0.50 | 0.50 | 0.50 | 0.50 |
| Monocalcium phosphate | 0.55 | 0.55 | 0.55 | 0.55 |
| Premix1 | 0.35 | 0.35 | 0.35 | 0.35 |
| Chlorine chloride | 0.10 | 0.10 | 0.10 | 0.10 |
| Salt | 0.35 | 0.35 | 0.35 | 0.35 |
| Unripe banana flour | - | 5.00 | - | 5.00 |
| Calculated chemical constituents | - | - | - | - |
| ME (kcal/kg)2 | 2,901 | 2,900 | 3,001 | 3,000 |
| Crude protein, % | 22.0 | 22.0 | 19.0 | 19.0 |
| Crude fibre, % | 5.65 | 5.61 | 5.75 | 5.71 |
| Ca, % | 0.77 | 0.76 | 0.75 | 0.74 |
| P (available), % | 0.45 | 0.42 | 0.46 | 0.44 |
2ME (metabolizable energy) was determined based on formula [30]: 40.81 {0.87 [crude protein + 2.25 crude fat + nitrogen‐free extract] + 2.5}.
CONT: birds provided with control feed, UBF: birds provided with 5% unripe banana flour in feed, UBFPRO: birds provided with 5% unripe banana flour in feed plus 0.05% probiotics, UBFZYM: birds provided with 5% unripe banana flour plus 0.05% multienzyme.
The birds were reared in a broiler houses with an open house system using rice husks as litter. During the trial, the lighting was provided continuously throughout the day. The feed (mashed form; Table 1) was rationed for isocaloric and isonitrogenous, and complied with the Indonesian National Standard for broiler growers (days 8-21) and finisher feeds (days 22-38). Chickens were vaccinated with Newcastle disease (ND) and Infectious Bronchitis (IB) vaccines by spraying immediately after hatching. The ND vaccination was also carried out by drinking water to chickens at the age of 18 days. Body weight (weighed individually), feed consumption and feed efficiency were recorded weekly. On day 21, blood was drawn from the wing vein of two chicks (one male and one female) from each pen (14 chickens per treatment group). Seven birds per treatment group of identical male chicks from whose blood had been drawn were then slaughtered. The intestines were then removed, and the duodenum, jejunum, and ileum were put in 10% buffered formalin for evaluation of the small intestinal morphology (villi height and crypt depth). To count the population of selected bacteria in the intestine, digesta was taken from the ileum and cecum. Additionally, a digital pH meter (Portable pH Meter OHAUS ST300) was used to measure the pH of the digesta in the duodenum, jejunum, ileum, and cecum. At the end of the trial, blood was collected from the wing veins of two chickens (one male and one female in each pen; a total of 14 birds per treatment group) and one male chick as blood sampled from each pen was slaughtered. As was done on day 21, samples were taken for the evaluation of intestinal bacterial counts, morphology, and pH.
Blood was placed in a vacutainer containing ethylenediaminetetraacetic acid (EDTA) for blood profile analysis, and the remaining blood was placed in a vacutainer without anticoagulant to produce serum. The Prima Fully-Auto Hematology Analyzer (PT. Prima Alkesindo Nusantara, Jakarta, Indonesia) was used to analyze a complete blood profile based on the manufacturer’s description. For serum production, the collected blood was left at room temperature for about 2 hours. To separate serum and blood clots, the blood was centrifuged for 10 minutes at 5,000 rpm. The serum was then stored in a freezer (at -10°C) until analysis. The profile of lipids (total triglycerides, total cholesterol, low-density lipoprotein and high-density lipoprotein) and the concentrations of uric acid, and creatinine were determined on the basis of enzyme-based colorimetric techniques. Spectrophotometric/photometric techniques were used to determine the levels of total protein, albumin, glucose, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) in broiler chicken serum. To obtain the values of globulin concentration, the total protein value was subtracted from the albumin value in serum. All biochemical analyzes of serum samples were performed according to the manufacturer’s description (DiaSys Diagnostic System GmbH, Holzheim, Germany). Hemagglutination inhibition (HI) test was used to determine the antibody titer against NDV, and the results were presented as geometric mean titers (log2). Coliform and lactose-negative Enterobacteriaceae populations were counted as red and colourless colonies on MacConkey agar (Merck KGaA, Darmstadt, Germany) after 24 hours of aerobic incubation at 38°C. The sum of coliform and lactose-negative Enterobacteriaceae was considered as the number of Enterobacteriaceae. After anaerobic incubation at 38°C for 48 hours on de Man Rogosa and Sharpe (MRS; Merck KGaA) agar, the number of lactic acid bacteria was counted.
3. RESULTS
3.1. Complete Blood Counts of Broiler Chickens
Data on complete blood counts of broiler chickens measured on days 21 and 38 are presented in Table 2. On day 21, the values of mean corpuscular volume (MCV) were lower (p < 0.05) in UBF than that of UBFPRO, but did not differ from that of CONT and UBFZYM. On day 38, the values of haemoglobin were higher (p < 0.05) in UBFZYM than that in CONT and UBF, but was not different from that of UBFPRO. The levels of mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC) were higher (p < 0.05) in UBFZYM than those in other treatment groups. Moreover, UBFZYM had greater (p < 0.05) numbers of leukocytes than CONT, but did not substantially differ from UBF and UBFPRO groups.
3.2. Biochemical Parameters of Serum of Broiler Chickens
The data on serum biochemistry and antibody titers of broilers toward NDV are listed in Table 3. At the measurement of day 21, the levels of uric acid were lower (p < 0.05) in UBFZYM than that in CONT, but not different from UBF and UBFPRO. The other serum biochemical parameters did not differ (p > 0.05) across the treatment groups for the measurement on days 21 and 38.
3.3. pH Values and Bacterial Populations of Intestine of Broiler Chickens
Data on the pH values across the intestinal segments are presented in Table 4. There was no effect (p > 0.05) of dietary treatments on the intestinal pH values at the measurement of day 21. On day 38, UBFPRO chicks tended to have lower (p = 0.05) pH values in duodenum than that of other treatment groups. In the ileum, UBFZYM tended to have higher (p = 0.05) pH, while in the caecum UBFPRO had higher (p = 0.05) pH values than that of CONT groups.
| Items | CONT | UBF | UBFPRO | UBFZYM | SEM | p value |
|---|---|---|---|---|---|---|
| Day 21 | - | - | - | - | - | - |
| Erythrocytes (1012/L) | 2.30 | 2.47 | 2.55 | 2.34 | 0.07 | 0.55 |
| Haemoglobin (g/dL) | 9.46 | 9.88 | 11.0 | 11.2 | 0.56 | 0.67 |
| Haematocrits (%) | 30.8 | 32.3 | 37.0 | 31.0 | 1.13 | 0.16 |
| MCV (fl) | 135ab | 132b | 136a | 135ab | 0.59 | 0.04 |
| MCH (pg) | 43.1 | 47.5 | 37.4 | 42.3 | 2.69 | 0.62 |
| MCHC (g/dL) | 32.5 | 36.7 | 27.7 | 32.6 | 2.19 | 0.54 |
| RDW-SD (fl) | 58.2 | 52.3 | 55.8 | 58.2 | 1.23 | 0.29 |
| RDW-CV (%) | 11.4 | 10.5 | 10.8 | 11.4 | 0.24 | 0.45 |
| MPV (fl) | 10.1 | 9.45 | 9.54 | 10.1 | 0.17 | 0.47 |
| Leukocytes (109/L) | 85.8 | 89.2 | 89.1 | 117 | 6.99 | 0.39 |
| Heterophils (109/L) | 4.73 | 4.69 | 5.03 | 5.67 | 0.29 | 0.64 |
| Lymphocytes (109/L) | 81.1 | 84.5 | 78.4 | 81.6 | 2.35 | 0.84 |
| Thrombocytes (109/L) | 12.2 | 13.2 | 13.0 | 15.1 | 1.03 | 0.81 |
| Day 38 | - | - | - | - | - | - |
| Erythrocytes (1012/L) | 2.88 | 2.78 | 3.25 | 3.34 | 0.13 | 0.37 |
| Haemoglobin (g/dL) | 10.6b | 10.3b | 12.0ab | 14.8a | 0.62 | 0.04 |
| Haematocrits (%) | 36.3 | 34.7 | 40.6 | 46.8 | 2.10 | 0.17 |
| MCV (fl) | 128 | 125 | 126 | 128 | 0.71 | 0.34 |
| MCH (pg) | 36.9b | 37.0b | 37.1b | 41.3a | 0.54 | <0.01 |
| MCHC (g/dL) | 29.3b | 29.8b | 29.8b | 32.5a | 0.44 | 0.03 |
| RDW-SD (fl) | 47.4 | 49.4 | 48.3 | 48.8 | 0.60 | 0.69 |
| RDW-CV (%) | 9.79 | 10.4 | 10.2 | 10.1 | 0.13 | 0.33 |
| MPV (fl) | 9.15 | 9.27 | 9.26 | 9.29 | 0.08 | 0.92 |
| Leukocytes (109/L) | 78.4b | 94.5ab | 102ab | 127a | 5.94 | 0.03 |
| Heterophils (109/L) | 7.11 | 10.4 | 7.25 | 8.36 | 0.58 | 0.15 |
| Lymphocytes (109/L) | 83.0 | 84.0 | 94.9 | 118 | 5.72 | 0.10 |
| Thrombocytes (109/L) | 16.8 | 15.2 | 15.6 | 18.9 | 0.84 | 0.40 |
MCV: mean corpuscular volume, MCH: mean corpuscular haemoglobin, MCHC: mean corpuscular haemoglobin concentration, RDW-SD: red blood cell distribution width-standard deviation, RDW-CV: red blood cell distribution width-coefficient variation, CONT: birds provided with control feed, UBF: birds provided with 5% unripe banana flour in feed, UBFPRO: birds provided with 5% unripe banana flour in feed plus 0.05% probiotics, UBFZYM: birds provided with 5% unripe banana flour plus 0.05% multienzyme, SEM: standard error of the means
Table 3.
| Items | CONT | UBF | UBFPRO | UBFZYM | SEM | p value |
|---|---|---|---|---|---|---|
| Day 21 | - | - | - | - | - | - |
| Total cholesterol (g/dL) | 99.5 | 103 | 104 | 108 | 2.86 | 0.76 |
| Total triglyceride (g/dL) | 53.5 | 59.8 | 54.6 | 65.0 | 2.87 | 0.48 |
| LDL (g/dL) | 16.4 | 16.8 | 18.2 | 16.3 | 0.87 | 0.87 |
| HDL (g/dL) | 55.9 | 63.1 | 60.4 | 62.0 | 1.51 | 0.36 |
| Total protein (g/dL) | 2.65 | 2.54 | 2.58 | 2.74 | 0.05 | 0.59 |
| Albumin (g/dL) | 1.21 | 1.20 | 1.18 | 1.23 | 0.02 | 0.92 |
| Globulin (g/dL) | 1.45 | 1.34 | 1.40 | 1.52 | 0.04 | 0.37 |
| Uric acid (mg/dL) | 10.7a | 8.62ab | 9.60ab | 7.33b | 0.44 | 0.04 |
| Creatinine (mg/dL) | 0.06 | 0.05 | 0.06 | 0.06 | <0.01 | 0.35 |
| AST (U/L) | 203 | 264 | 253 | 222 | 16.2 | 0.54 |
| ALT (U/L) | 3.19 | 2.84 | 2.66 | 2.73 | 0.29 | 0.93 |
| Antibody titers (Log2 GMT) | 2.07 | 2.07 | 2.00 | 2.07 | 0.09 | 0.99 |
| Day 38 | - | - | - | - | - | - |
| Total cholesterol (g/dL) | 89.5 | 79.9 | 95.3 | 96.2 | 2.78 | 0.14 |
| Total triglyceride (g/dL) | 48.6 | 48.9 | 57.8 | 50.1 | 2.59 | 0.57 |
| LDL (g/dL) | 24.3 | 25.9 | 21.4 | 20.2 | 1.19 | 0.31 |
| HDL (g/dL) | 69.6 | 63.9 | 65.8 | 68.6 | 2.04 | 0.74 |
| Total protein (g/dL) | 2.18 | 2.15 | 2.33 | 2.38 | 0.06 | 0.54 |
| Albumin (g/dL) | 0.86 | 0.91 | 0.96 | 0.88 | 0.03 | 0.77 |
| Globulin (g/dL) | 1.32 | 1.24 | 1.37 | 1.50 | 0.06 | 0.53 |
| Uric acid (mg/dL) | 4.80 | 4.53 | 5.06 | 5.40 | 0.25 | 0.66 |
| Creatinine (mg/dL) | 0.09 | 0.08 | 0.08 | 0.08 | <0.01 | 0.31 |
| AST (U/L) | 236 | 220 | 283 | 228 | 14.8 | 0.44 |
| ALT (U/L) | 1.18 | 1.16 | 1.31 | 1.02 | 0.09 | 0.73 |
| Antibody titers (Log2 GMT) | 1.14 | 1.71 | 0.79 | 1.36 | 0.20 | 0.44 |
LDL: low-density lipoprotein, HDL: high-density lipoprotein, A/G ratio: albumin to globulin ratio, AST: aspartate aminotransferase, ALT: alanine aminotransferase, GMT: geometric mean titers, CONT: birds provided with control feed, UBF: birds provided with 5% unripe banana flour in feed, UBFPRO: birds provided with 5% unripe banana flour in feed plus 0.05% probiotics, UBFZYM: birds provided with 5% unripe banana flour plus 0.05% multienzyme, SEM: standard error of the means.
| Items | CONT | UBF | UBFPRO | UBFZYM | SEM | p value |
|---|---|---|---|---|---|---|
| Day 21 | - | - | - | - | - | - |
| Duodenum | 6.68 | 6.43 | 6.48 | 6.70 | 0.08 | 0.54 |
| Jejunum | 5.83 | 5.83 | 6.15 | 5.92 | 0.14 | 0.86 |
| Ileum | 6.39 | 5.79 | 5.74 | 5.77 | 0.15 | 0.38 |
| Cecum | 6.77 | 6.55 | 6.67 | 6.68 | 0.18 | 0.98 |
| Day 38 | - | - | - | - | - | - |
| Duodenum | 6.03 | 6.02 | 5.68 | 6.04 | 0.06 | 0.05 |
| Jejunum | 5.71 | 5.71 | 5.64 | 5.55 | 0.11 | 0.96 |
| Ileum | 4.94 | 5.46 | 5.37 | 5.98 | 0.14 | 0.05 |
| Cecum | 6.47 | 6.53 | 6.72 | 6.53 | 0.04 | 0.05 |
The data on the selected bacterial populations in the intestine of broilers obtained on days 21 and 38 presented in Table 5. At the measurement of day 38, the numbers of lactic acid bacteria were higher (p < 0.05) in the ileum of UBFPRO and UBFZYM than that of CONT, but did not differ from that of UBF. The lactic acid bacteria to coliform ratio also tended to be higher (p = 0.07) in the ileum of UBFPRO and UBFZYM than that of CONT. There was no notable effect of dietary treatments on the bacterial populations on the cecum of broilers on day 38. Moreover, no significant effect of dietary treatments on the bacterial populations in the ileum and caecum of broilers on day 21.
3.4. Morphology of Intestine of Broiler Chickens
The data on the intestinal morphology of broilers are presented in Table 6. On day 21, there was a tendency (p = 0.06) for crypt depth to be greater in UBF than in CONT, but no significant difference in intestinal morphology in broiler jejunum and ileum was observed. At the measurement on day 38, crypt depth of duodenum was lower (p < 0.05) in UBFZYM than in CONT, but did not differ from that of UBF and UBFPRO. The villus height to crypt depth ratio of the duodenum was higher in UBFZYM (p < 0.05) than in the other treatment groups. While there was no significant difference in jejunal morphology, the villus height to crypt depth ratio was higher (p < 0.05) in the UBF ileum than in the CONT.
| Items (log cfu/g) | CONT | UBF | UBFPRO | UBFZYM | SEM | p value |
|---|---|---|---|---|---|---|
| Day 21 | ||||||
| Ileum | - | - | - | - | - | - |
| LAB | 5.88 | 5.26 | 4.60 | 6.64 | 0.46 | 0.45 |
| Coliform | 3.85 | 3.51 | 2.61 | 3.89 | 0.29 | 0.39 |
| LNE | 2.19 | 2.64 | 2.00 | 2.60 | 0.19 | 0.58 |
| Enterobacteriaceae | 3.94 | 3.72 | 2.82 | 4.03 | 0.28 | 0.41 |
| LAB/coliform | 1.52 | 1.69 | 1.71 | 1.99 | 0.15 | 0.76 |
| Cecum | - | - | - | - | - | - |
| LAB | 8.12 | 7.73 | 7.91 | 7.57 | 0.23 | 0.87 |
| Coliform | 5.07 | 5.54 | 5.89 | 5.28 | 0.21 | 0.55 |
| LNE | 3.87 | 4.01 | 3.76 | 3.84 | 0.26 | 0.99 |
| Enterobacteriaceae | 5.47 | 5.58 | 5.91 | 5.37 | 0.21 | 0.82 |
| LAB/coliform | 1.77 | 1.42 | 1.35 | 1.41 | 0.08 | 0.25 |
| Day 38 | ||||||
| Ileum | - | - | - | - | - | - |
| LAB | 3.81b | 4.66ab | 6.04a | 6.18a | 0.34 | 0.03 |
| Coliform | 2.36 | 2.21 | 2.72 | 2.33 | 0.18 | 0.79 |
| LNE | 2.64 | 2.24 | 2.00 | 2.66 | 0.23 | 0.72 |
| Enterobacteriaceae | 3.22 | 2.53 | 2.94 | 3.20 | 0.25 | 0.77 |
| LAB/coliform | 1.43 | 1.84 | 2.19 | 2.41 | 0.14 | 0.07 |
| Cecum | - | - | - | - | - | - |
| LAB | 7.55 | 8.26 | 8.35 | 7.28 | 0.30 | 0.53 |
| Coliform | 4.29 | 4.25 | 5.34 | 4.44 | 0.25 | 0.39 |
| LNE | 3.01 | 3.12 | 3.32 | 3.29 | 0.24 | 0.97 |
| Enterobacteriaceae | 4.76 | 4.47 | 5.48 | 4.80 | 0.22 | 0.45 |
| LAB/coliform | 1.87 | 2.20 | 1.58 | 1.97 | 0.15 | 0.57 |
LAB: lactic acid bacteria, LNE: Lactose negative Enterobacteriaceae, CONT: birds provided with control feed, UBF: birds provided with 5% unripe banana flour in feed, UBFPRO: birds provided with 5% unripe banana flour in feed plus 0.05% probiotics, UBFZYM: birds provided with 5% unripe banana flour plus 0.05% multienzyme, SEM: standard error of the means.
| Items | CONT | UBF | UBFPRO | UBFZYM | SEM | p value |
|---|---|---|---|---|---|---|
| Day 21 | ||||||
| Duodenum | - | - | - | - | - | - |
| Villi height (µm) | 961 | 1,194 | 972 | 1,204 | 62.0 | 0.33 |
| Crypt depth (µm) | 179 | 241 | 184 | 213 | 9.38 | 0.06 |
| VH/CD ratio | 5.44 | 5.07 | 5.40 | 5.65 | 0.28 | 0.92 |
| Jejunum | - | - | - | - | - | - |
| Villi height (µm) | 544 | 522 | 479 | 540 | 47.1 | 0.97 |
| Crypt depth (µm) | 113 | 153 | 112 | 123 | 8.42 | 0.28 |
| VH/CD ratio | 4.66 | 3.54 | 4.80 | 4.50 | 0.34 | 0.57 |
| Ileum | - | - | - | - | - | - |
| Villi height (µm) | 456 | 516 | 435 | 412 | 19.8 | 0.29 |
| Crypt depth (µm) | 121 | 145 | 123 | 129 | 7.07 | 0.65 |
| VH/CD ratio | 4.10 | 3.72 | 3.63 | 3.42 | 0.23 | 0.79 |
| Day 38 | ||||||
| Duodenum | - | - | - | - | - | - |
| Villi height (µm) | 1181 | 1191 | 1090 | 1282 | 36.9 | 0.35 |
| Crypt depth (µm) | 339a | 314ab | 293ab | 243b | 12.0 | 0.02 |
| VH/CD ratio | 3.53b | 3.84b | 3.93b | 5.51a | 0.25 | 0.01 |
| Jejunum | - | - | - | - | - | - |
| Villi height (µm) | 1060 | 1096 | 974 | 1072 | 34.2 | 0.64 |
| Crypt depth (µm) | 298 | 299 | 328 | 248 | 15.5 | 0.34 |
| VH/CD ratio | 3.63 | 3.88 | 3.16 | 4.78 | 0.25 | 0.13 |
| Ileum | - | - | - | - | - | - |
| Villi height (µm) | 831 | 1029 | 895 | 1025 | 51.3 | 0.45 |
| Crypt depth (µm) | 198 | 166 | 194 | 207 | 10.9 | 0.60 |
| VH/CD ratio | 4.19b | 6.81a | 5.03ab | 5.04ab | 0.35 | 0.04 |
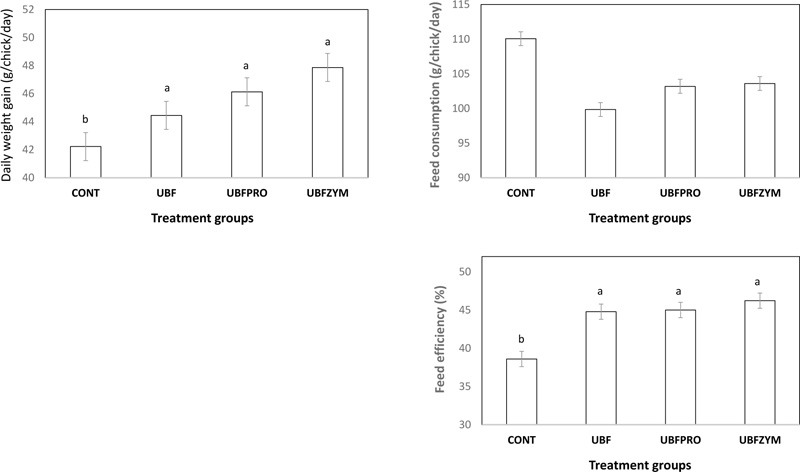
3.5. Performance of Broilers
Daily weight gain and feed efficiency were higher (p < 0.05) in UBF, UBFPRO and UBFZYM when compared with that CONT broilers. There was no significant difference in the feed consumption of broilers among treatment groups (Fig. 1).
4. DISCUSSION
On day 21, the chicks in UBF group had relatively lower levels of MCV as compared to other treatment groups. In most conditions, the low MCV is generally attributed to anemia condition in chickens. However, the anemia seemed not to occur in our current study as the levels of MCV in all treatment groups were within the normal range (normal MCV values in broilers are 90-140 fl [11]). Also, the difference in MCV levels was no longer observed on day 38 across the treatment groups. On the measurement of day 38, the values of haemoglobin, MCH and MCHC were higher in UBFZYM than those in CONT and UBF chicks. A previous study by Attia et al. [12] reported that multienzymes supplementation was associated with the enhanced levels of haemoglobin, MCH and MCHC of broilers. Literature further suggested that multienzymes treatment improved the apparent nutrient digestibility of broilers, and thus enhanced the availability of nutrients for energy metabolism to support the growth [12, 13]. Considering the general role of haemoglobin in supporting the metabolic activity of chickens, the increased levels of haemoglobin, MCH and MCHC due to multienzymes supplementation may therefore be a response of broilers to supply more oxygen to cells for the metabolic activity of chickens. Our inference was actually supported by the fact that UBFZYM chickens had the highest final body weight among the groups of chickens. With regards to the absence of effect of unripe banana flour (source of prebiotics) and probiotics on the haemoglobin profile of broilers, the corresponding results were also reported by other authors in which administration of probiotics Lactobacillus paracasei [14] and Bacillus coagulans or Lactobacillus sp. [15]. resulted in no impact on erythrocyte profiles including haemoglobin, MCH and MCHC of broilers. Moreover, Al-Khalaifa et al. [15] documented no effect of prebiotics (mannan-oligosaccharide and fructo-oligosaccharide) on haemoglobin, MCH and MCHC of broilers.
It was shown in this present study that the numbers of leukocytes were higher in UBFZYM than that in CONT, while the effect of unripe banana flour was not substantial on the numbers of leukocytes. This may suggest that irrespective of the unripe banana flour effect, the multienzyme supplementation resulted in improved immune competencies of broilers. Our result was in accordance with Attia et al. [13] showing an increased number of leukocytes and lymphocytes with multienzyme+phytase supplementation in broiler diets. In line with this, Attia et al. [12] documented that enzyme supplementation improved immune competencies as indicated by the improvement in lymphoid organ development of broilers. They further confirmed that the increased follicular diameter of the bursa of Fabricius due to exogenous enzymes resulted in increased lymphocyte cell production. In accordance, the higher number of lymphocytes was also observed in the UBFZYM group as compared to other groups in this study. Attia et al. [12] further suggested that the improved immune system of enzyme-treated broilers was also due to the increased nutrient available for the production of immune cells.
It was clear in this present study that the levels of uric acid were lower in UBFZYM than in the control group. In most conditions, the increased blood uric acid levels may be attributed to muscle proteolysis (protein degradation) in poultry [16]. One of the factors that can induce protein degradation is low nutrient digestibility so that the availability of nutrients, especially protein, decreases. To address the lack of protein needed for physiological processes, the body will increase muscle proteolysis, as a result, uric acid production increases [16]. Considering the role of multienzyme in enhancing nutrient digestibility especially protein [12, 13], the low levels of uric acid in the serum of broilers treated with multienzyme may therefore be attributed to the less muscle protein degradation or sufficient protein supply in the body to support physiological processes of broilers. Our latter inference was actually supported by the highest final body weight of multienzyme-treated broilers as compared to other chicken groups. In this study, the effect of unripe banana flour was not substantial on the levels of uric acid. With regard to the prebiotic content of unripe banana flour, a study by Biswas et al. [17] noticed that prebiotic (fructo-oligosaccharides and mannan-oligosaccharides) treatment decreased uric acid concentration in the blood of broilers. In contrast, a study by Tang et al. [18] revealed that prebiotic treatment did not affect the levels of uric acid in laying hens. The differences in the nature and level of prebiotics administrated to poultry feed, feed composition (protein content), as well as the species and age of poultry were most likely to be responsible for the inconsistent impact of prebiotics on the protein metabolism and hence uric acid levels in the blood.
It was shown in this present study that on day 38 the pH values were lower in the duodenum of UBFPRO broilers as compared to other groups. Considering the absent difference in duodenal pH between CONT and UBF, the lower duodenal pH values of UBFPRO could be accounted to the role of probiotics administrated in the diets. This inference was supported by Machado et al. [19] who reported that the low pH values in the intestine of probiotic-treated chicks were attributed to the high population of lactic acid bacteria which produce lactic acid, and thus lowering the pH values of the intestine. In contrast to that of occurring in the duodenum, the pH values were higher in the caecum of UBFPRO as compared especially to control broilers. This finding was different from Machado et al. [19] showing the lowest pH values in the caecum of broiler receiving probiotics. In this study, the rationale for the higher caecal pH values due to probiotic treatment remains unclear. However, the improved nutrient digestibility and absorption in the upper intestinal part due to probiotics [20] may reduce the amount of feed reaching the caecum. The latter condition may therefore reduce the amount of substrates available for the growth of lactic acid producing bacteria and thus having an impact on the higher pH value in the cecum. With regard to the condition in ileum, the pH values were higher in UBFZYM than that in CONT broilers. In line with our finding, Gao et al. [21] reported that the pH of the digesta in the intestine (duodenum and jejunum) increased following the exogenous enzyme (xylanase) supplementation. Similar to the conditions in this present study, the elevated pH values in the ileum of broilers supplemented with multienzyme appeared to be related to more complete nutrient digestion (due to greater enzymatic activity of multienzyme), which therefore limited the amount of substrate available to support the growth of lactic acid bacteria in the ileum of broilers [19, 21].
It was shown in this study that the effect of unripe banana flour was not significant on the numbers of selected bacteria in the ileum and caecum of broilers at the measurements of day 21 and 38. Being considered as prebiotic source, the unripe banana flour neither increased the numbers of lactic acid bacteria, nor lowered coliform, lactose negative Enterobacteriaceae and Enterobacteriaceae. Previous study by Al-Khalaifa et al. [15] did not observe any influence of dietary administration of fructo-oligosaccharides (FOS) and mannan-oligosaccharides (MOS) on the numbers of lactic acid bacteria and Escherichia coli in the intestine of broilers. The inconsistent results of prebiotics on broiler chickens were also reported by Yang et al. [22]. They suggested that the inconsistent results when using prebiotics as feed additive may be attributed to the different natures and levels of prebiotics, species or strains of broilers and diets used during the study, as well as rearing conditions. In this study, the numbers of lactic acid bacteria and lactic acid bacteria to coliform ratio were higher in UBFPRO and UBFZYM than that of CONT. In respect of the lack effect of unripe banana flour, the administration of probiotics or multienzyme was therefore able to increase the growth of lactic acid bacteria in the ileum of broilers. Sugiharto [20] previously revealed that probiotics may create a more comfortable condition in the intestine (by producing organic acid and excluding potential pathogenic bacteria) and thus improve the growth of beneficial bacteria including lactic acid bacteria. In term of enzyme, Sugiharto [20] also suggested that exogenous enzyme may increase the production of lactic and other organic acids, which are very beneficial for the growth of lactic acid bacteria and other commensal bacteria in the intestine of broilers. The capability or enzyme in improving the availability of nutrients seemed also to be responsible for the availability of substrates needed by commensal bacteria to grow. The ratio of lactic acid bacteria to coliform bacteria in the intestine has been proposed as an indicator of gut health, with higher lactic acid bacteria to coliform ratio being associated with better intestine health in broilers (Vimon et al., 2020). Indeed, a higher lactic acid bacteria count is expected to inhibit the colonization of pathogenic bacteria such as coliform in broiler intestines [20, 23]. In line with the lactic acid bacteria population, broilers given probiotics or multienzyme had higher lactic acid bacteria to coliform ratio levels in their ileum than that of controls.
A study revealed that prebiotics improved the intestinal morphology of broilers [20]. In line with this, Alyileili et al. [24] found that using degraded date pits as a prebiotic source increased villus height, villus height to crypt depth ratio, and decreased crypt depth in broiler intestine. Although it was not significantly observed in the small intestinal segments at day 21 or the duodenum and jejunum at day 38, the ileum of UBF chicks had a higher villus height to crypt depth ratio than the control at day 38. This may therefore suggest that unripe banana flour can act as a prebiotic, which can help improve the morphology of broiler ileal segments. In poultry, a higher villous height to crypt depth ratio indicates a greater capacity for nutrient digestibility and absorption [25]. According to observations on the duodenal segment on day 38, the ratio of villous height to crypt depth in UBFZYM was significantly higher than in other birds. Given the importance of the duodenum in digestive and absorptive functions, the higher ratio of villous height to crypt depth may indicate that UBFZYM had better digestive and absorptive capacity than the birds in the other treatment groups. Indeed, the fact that the UBFZYM had the highest average body weight gain and feed efficiency among the bird groups supported our inference. In this present study, the higher ratio of villous height to crypt depth in the duodenum of UBFZYM broilers was attributed to the lower crypt depth in the respective chicks. Longer villi and shallower crypts, according to Alyileili et al. [24], promote nutrient absorption by activating intestinal cell maturation and digestive enzyme activity. In this regard, the lower crypt depth of UBFZYM may be attributed to the chicks’ improved nutrient absorption as well as a reduction in the metabolic cost of intestinal epithelium turnover [26]. In this study, the higher duodenal villus height to crypt depth ratio of the UBFZYM chicks appeared to be attributed to the role of enzymes in improving the morphology of the broiler small intestine. Previously, Yang et al. [27] confirmed that xylanase supplementation of wheat-based diets did not change villus height but did reduce jejunal crypt depth in 7-day-old broilers. Moreover, Mathlouthi et al. [28] found that 22-day-old broilers fed diets based on corn or wheat with β-glucanase + xylanase had higher villus height and villus height to crypt depth ratio in the ileum than those fed a wheat-based diet with no enzyme supplementation.
The current study found that using unripe banana flour improved broiler growth performance and feed efficiency of broiler chickens. These improvements could be attributed to the functional properties of unripe banana flour, such as prebiotic activity [4]. Indeed, research has shown that prebiotics can improve the digestive and absorptive functions of the broiler intestine [20], as evidenced by a higher villus height to crypt depth ratio, especially in the ileal segment in the present study. The antioxidative property of unripe banana flour [4] also appeared to be responsible for the improvement in physiological conditions, which ultimately increased energy allocation for growth. The combination of unripe banana flour and probiotic or multienzyme was expected to exert a synergistic effect on broiler growth performance. However, such a synergistic effect on broiler growth performance did not appear in the current study. Several factors, such as enzyme types and levels, prebiotic types, probiotic levels and strains, and so on, may influence the magnitudes of synergism among functional properties as revealed by El-Sayed et al. [29] (Table 1).
CONCLUSION
The broiler growth performance was improved when UBF was given alone or in combination with probiotics or multienzymes. The use of probiotics or multienzymes in combination with UBF improved intestinal bacterial population, while multienzymes improved broiler physiology and intestinal morphology.
LIST OF ABBREVIATIONS
| UBF | = Unripe Banana Flour |
| MCV | = Mean Corpuscular Volume |
| MCHC | = Mean Corpuscular Haemoglobin Concentration |
| AGP | = Antibiotic Growth Promoters |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The in vivo trial was approved by the Animal Ethics Committee of the Faculty of Animal and Agricultural Sciences, Universitas Diponegoro (No. 58-04a/A-5/KEP-FPP).
HUMAN AND ANIMAL RIGHTS
No humans were used in this study. All the reported experiments were in accordance with The US National Research Council's “Guide for the Care and Use of Laboratory Animals”.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data of current study are available from author, [S.S], on a reasonable request.
FUNDING
The study was financed by Universitas Diponegoro through World Class Research Universitas Diponegoro (WCRU) No. 118-06/UN7.6.1/PP/2021.
CONFLICT OF INTEREST
The authors had no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


