All published articles of this journal are available on ScienceDirect.
The Growth-promoting and Antipathogenic Effects of Microorganisms Isolated from Solanum nigrum L. and Inoculated in Solanum lycopersicum L.
Abstract
Background:
In recent years, there has been a growing scientific interest in the biodiversity and function of endophytic bacteria, as well as the prospects for their practical use.
Objective:
The purpose of this work was to isolate endophytic microorganisms from generative organs of the European black nightshade (Solanum nigrum L.), assess their biological activity, and test their growth-promoting/protective effects in tomato (Solanum lycopersicum L.) plants.
Methods:
From the tissues of generative organs of S. nigrum plants, 14 strains of endophytic microorganisms were isolated. Most of them belonged to Bacillus sp. The physiological and biochemical properties and enzymatic and oxidative metabolism of some of them were studied. Sequence analysis of 16S rRNA fragments revealed 99,65% similarity of BA1s-OSN-0820 and BAXS-OSN-0820 isolates to B. amyloliquefaciens strains, while the ITS sequence of the RHC-OSN-0820 isolate showed 99,65% of similarity to Rhodotorula kratochvilovae.
Results:
The actions of these endophytes against tomato pathogenic bacteria and fungi were then tested. The isolates had a significant bacteriostatic effect against Xanthomonas campestris, the causative agent of black bacterial spotting of leaf, and Clavibacter michiganensis, the causative agent of bacterial wilt, with a lower effect against Pseudomonas syringae, the causative agent of bacterial spotting. Isolates also showed selectivity against micromycetes that cause mycosis in tomatoes grown indoors, such as Fusarium oxysporum, Alternaria solani, Botrytis cinerea, Sclerotinia sclerotiorum, and F. acuminatum. In particular, the highest antifungal activity was detected against S. sclerotiorum and fungi of the Fusarium genus. Inoculation of tomatoes with endophytic microorganisms revealed a positive effect on seed germination efficiency and the stimulation of seedling growth. Thus, the effectiveness of interspecific transfer of endophytic microorganisms from a wild, S. nigrum, to a cultivated Solanum species, S. lycopersicum, was reported.
Conclusion:
A consortium of plant-associated microorganisms isolated from a wild relative has a positive effect on the germination of tomato seeds, stimulating the formation of the root system and nutrition of seedlings. The antagonism of the isolates against phytopathogenic fungi and bacteria provides long-term protection during the most critical stages of plant development and has prospects for the development of microbial biologics for cultivated plants of the Solanaceae family.
1. INTRODUCTION
An important component of the adaptive properties of plants is a complex of endophytic microorganisms, which, together with epiphytes, form the plant microbiome [1]. Endophytes are found in various plant organs, both in the root and aboveground structures, including flowers and seeds [2-7]. Endophytes take part in the protection of plants from diseases [8], show antagonistic activity against pathogens [9-11], are able to fix nitrogen [12], and synthesize plant growth regulators [13-18].
In recent years, there has been a growing scientific interest in the biodiversity and function of endophytic bacteria, as well as the prospects for their practical use [19-23]. Much attention is paid to the study of taxonomic groups of endophytes that colonize the generative organs of plants, potential pathways of their colonization, and plant-microbial interactions [23-25]. Microscopic analysis by in situ fluorescent hybridization showed the presence of Gammaproteobacteria (including Pseudomonas spp.) and Firmicutes (including Bacillus spp.), which were visualized inside the epidermis and xylem of the ovary and/or inside the seminal germ of many plant species. Firmicutes, mainly Bacillus spp., were further visualized inside the berries, in the intercellular spaces of pulp cells and/or cellulose xylem, as well as along some cell walls inside the seed of some species [25].
It has been studied that in some cases, endophytes are much more effective than some rhizospheric bacteria in stimulating plant growth and productivity, protecting them against diseases, pests and the effects of pollutants, and providing plants with mineral elements nutrition [26].
The ecological role of endophytic microorganisms in generative organs of plants of the Solanaceae family, their participation in the regulation of growth and development of tomato plants, pathogen resistance reactions and adaptation to changing living conditions are all issues insufficiently studied so far. In addition, a variety of factors, such as chemical treatments, and cultivation under artificially created conditions, significantly influence the balance of species composition and function of plant-associated bacteria [27, 28]. The loss of symbionts can be an undesirable and sometimes catastrophic event. It is possible that in the tissues and organs of each plant species, a specific and fairly balanced complex of endophytic bacteria and fungi is present as a result of a long-term co-evolutionary process. It is therefore conceivable that such a consortium of microorganisms is adapted to metabolism specific to individual plant species. However, it is also conceivable that wild relatives of crops cultivated nowadays are a reservoir of potentially useful microorganisms. Under this assumption, it would be interesting to explore the effects of the inoculation of crops with microorganisms associated with their wild relatives.
In this regard, the purpose of this work was to: a) isolate endophytic microorganisms from the generative organs of Solanum nigrum L., known as black nightshade, b) screen some of them for their biological activity, and c) transfer them in a noninvasive way to Solanum lycopersicum L. plants to test for their effectiveness in promoting the growth and protection of tomato plants from bacterial and fungal diseases.
2. MATERIALS AND METHODS
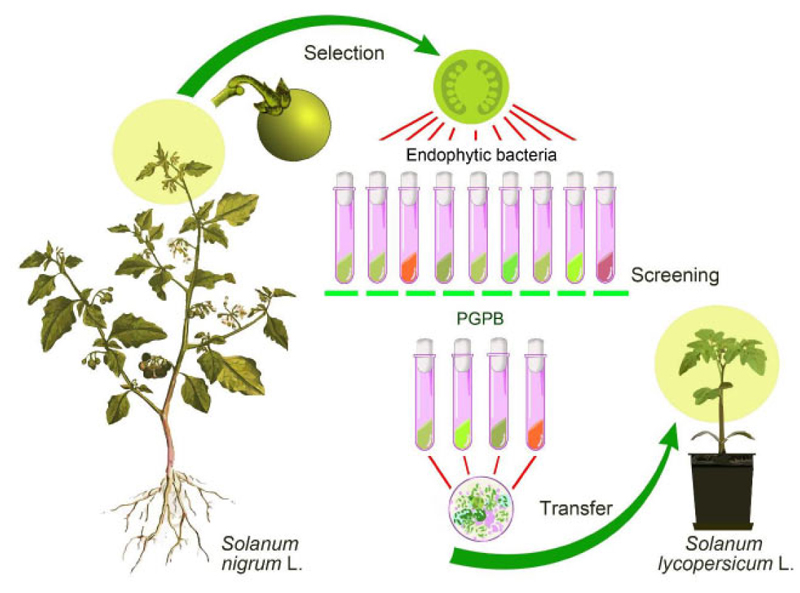
The procedure for isolating endophytic bacteria, their screening, and the interspecies transfer was carried out sequentially, as shown in Fig. (1).
The isolates of endophytic microorganisms from the tissues of generative organs of black nightshade (S. nigrum L.) were used in this research. Plant material (tops of shoots with buds) was collected in the second half of June, 2020, from the territory of the Boyarka forestry (block 140, allocation 4) on the site after reforestation. The geographical coordinates of the sampling site are 50°18'09.7”N 30°19'01.4”E.
The objectives of this research are to isolate endophytic microorganisms from the tissues of the seed bud of black nightshade flowers, gain their functional evidence, and assess their role in tomato plants (determinate tomato variety Sanka, and parthenocarpic hybrid tomato Ephemer F1, produce the early season fruits, Ukrainian selection).
The isolation of endophytic microorganisms, their growth-stimulating activity, and their ability to macerate plant tissues was performed as reported in Borkar [29]. Morphometric measurements of seedlings and seed germination assessment were carried out in accordance with State Standard of Ukraine DSTU 4138 2002 “Seeds of agricultural crops; Methods for quality determining”. Isolates of endophytic microorganisms were identified by morphological and cultural properties according to generally recognized methods in bacteriology and mycology [30]. The bacteria were Gram-stained and used to study the morphological features of bacteria. Also, spore formation, glucose fermentation, and oxygen requirements were studied. The cell morphology, inability to form spores and pseudomycelia, and the type of culture propagation of the yeasts were studied. The growth parameters on solid and liquid media of the endophytic microorganisms were studied [30].
The NEFERMtest24 test system (MikroLaTEST®, ErbaLachema, Czech Republic) was used to study the physiological and biochemical properties of bacterial isolates. Enzymatic and oxidative glucose metabolism (OF-test) was determined using a microplate (OFtest, Erba Lachema).
The antagonistic activity of endophytic bacterial isolates was determined by perpendicular streak technique by using as test cultures the following phytopathogenic bacteria: Pseudomonas syringae pv. syringae (van Hall) UKM В-1027, P. fluorescens Migula 7769, Pectobacterium carotovorum subsp. carotovorum (Jones) UKM В-1075, Xanthomonas campestris pv. campestris (Pammel) Dowson UKM В-1049, Clavibacter michiganensis subsp. michiganensis (Spieckermann & Kothoff) (102 Davis et al.), and Rhizobium vitis (Ophel and Kerr) Young et al. UKM В-1000. The strains of these phytopathogenic bacteria were from the Ukrainian Сollection of Microorganisms at D. K. Zabolotny Institute of Microbiology and Virology of the National Academy of Sciences of Ukraine (http://ucm.org.ua/). The degree of sensitivity of phytopathogenic bacteria to endophytic bacteria was determined by the width of the zone of no growth [29].
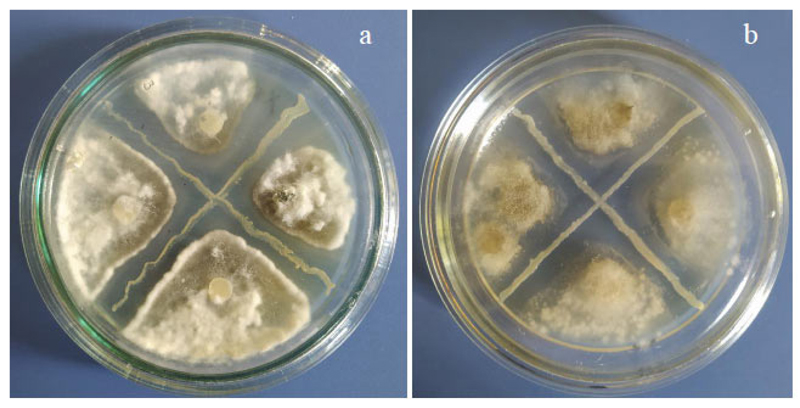
Determination of the antifungal activity of isolates against pathogens of mycoses of tomato plants (micromycetes, F. oxysporum Schlecht., Alternaria alternata (Fr.) Keissl., Botrytis cinerea Pers., Sclerotinia sclerotiorum (Lib.) de Bary, and F. acuminatum Ellis and Everhart) was performed using a perpendicular lines method [31]. Colonies of phytopathogenic fungi were placed between perpendicular strokes of bacterial colonies. The degree of antifungal activity was assessed according to a 4-point scale: “+++” - strong activity (inhibition zone > 10 mm), “++” - moderate activity (inhibition zone between 5 and 10 mm), “+” - weak activity, “-” - no activity (inhibition zone > 5 mm). Plant interaction with microbial phytopathogenic micromycetes was examined as described previously [31].
To perform molecular identification, the isolates were grown on potato glucose agar (PGA) for 48 hours, and then genomic DNA was isolated from the cell suspension using GeneJet Genomic DNA Purification Kit (ThermoScientific), according to the manufacturer's instructions. The PCR mixture to amplify the 16S rRNA gene and the ІТS region was performed in a volume of 25 µL containing 12.5 µL of the 2х DreamTaq PCR Master Mix (ThermoScientific), 30 pmoles of each primer, and 50 ng of DNA. Amplification of the 16S rRNA gene was performed using the primers 27f (5׳-AGAGTTTGATCMTGGCTCAG-3׳) and 1492r (5׳-CGGTTACCTTGTTACGACTT-3׳), that of ІТS with the primers ITS1 (5’-TCCGTAG-GTGAACCTGCGG-3׳) ITS4 (5’-TCCTCCGCTTATTGATATGC-3’). The concentration of the resulting amplicons was determined with a DS -11 FX+ spectrophotometer (DeNovix, USA). The purified PCR product was sequenced on a Genetic Analyzer 3130 (Applied Biosystems, USA) using the “BigDye Terminator v 3.1 Cycle Sequencing Kit” reagent kit. The resulting nucleotide sequence was compared with those present in the GenBank database using the NCBI Blastn program.
Phylogenetic analysis and alignment of nucleotide sequences were performed using the MEGA 6 program [32]. A dendrogram of phylogenetic connections was constructed using the neighbor-joining method utilizing a 2-parametric Kimura model based on 1000 bootstrap replicas. The 16S rRNA and ITS sequences of reference cultures of the genera Bacillus and Rhodotorula were retrieved from the GenBank database. Nucleotide sequences of the 16S rRNA gene of strains B. amyloliquefaciens BA1S-OSN-0820 and BAXS-OSN-0820 were stored in the GenBank database under the numbers MW255061.1 and MW255060.1, and the ITS-sequence fragment R.kratochvilovae RHC-OSN-0820 under the number MW255028.1.
The experiments of interspecies transfer of strains were carried out in the greenhouses of the National University of Life and Environmental Sciences of Ukraine. Seedlings were planted in a greenhouse in the development phase of the nine leaves with a density of 4 plants/m2. Plants were mainly cultivated in greenhouses during the spring-summer seasons in the plastic-covered greenhouse.
Working suspensions were prepared as complex of B. amyloliquefaciens BA1S-OSN-0820 and BAXS-OSN-0820 (2×109 CFU/ml), R. kratochvilovae RHC-OSN-0820 (0.8×109 CFU/ml) (in the proportion of 1:1:1, application rates 5-10 ml per 10 L of water). Throughout the ontogenesis of plants, 3-4 watering of the seedlings (30 plants treated) with a working suspension of strains with a dose of 1 L/m2 was performed.
Visual damage was recorded on ten randomly selected tomato plants on a scale from 0 to 5, with level 0 – healthy plants, no symptoms; level 1 – number of leaves with damage symptom to 10%; level 2 – from 11 to 25%; level 3 – from 26 to 50%; level 4 – from 51 to 75%.
Photo documentation and digital image processing were performed in a specialized program, Image-Pro Premier 9.0. The significance of differences (length of roots and shoots) between p-values (p < 0.05) was determined by the analysis of variance (ANOVA) method in the XLSTAT (Addinsoft Inc., USA, 2010). The data were compared using Tukey’s test. SigmaPlot 12.0 was used for regression analysis.
| Test | Isolates | ||||||
|---|---|---|---|---|---|---|---|
| B1S | BХS | B3S | B4S | B5S | B9S | B14S | |
| The form of cells | Rods | Rods | Rods | Rods | Rods | Rods | Rods |
| Spore formation | + | + | + | + | + | + | + |
| Gram reaction | + | + | + | + | + | + | + |
| Fermentation of glucose | |||||||
| aerobic | - | - | + | - | - | - | - |
| Anaerobic | - | - | - | - | - | - | - |
| Activity of pectolytic enzymes | - | - | + | + | + | + | + |
| NEFERMtest24 (MikroLaTEST®, ErbaLachema) | |||||||
| Urease | - | - | - | + | - | - | - |
| Arginine | - | - | + | - | - | - | - |
| Ornithine | + | + | - | + | + | - | - |
| Lysine | + | + | - | + | + | + | + |
| Acetamide | - | - | + | - | - | - | - |
| β-glucosidase | + | + | + | + | + | + | + |
| N-acetyl-β-D-Glucosamididase | - | - | - | - | - | - | - |
| Simpson Citrate | - | - | + | + | - | - | - |
| Lactose | - | - | - | - | - | - | - |
| Mannitol | - | - | - | + | - | - | - |
| Trehalose | - | - | + | + | - | - | - |
| Xylose | - | - | + | - | - | - | - |
| Arabinose | - | - | - | - | - | - | - |
| α-galactosidase | + | + | + | - | + | + | + |
| β-galactosidase | - | - | + | - | - | - | - |
| Malonat | - | - | + | - | - | - | - |
| Galactose | - | - | + | - | - | - | - |
| Maltose | - | - | + | - | - | - | - |
| Cellobiose | - | - | - | + | - | - | + |
| Sucrose | - | - | - | + | - | - | + |
| Inositol | - | - | - | - | - | - | - |
| γ- glutamyltransferase | + | + | + | - | + | + | - |
| Phosphatase | + | + | - | - | + | + | + |
| Esculin | + | + | + | + | + | + | + |
3. RESULTS AND DISCUSSION
A total of 14 isolates of endophytic microorganisms were obtained from the seed germ tissues of S. nigrum plants, among which dominant morphotypes were found (Table 1). Typical representatives of all morphotypes were selected to identify and study their properties. These were the bacterial isolates B1S, B3S, B4S, B5S, B9S, B14S, BХS and yeast isolate Y8S.
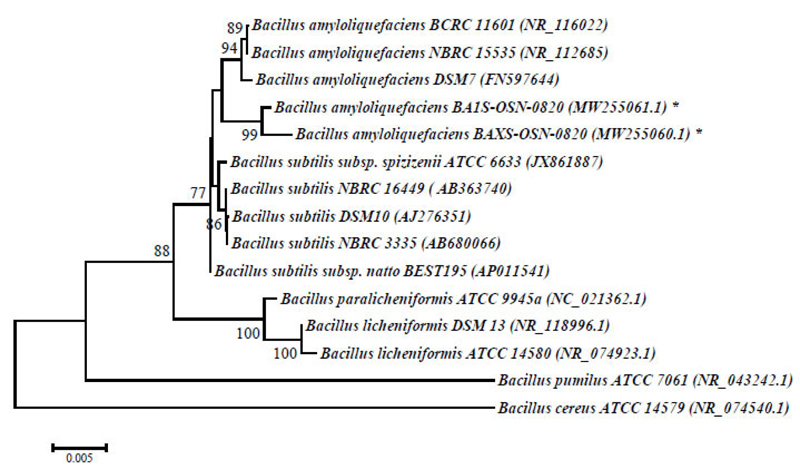
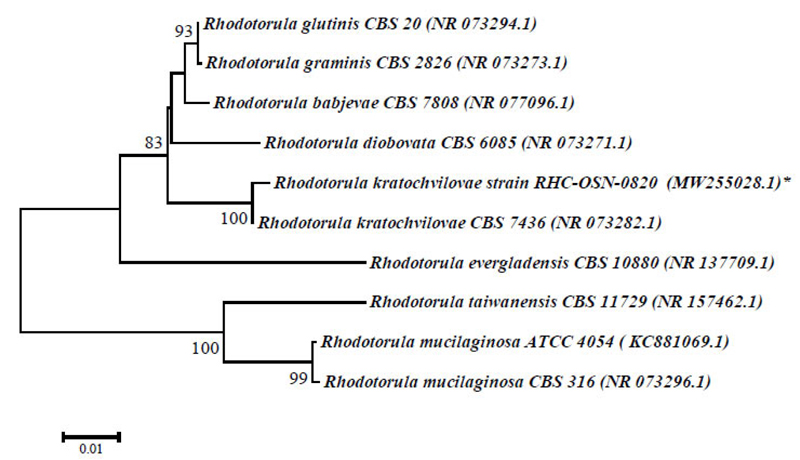
The physiological and biochemical properties and enzymatic and oxidative metabolism of bacterial isolates were studied. Based on the results of biochemical analyses and morphological and cultural properties, isolates В1S, B5S, B9S, B14S, ВXS were identified as B. amyloliquefaciens and isolates B3S, B4S as Bacillus sp., whereas the isolate Y8S was selected for further genetic identification (Table 1). The 1454-nt long sequences of the 16S rRNA gene of strains BA1S-OSN-0820 (B1S isolate) and BAXS-OSN-0820 (BXS isolate) showed 99,65% similarity to Bacillus amyloliquefaciens strains, and the 578-nt long ITS from RHC-OSN-0820 strain (Y8S isolate) showed 99,65% of similarity to Rhodotula kratochvilovae. The species identification of the selected strains was confirmed by phylogenetic analyses. As shown in Fig. (2), the BA1S-OSN-0820 and BAXS-OSN-0820 strains formed a cluster with representatives of species B. amyloliquefaciens, which is clearly different from the cluster of B. subtilis strains (Fig. 2), whereas, from Fig. (3), it can be seen that the RHC-OSN-0820 strain formed a group with the typical R. kratochvilovae CBS 7436 strain, which is reliably distinguished from other representatives of the genus Rhodotorula.
The ability of endophytic bacteria to biocontrol phytopathogens makes it possible to use them as biologics, so the antimicrobial activity of the isolates was screened. Their bacteriostatic and bactericidal actions and selectivity against strains of phytopathogenic bacteria P. syringae pv. syringae UKM В-1027, P. fluorescens 7769, P. carotovorum subsp. carotovorum UKM В-1075, X. campestris pv. campestris UKM В-1049, C. michiganensis subsp. michiganensis 102, and R. vitis UKM В-1000 were tested (Table 2).
| Phytopathogenic Bacteria | The Width of the Zone of No Growth, mm | |||
|---|---|---|---|---|
| B3S | B4S | B1S | BХS | |
| P. syringae pv. syringae UKM В-1027 | – | 5,2 ± 0,12 | 8,9 ± 0,21 | 7,2 ± 0,3 |
| P. fluorescens 7769 | – | – | >30 | – |
| P. carotovorum subsp. carotovorum UKM В-1075 | – | – | – | 25,6 ± 1,5 |
| X. campestris pv. campestris UKM В-1049 | – | 18,5 ± 1,3 | 18,7 ± 1,1 | >30 |
| C. michiganensis subsp. michiganensis 102 | – | 18,4 ± 1,1 | 18,6 ± 1,2 | 15,7 ± 1,1 |
| R. vitis UKM В-1000 | – | – | – | – |
The strains isolated did not show an antagonistic effect against the causative agent of the crown gall of tomato R. vitis, a disease first described in indoor tomatoes in the CIS countries in 2013, causing a significant decrease in commercial yield by 15-50%. The lack of antagonistic action can be explained by the fact that the causative agent of the disease is rhizogenic bacteria, and the isolated strains were from the tissues of the seminal rudiments. This suggests the importance of considering the organ origin of the isolates as a driver for their antagonistic action against pathogens.
BXS isolate was the only one among all the isolates tested to show antagonistic activity against the causative agent of soft rot of tomatoes, P. carotovorum subsp. carotovorum UKM В-1075. At the same time, BXS had the highest antagonistic activity against the causative agent of black bacterial spotting of tomato X. campestris (Table 2).
B1S isolate was the only one to show antagonistic activity towards P. fluorescens 7769. B4S and B1S isolates had a high bacteriostatic effect against X. campestris, B4S, B1S and BХS demonstrated high activity against the causative agent of bacterial wilt of tomatoes C. michiganensis subsp. michiganensis 102 (15,7–18,6 mm), which is particularly dangerous to tomato plants during their fruiting period and has a low bacteriostatic effect against P. syringae pv. syringae (5,2–8,9 mm).
Deterioration of the phytopathological situation of tomatoes due to the increased prevalence (up to 35–45%) and harmfulness of root rot, bacteriosis, and fusarium and verticillium wilts has been observed in recent years [33]. These diseases can often have epiphytotic development and induce losses at the end of the growing season, from 25 to 50% of plants. Based on long-term monitoring of tomato diseases in greenhouses, it was found that the phytopathological complex is represented by a wide range of diseases. Among them, root and foot rot (34,3%), verticillium and fusarium wilt (6,5 and 4,6%), bacterial rot (stem core necrosis and bacterial cancer 13,1%), white and gray rot (12,5 and 9.8%) are the widespread diseases. In the second half of the growing season, tomato plantings were affected by alternariosis and brown leaf spotting, i.e., 5,8 and 8,3% [33].
The analysis of antifungal activity has shown the selectivity of the isolates under investigation against phytopathogenic micromycetes responsible for mycosis of the tomatoes in the covered soil, namely Fusarium oxysporum, Alternaria solani, Botrytis cinerea, Sclerotinia sclerotiorum, and Fusarium sambucinum (Table 3). The highest antifungal activity was found in B1S and BХS isolates against S. sclerotiorum and fungi of the genus Fusarium, and slightly lower against B. cinerea (Fig. 4). All studied isolates showed a fungistatic effect against A. solani, the causative agent of alternariosis of tomatoes in covered soil, one of the most dangerous plant diseases. Isolates B3S and Y8S showed little activity against F. oxysporum and were not active against B. cinerea and S. sclerotiorum.
According to data on the frequency of antagonistic activity in endophytic bacteria in the scientific literature, the ratio of phytopathogen antagonists to the total number of endophytic bacteria varies significantly depending on the type of phytopathogen and host plant: in potato plants from 0 to 43% and in rice plants from 25 to 75% [34]. In our study, this value was 21,4%. Determination of the activity of pectolytic enzymes showed the presence of a certain maceration of tissues under the action of B4S, B5S, and B14S isolates and its absence in B1S, Y8S, and BXS (Table 1).
| Phytopathogenic Mycromycetes | Isolates of Endophytic Bacteria | |||
|---|---|---|---|---|
| B1S | B3S | Y8S | BХS | |
| Fusarium oxysporum | +++* | + | + | +++ |
| Fusarium acuminatum | ++ | ++ | ++ | +++ |
| Alternaria solani | ++ | ++ | ++ | ++ |
| Sclerotinia sclerotiorum | +++ | – | – | +++ |
| Botrytis cinerea | ++ | – | – | ++ |
An increase in the resistance of tomato plants to pathogens by inoculation with endophytic microorganisms was established in model experiments. Analysis of morphometric parameters of inoculated plants showed that in response to inoculation with endophytic bacteria and infection with pathogens, plants showed a different strategy. Plants of the Sanka variety and Ephemer hybrid inoculated with B1S strains even when affected by pathogens (B. cinerea) were characterized by an increase in the length of roots and shoots compared to the control, whereas when inoculated with BXS, the increment in root length was not observed in Sanka variety (Fig. 5).
In seedlings of Ephemer F1 hybrid infected by A. solani, the inoculation with either BXS or B1S induced a significant increase in both root and shoot length by using B1S and BXS isolates. Conversely, in those of the Sanka variety infected by the same pathogen, BXS triggered an increase in shoot length (42,5%), whereas B1S induced a significant increase in root length (2,2 times) (Fig. 6).

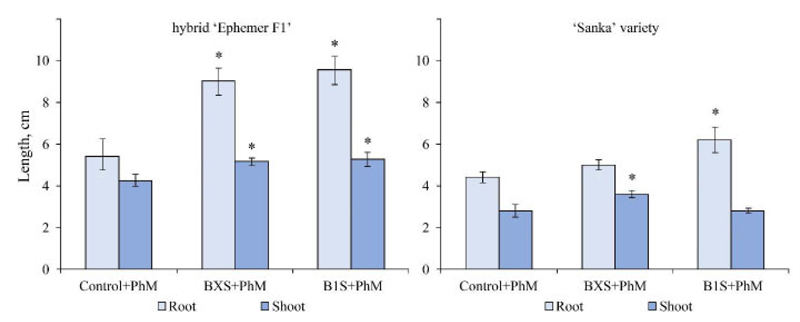
Note: the significance of differences compared to the control was assessed by one-way ANOVA;
* – significant differences at р < 0.05 (Tukey’s test)
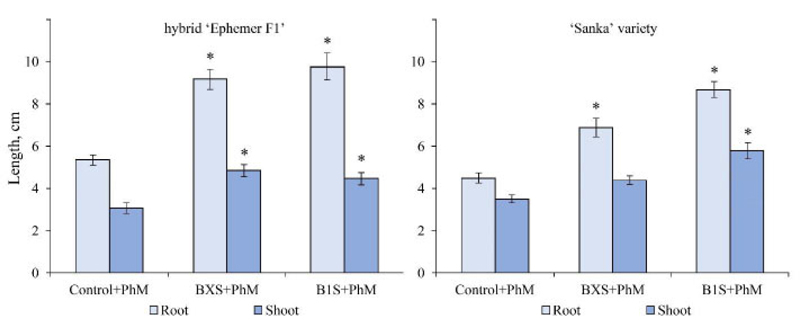
Note: the significance of differences compared to the control was assessed by one-way ANOVA;
* – significant difference at р < 0.05(Tukey’s test)
In seedlings of Ephemer F1 hybrid infected by F. oxysporum, treatment with either BXS or B1S induced a significant increase in both root and shoot length, whereas, in Sanka seedlings infected by the same pathogen, B1S triggered an increase in both root and shoot length, whereas BXS triggered growth only in the roots (Fig. 7).
Thus, some differences were found in the response of different tomato genotypes to endophyte inoculation.
A similar improvement in the morphometric parameters of tomato plants inoculated with the endophytic bacteria B. amyloliquefaciens RWL-1, isolated from seeds and artificially infected with F. oxysporum f. sp. Lycopersici, was observed by Shahzad et al. (2017). The plants showed increased metabolism of cell wall amino acids (for example, aspartic acid, glutamic acid, serine, and proline) and an increase in the content of salicylic acid compared to control plants [11]. Increased activity against B. cinerea in B. amyloliquefaciens was reported with the ech42 gene coding for an endochitinase from Clonostachysrosea [35]. It was established that endophytic microorganisms, due to their mutualistic interaction with plants, are able to trigger the protective mechanism of plants, stimulating systemic induced resistance against pathogens and pests in them [20, 36].
The growth-stimulating effect on biometric indicators throughout the vegetation period of greenhouse-grown plants using a complex of endophytic strains was obtained. Thus, the length of the stem reached 86,7-90,3 cm, while in control, it did not exceed 70,2-72,5 cm. The leaf area was 17.0-19.2% higher than the control plants. The area of tomato leaves infected by late blight, in the variant of using a complex of endophytic strains, decreased by 3,1 times compared to untreated plants, and the number of plant leaves with damage symptoms did not exceed 10%.
Analysis of data on the combination of pathogen antagonism properties and growth–stimulating activity in potato endophytic bacteria showed that the antifungal activity was low, and the ability to produce biologically active substances was quite high [37]. Of the 77 isolates isolated from Solanum nigrum, 37 significantly increased plant growth rates, of which 22 increased seed germination by up to 100% compared to the control [38]. Of the 336 tomato plant isolates, 61% of the isolates stimulated plant growth and increased biomass from 50 to 64% [39].
The ability to stimulate plant growth in combination with antagonistic activity against fungal phytopathogens occurred in 60% of endophytic bacterial isolates, while high rates of activity of the manifestation of the two properties were observed in 19% of bacterial isolates [40].
Thus, plant-associated endophytic microorganisms increase the adaptive capabilities of plants to adverse biotic and abiotic factors and are promising for the development of microbiological and environmentally friendly plant protection measures.
CONCLUSION
The generative system of angiosperms includes specific tissue barriers that reduce the risk of damage to seed germs and embryos from the penetration of viruses, phytopathogenic bacteria, and fungi. In this study, 14 strains of microbial isolates were isolated from the tissues of the generative organs of black nightshade. The genus of Вacillus were predominant among the 14 strains. By analyzing the sequences of the 16S rRNA gene and ITS-sequences of typical cultures among the isolated endophytes, they were assigned to B. amyloliquefaciens (strains BA1S-OSN-0820 and BAXS-OSN-0820) and R. kratochvilovae (strain RHC-OSN-с0820).
Tomato plants inoculated with endophytic microorganisms showed higher resistance to phytopathogenic fungi after artificial infection in model laboratory experiments. Antagonism against phytopathogenic fungi and bacteria from isolated isolates provides long-term protection during the most critical stages of plant development and has prospects for the development of microbial biologics for cultivated plants of the family Solanaceae.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article are available from the author upon request.
FUNDING
This study was supported by the project “Induced resistance and control of phytopathogenic bacteria in novel biotechnologies for the growth of vegetable crops using growth stimulators with elicitor activity” (2020–2023), state registration no. 0120U102106.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


