All published articles of this journal are available on ScienceDirect.
Chlororespiration as a Protective Stress-inducible Electron Transport Pathway in Chloroplasts
Abstract
Chlororespiration is the uptake of oxygen into the respiratory electron transport chain (ETC) localized in the thylakoid membranes of chloroplasts. The chlororespiratory ETC interacts with photosynthetic electron transport and participates in the non-photochemical reduction/oxidation of the plastoquinone pool (PQP) accompanied by O2 consumption. The two key thylakoid enzymes in chlororespiration are the plastid-encoded NAD(P)H dehydrogenase complex (NDH) and the nucleus-encoded terminal plastoquinol oxidase (PTOX). The contribution of chlororespiratory electron flux to the total electron flow in non-stressed plants is considered insignificant. In contrast, under abiotic stresses, chlororespiration appears to be triggered, at least in some photosynthetic organisms, acting as a protective alternative electron transport pathway. There is evidence of NDH complex and PTOX increasing their activity and/or abundance when plants experience high light, drought, heat, or low-temperature stresses. Alternative electron transfer to oxygen via PTOX protects PQP from over-reduction under stress conditions. For instance, it was shown that PTOX-dependent electron drainage accounted for up to 30% of total PSII electron flow in salt-stressed plants. PTOX is not bound to the thylakoid membrane in dark-adapted leaves but is associated with it at intense illumination and high transmembrane proton gradient (ΔpH) or membrane potential (Δψ). It was also shown that PTOX is capable of lateral translocation from stromal lamellae to granal thylakoid stacks under salt stress. Such changes in PTOX localization increase the accessibility of the substrate (plastoquinol) and the turnover rate of the enzyme. The available data allow considering PTOX as a possible target for manipulation to increase stress tolerance in sensitive plants.
1. INTRODUCTION
During oxygenic photosynthesis, the sequential operation of photosystems I and II (PS I and II, respectively) localized in thylakoid membranes drive linear (non-cyclic) electron transport from H2O to NADP+, resulting in the formation of NADPH, the reducing agent mainly used for CO2 fixation in the Calvin-Benson-Bassham (CBB) cycle. In the linear electron transport chain (ETC), electrons extracted by PSII from water are transferred via the plastoquinone pool (PQP), the cytochrome b6f complex (Cyt b6f), and plastocyanin (PC) to PSI and ultimately to ferredoxin (Fd), which is oxidized by the membrane-associated flavoprotein Fd-NADP+ reductase (FNR) with reduction of NADP+ to NADPH [1]. Photosynthetic electron transport is coupled to transmembrane proton transport, creating an electrochemical proton gradient (ΔμH+) used in ATP synthesis. PQP acts as the hub in the electron and proton transfer processes, with its redox state playing a critical role. The oxidation of plastoquinol (PQH2) by the Cyt b6f complex is the rate-limiting step of the linear electron transport pathway, as it depends on the PQ/PQH2 diffusion rate in the thylakoid membrane.
In a dynamic environment, the metabolic needs of a plant cell in ATP and NADPH are constantly changing [2]. Under conditions limiting CO2 fixation, such as drought, salt, temperature, and/or high light stress, when NADPH production by ETC exceeds the capacity of its utilization in the CCB cycle, carriers in the ETC get over-reduced. In this case, the efflux of electrons from the ETC to O2 will inevitably produce reactive oxygen species (ROS), which can severely impair the photosynthetic machinery [2-6]. Chloroplast redox metabolism, including photosynthetic electron transport and CO2 assimilation, is most vulnerable under stress, leading to excessive excitation pressure, photodamage, and propagation of ROS [7]. Although PS I and PS II are the major sources of ROS in chloroplasts [6], both are vulnerable to over-reduction-induced mass production of ROS. It was shown that ROS production is enhanced when electron carriers are excessively reduced [8, 9]. Plants have evolved multiple mechanisms balancing light and dark reactions to protect the photosynthetic apparatus from light-induced damage [10, 11]. In addition to enzymatic and non-enzymatic ROS detoxification systems [12], alternative pathways of photosynthetic electron transport may serve as sinks for excess electrons, thereby helping to prevent over-reduction of electron carriers [3, 13, 14]. Thus, the contribution of alternative electron transport pathways around PSI to energetic and metabolic balance increases under stress conditions [15]. Under certain circumstances, three alternative pathways of electron transport can operate in the thylakoids [16], namely, PS I-mediated cyclic electron transport (CET) [17-21], chlororespiration [22-25], and pseudo-cyclic electron transport. The latter, also known as the water-water cycle [26], leads to the reduction of oxygen in water via the Mehler reaction [16]. These alternative pathways alleviate the over-reduction of the PQ pool, simultaneously enabling luminal acidification that triggers energy dissipation in the PSII antennae [10]. Each of these alternative ET pathways was shown to be dispensable but contributing to the maintenance of PSI function even under non-stress conditions [16].
This review focuses on the organization and physiological role of the chloroplast respiratory chain. The data are discussed on the activation and probable adaptive role of chlororespiration under heat, cold, salt, and drought stresses that cause increased ROS production and oxidative stress in plants. The ΔμH+-dependent dynamics of plastid terminal oxidase (PTOX) and possible ways of genetic transformation of plants to increase their stress tolerance are reviewed.
2. CHLORORESPIRATION AS AN ALTERNATIVE ELECTRON TRANSPORT PATHWAY
In 1982, Bennoun [22] showed that the redox state of the PQP can change in the dark, leading him to speculate that PQ can be reduced by NAD(P)H dehydrogenase (NDH) and oxidation of PQH2 occurs by oxidase-mediated electron transfer to O2. Bennoun introduced the term chlororespiration to describe this electron pathway by analogy with respiratory electron flux in mitochondria. In support of this hypothesis, homologs of genes encoding subunits of mitochondrial complex I were found in the chloroplast [27, 28]. Ndh genes encode at least 15 subunits (Ndh-A–O), among which NdhA–NdhK are plastid-encoded and the rest are nucleus-encoded [29]. In addition, PTOX protein, homologous to alternative oxidases of plant mitochondria, was identified in chloroplasts [30, 31].
It is well established now that the PQP in chloroplasts can be reduced in the dark by stromal reducing equivalents. Electrons from NAD(P)H generated in the catabolic pathways of glycolysis, pentose phosphate pathway and the Krebs cycle are transferred to PQ. PQ reduction by NADH and/or NADPH in chloroplasts is mediated by NDH, similar to complex I of the mitochondrial respiratory chain. The NDH is the entry point of electrons into the photosynthetic electron transport chain, performing the non-photochemical reduction of PQ [32, 33]. It has been reported that reduced Fd could be the initial electron donor for the NDH complex [34, 35]. PTOX is the point of electron transfer from PQH2 to molecular oxygen, leading to water formation in the stroma and mitigating ROS formation when PQP is over-reduced (Fig. 1) [24, 36-38]. Thus, chlororespiration may serve to poise the redox level of the photosynthetic electron-transport chain for more efficient photosynthesis after over-excitation.
In unstressed or not stress-adapted plants, the proportion of chlororespiration in the total electron flow through PQ in thylakoids was calculated to be in the range of 0.05–0.3% [39-41]. However, under abiotic stress, chlororespiration may be triggered, at least in some photosynthetic organisms, acting as an alternative electron transport pathway. Many results suggest that chlororespiratory enzymes play a role in the protection of plants under unfavourable conditions that are accompanied by increased ROS formation and oxidative stress [42-52]. For instance, there is evidence of increasing activity and/or abundance of PTOX when plants experience heat, high light [42, 43, 46, 47], salinity [48, 49], water stress [42, 43, 52] and low temperature [44, 45, 53]. It was observed that electron transfer from thylakoid ETC to oxygen is activated at low temperatures [14, 50], which may be related to increased chlororespiration [14, 45, 54].
As shown in a number of studies [40, 55, 56], the level of PQ pool reduction increases under stress conditions. NAD(P)H consumption in respiration decreases due to oxygen deficiency or destruction of respiratory enzymes under conditions of drought [57], low temperature [54], or hypoxia [46]. As a result, the concentration of reducing equivalents in the chloroplast stroma increases, which suppresses photosynthetic activity, induces over-reduction of the PQ pool, and activates chlororespiration.
Therefore, chlororespiration may be considered a way of PQP redox state regulation when NAD(P)H/NAD(P)+ ratio is high [55]. Also, under stress conditions or low photosystem II activity, chlororespiration is probably essential for balancing excitation-energy transfer between the two photosystems [13, 15] and supporting cyclic electron transport in chloroplasts [15, 58].
3. COOPERATION WITH CYCLIC ELECTRON FLOW AROUND PS I
Chlororespiration and CET share PQ and the NDH complex (Fig. 1) and have closely related functions. CET is an important alternative ET pathway able to fine-tune the redox state of the photosystems [17, 19, 34, 59-61]. In recent years, CET was identified to be essential for plant growth and development during stress periods [62]. The NDH-mediated CET can tackle the over-reduction of stromal components balancing the redox state of electron carriers and providing the extra ATP for metabolic reactions.
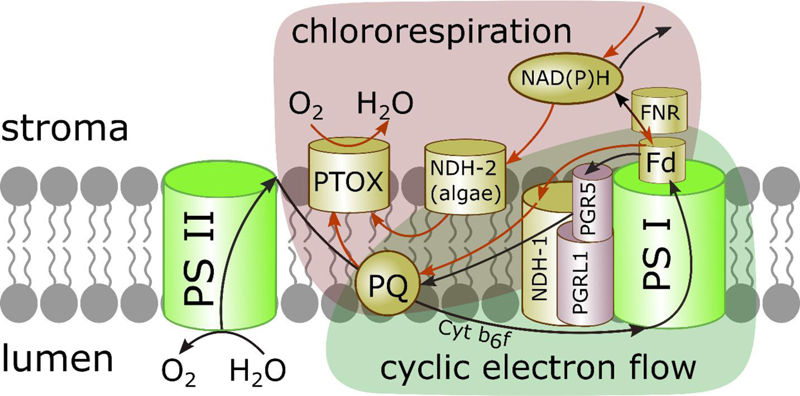
During chlororespiration, the NDH and PTOX cooperate to deliver and remove electrons from PQP, respectively, balancing the redox state of the electron carriers [11, 13, 63, 64]. In this regard, it was suggested that chlororespiration tightly controls CET rate in vivo by altering the redox state of the inter-system electron carriers [40]; in particular, CET and chlororespiration are coordinated to mitigate photoinhibition during heat stress [64].
Genetic analysis in Arabidopsis revealed two types of CET, specifically, two sets of genes involved in photosynthesis regulation [34, 35]. Key components of the main CET pathway in higher plants are PGR5 and PGRL1 proteins (abbreviation for “proton gradient regulation 5 and pgr5-like 1”, respectively) that regulate the proton gradient [40, 65]. PGR5-PGRL1-mediated CET plays a major role in ΔμH+ formation and ATP synthesis, increasing the ATP/NADPH ratio to meet metabolic needs. In addition, CET plays an important role in protecting the photosynthetic apparatus from photoinhibition by increasing proton gradient that triggers non-photochemical energy quenching – a mechanism for dissipation of excessive absorbed light energy [16, 34, 35]. This pathway is sensitive to the inhibitor antimycin A. The second CET pathway involves NDH homologous to the mitochondrial complex I, also participating in chlororespiration [34, 35, 46, 66-68].
Chloroplastic NDH is the last large photosynthetic membrane-protein complex with a mostly unknown structure [35, 66-69]. Similar to the respiratory NADH dehydrogenase complex I, chloroplast NDH catalyzes quinone pool reduction by transporting electrons from PS I with simultaneous pumping of protons across the thylakoid membrane, thus ensuring additional ATP synthesis [67, 69]. NDH includes 11 of the 14 subunits of mitochondrial complex I and several subunits specific for oxygenic photosynthesis conserved in cyanobacteria and plants [35, 70, 71]. Photosynthesis-specific subunits, in particular NdhS, are suggested to allow NDH to accept electrons from Fd [35, 64, 65]. Recently, the structure of a 0.42-MDa NDH complex isolated from the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1 was determined by single-particle cryo-electron microscopy at 3.1 Å resolution [70]. The arrangement of the photosynthesis-specific subunits suggests the existence of several electron transfer pathways maximizing PQ reduction and avoiding the harmful effects of semi-PQ radicals.
The two CET pathways function efficiently in C4 plants [72]. In C3 plants, CET is carried out mainly via PGR5 and PGRL1 complex, at least under optimal conditions [34, 35]. The rate of CET involving the NDH complex is very low, which may be associated with its low content in chloroplasts - only 0.2% of the total proteome of thylakoid membranes [51], and the NDH/PS II ratio is estimated as 1/50–1/100 [67]. Therefore, the contribution of this pathway to ΔμH+ and ATP synthesis may not be significant [20]. However, under stress conditions, the role of the NDH complex greatly increases.
Recently, it was shown that ATP at physiologically relevant concentrations inhibits both NDH- and PGR5/PGRL1-dependent CET [73]. In that work, a simple model of CET regulation in chloroplasts was proposed in which CET is activated at low but inhibited at high levels of ATP, thus maintaining efficient ATP homeostasis. In non-stressed C3 plants, under stable conditions, when the metabolic demand for ATP is almost met by LET, CET may be activated only gradually. It was suggested that the role of CET increases under conditions of increased ATP demand, such as under environmental stress or during the induction of photosynthesis in plants adapted to darkness or in mutants with altered ATP/NADPH requirements [74]. CET probably plays a more important role in replenishing ATP required for the operation of CO2 concentrating mechanisms in algae or in C4 photosynthesis [18, 73].
4. ACTIVATION OF CHLOROPLAST RESPIRATORY PATHWAY UNDER ABIOTIC STRESSES
As sessile organisms, plants must cope with extreme and varying environmental factors in both wild nature and agriculture. Several studies suggested that components of the chlororespiratory pathway may be involved in the defense or adaptive mechanisms of plant response to environmental stresses such as heat, cold, high light, or drought. Those findings were based on physiological studies showing the increased intensity of non-photochemical reduction of PQs and expression of NDH complex and PTOX under stress. Under temperature and water stress or high light intensity, as well as under conditions of CO2 limitation of photosynthesis, photosynthetic CO2 fixation activity decreases, and excessive amounts of reduced Fd and NADPH accumulate in the stroma [39, 51, 60, 75-77]. Under such conditions, due to a significant deficit of stromal electron acceptors (NADP+ and oxidized Fd), the probability of electron dumping to oxygen increases with oxidative degradation of electron transport chain components and membrane lipids. In this case, the NDH complex prevents “over-reduction” of stromal electron acceptors [24, 39] by regulating the redox state of the PQ pool and, thus, contributes significantly to the protection of the photosynthetic apparatus from oxidative stress [39, 51, 60, 75-77].
Chlororespiration was shown to be involved in the acclimation of plants to unfavorable environmental conditions [46]. Two species of Brassica, a wild species Brassica fruticulosa and an agricultural one Brassica oleracea, have been studied under high light and elevated temperature stress [46]. Although the photosynthetic efficiency of PS II was higher in the cultivated species, PS I activity was higher in B. fruticulosa than in B. oleracea, and in both species this activity increased when exposed to heat and high light. Immunoblot analysis of thylakoid membranes using specific antibodies grown against the NDH-K subunit of the thylakoid NADH dehydrogenase complex and against PTOX revealed higher amounts of both proteins in B. fruticulosa than in B. oleracea. In addition, PTOX activity in plastoquinone oxidation and NDH activity in thylakoid membranes were higher in the wild type than in the agricultural species. The results indicate that tolerance of photosynthetic activity to high light and heat was higher in wild species than in agricultural species, suggesting that plant adaptation to these stresses under natural conditions favors subsequent acclimation, and that the chlororespiration process is involved in adaptation to heat and high light in Brassica.
In most plant species tested, PTOX protein is present in low concentrations that can potentially account for less than 1% of the total electron transport activity [78]. However, at high light, heat, or cold, the PTOX level increases in various plant species [39, 47, 79]. Plants growing at high altitudes in the Alps must cope with extreme and variable environmental conditions, such as high or low solar radiation and temperatures ranging from freezing to more than 30 °C [78]. All alpine plant species tested for PTOX content showed elevated levels compared to lowland plants [13]. In leaves of Geum montanum, PTOX content increased with increasing altitude at which the plants were collected [13]. The highest PTOX content was found in Ranunculus glacialis leaves, exceeding that of every other plant species so far examined, even including Lycopersicon esculentum [13, 63] mutants. The PTOX content strongly declined during de-acclimation of R. glacialis, when these plants were grown at low elevation for several weeks, while their sensitivity to photoinhibition increased [13], suggesting the important role of PTOX in photoprotection.
Plants of many species of tropical and subtropical origin experience chilling stress when exposed to low non-freezing temperatures of 10–12 °C [44, 45, 50]. In the cold stress-sensitive tropical species Hibiscus rosa-sinensis [44], PS II was inhibited, as indicated by a decrease in the effective quantum yield of PSII and electron transport capacity. When the stem but not the roots of H. rosa-sinensis were cooled, the quantum yield of PSII and the relative electron transport rate were significantly lower than when the whole plant, root and stem, were cooled at 10°C. Moreover, when the whole plant was cooled, the amount of polypeptide PGR5, an important component of cyclic electron flux around PS I, increased, suggesting that under these conditions CET helps to protect photosystems. However, when the stem, but not the root, was cooled, the CET did not increase, and PSII was damaged as a result of insufficient dissipation of excess light energy. In contrast, chlororespiratory enzymes (NDH complex and PTOX) remained the same as in the control when the whole plant was chilled but increased when only the stem was chilled, indicating the involvement of chlororespiration in response to cold stress when other pathways, such as CET around PS I, are deficient. Studying the chilling stress on the tropical plant species, Spathiphyllum wallisii, Segura and Quiles [50] concluded that chlororespiration was induced only when the stem is significantly cool and the roots are simultaneously warmer compared to the stress-free control. According to the authors, PS II in stem chloroplasts is inhibited during stem chilling, causing a decrease in the PQ pool reduction and, as a result, inducing chlororespiration.
To further investigate the effects of stem cooling under high light conditions, plants with contrasting shade tolerance were studied – the sun-loving species Chrysanthemum morifolium and the shade-tolerant Spathiphyllum lanceifolium [45]. Under chilling in both species, only a slight decrease in the maximum quantum yield of PSII was observed, indicating the existence of mechanisms in the chloroplasts to dissipate excess excitation energy and prevent damage to the photosynthetic apparatus. In addition, changes were observed in the polypeptide PGR5, which is involved in cyclic electron flux around PS I, and in chlororespiratory enzymes (plastidial NDH complex and PTOX). PGR5 abundance increased significantly only in chilled C. morifolium plants, while the abundance of PTOX and NDH-H polypeptide, as well as NDH activity, increased significantly only in chilled S. lanceifolium plants. These results suggest that cyclic electron flux is more important in the light-loving specie C. morifolium, while in the shade-tolerant S. lanceifolium other mechanisms involving chlororespiratory enzymes are stimulated in response to chilling and high light. Also, activation of the chlororespiratory mechanism resulted in less H2O2 accumulation in S. lanceifolium leaves compared to C. morifolium [45].
The greater accumulation of H2O2 in leaves of C. morifolium compared to S. wallisii was previously reported by Ibanez et al. [42] in a study of the effects of drought, heat, and high irradiance. The stress conditions resulted in the downregulation of linear electron transport indicated by a gradual decrease in the photochemical efficiency of PS II. Under drought, heat, and high light stresses, only a slight decrease in maximum potential quantum yield was observed in both plant species, indicating the high efficiency of the defense mechanisms dissipating excess excitation energy to protect the photosynthetic apparatus. The changes similar to that under the chilling stress [45] were observed in the plastidial complex of NDH, PTOX, and PGR5. The abundance of PTOX and NDH-H, and NDH activity, were similar in control and increased under combined high light plus drought stress, particularly in S. wallisii. The involvement of chlororespiration and cyclic electron transport pathways in stress resistance differed between sun and shade plants, indicating different mechanisms of adaptation. The chlororespiratory pathway involving NDH and PTOX is stimulated in the shade species S. wallisii under drought, heat, and high light, reducing ROS accumulation.
The effect of water deficit on photosynthesis and chlororespiration was also studied in Rosa meillandina [42]. The plants are tolerant to heat and high light under sufficient water supply. However, under a combination of heat, high light, and limited irrigation conditions, the photochemical efficiency of PS II decreased and non-photochemical quenching increased. Changes in NDH, PTOX, and PGR5 were also observed. The activity and abundance of chlororespiratory enzymes increased in plants exposed to the combined stress. The authors suggested that the contribution of chlororespiration to the regulation of photosynthetic electron flux is significantly increased when PS II activity is inhibited under drought [42].
Li et al. [64] studied the effect of elevated temperatures on photochemical characteristics of wild type (WT) and NDH-knockout mutants of tobacco, a double mutant with disrupted ndhJ and ndhK genes (ΔndhJK) or a triple mutant with disrupted ndhC, ndhJ and ndhK genes (ΔndhCJK). Below 50°C, the maximum quantum yield of PS II decreased to a similar extent in WT and mutants. However, the decline at temperatures above 55°C was sharper in WT, indicating the involvement of chlororespiration in the downregulation of PS II. Heat-stimulated expression of both NdhK and PTOX was significantly higher in WT, while the PTOX was less expressed in ΔndhJK than in WT. PTOX inhibitor n-propyl gallate (n-PG) significantly increased the minimal chlorophyll fluorescence level (F 0) only in WT plants at elevated temperatures, indicating the operation of chlororespiration. In contrast, the inhibitor similarly affected F 0 in both WT and ΔndhCKJ at 20°C, suggesting no obvious function of chlororespiration under optimal physiological conditions. Also, n-PG enhanced the decrease in the maximum quantum yield of PS II in ΔndhCKJ. Thus, the tobacco was more sensitive to elevated temperature when ndhC, ndhJ, and ndhK genes were defective. The results suggest that chlororespiration and NDH-mediated cyclic electron flow may be coordinated to alleviate the over-reduction of stroma, thus keeping CO2 assimilation under heat stress [64].
Together with cyclic electron flow around PS I, high PTOX abundance and chlororespiratory activity may function as a protective valve under high irradiance and additional environmental stress. It is assumed that excess light energy absorbed by PS II is utilized in electron flow via PTOX to oxygen, and the energy absorbed by PS I is dissipated in cyclic electron flow [80].
The role of alternative electron pathways, CET and chlororespiration, in the acclimation of Chlamydomonas reinhardtii to N deprivation has been studied by Saroussi et al. [81]. The results suggest that photoautotrophic N deprivation is accompanied by increased chlororespiration that becomes apparent during light-to-dark transitions and acts as an alternative electron pathway where major electron sinks are limited. Using biochemical and biophysical analysis of wild-type and mutant strains of C. reinhardtii in photoautotrophic conditions, the authors showed that the key factor of acclimation is NDA2 controlling CET, chlororespiration, and the formation of H+ gradient across the thylakoid membrane. Nitrogen deficiency led to doubling of the NDA2-dependent CET rate, while the contribution of PGR5/PGRL1-dependent CET was negligible. The H+ gradient generated by CET is essential for maintaining the NPQ level, whereas increasing the PQP reduction level promotes state transition; both are necessary to reduce PSII activity. Moreover, stimulation of NDA2-dependent chlororespiration provides alleviation of the increased reduction state via PTOX-dependent water synthesis. Overall, the redirection of electrons through NDA2 in response to nitrogen deficiency supports cell viability by facilitating the dissipation of excess excitation energy through the quenching and chlororespiratory processes.
An increased role of chlororespiration in maintaining the energy state of the photosynthetic cell in darkness was shown in chlorobionts, chlorophyte symbionts in lichens. When both light-adapted and dark-adapted lichen Asterochloris ericii was exposed to darkness, NDH-2 activity was detected by a transient and reversible increase in F 0, observed after switching off the light [82]. This increase was enchanced by n-PG, which enables discrimination between plastoquinone reduction and oxidation activities after switching from light to darkness. After 24 h in darkness, there was a marked increase in the post-illumination fluorescence rise in n-PG-treated samples. Preincubation with n-PG did not affect the effective quantum yield of PSII after 15 min but significantly affected it after 24 h of darkness, which indicates PTOX is an important electron sink from PSII in 24 h dark-adapted cells. These results suggest that the chlororespiratory chain is present in A. ericii and enhanced during the dark period and by the availability of an exogenous carbon source (glucose or amino acid). Darkness induced the plastoquinone reductase activity and the phosphorylation of the light-harvesting complex (LHC). This mechanism probably serves an ecophysiological function in lichens to prevent damage at dawn or under strong fluctuating light conditions when lichens are in the hydrated state.
5. PTOX AS A “SAFETY VALVE” FOR EXCESS ELECTRONS IN THE PHOTOSYNTHETIC ELECTRON TRANSPORT CHAIN
PTOX, a nucleus-encoded plastid PQ oxidase, is a key chlororespiratory enzyme [37], catalyzing the transfer of four electrons from PQH2 to an oxygen molecule with water formation:
2 PQH2 + O2 → 2 PQ + 2 H2O
The involvement of PTOX in PQ oxidation using molecular O2 as a terminal electron acceptor has been demonstrated by analysis of AtPTOX-overexpressing transgenic tobacco plants [40]. PTOX was firstly identified in variegated plants, immutans mutant (im) of A. thaliana [37, 83] and the tomato PTOX-deficient mutant ghost (gh) [84]. The gh shows many phenotypic similarities with im, supporting the notion that IM and GH are orthologous genes [84, 85]. In the variegated phenotype with green and white sectors in tissues that normally would be green, the PTOX gene is repressed. The cells of white sectors possess abnormal plastids without pigments. The white im spots accumulate phytoene, indicating that the mutant is defective in carotenogenesis [86, 87] due to a deficiency in phytoene desaturation, a key step in carotenoid biosynthesis [83, 88-90]. Leaves of im plants are very sensitive to photo-oxidative stress. On exposition on light, white spots appear on them, indicating a lack of pigments and photo-damage of the tissues. This effect increases with increasing temperature and light intensity. The main reason is the inhibition of carotenoid synthesis in the absence of PTOX, so the protective functions of chloroplast carotenoids such as the violaxanthin cycle or triplet chlorophyll quenching cannot be fulfilled [91].
Unlike the multimeric NDH complex, PTOX is a relatively small protein. The IM gene encodes a 347 amino acid protein with a calculated molecular mass of 40.5 kDa [29]. In eukaryotes, it also possesses a chloroplast targeting the N-terminal transit sequence of 50 amino acids [29, 58]. The molecular mass of the mature IM protein after cleavage is 35 kDa. Further analysis revealed that IM is located on the stromal side of stroma lamellae. No PTOX was found in chloroplast envelope membranes [92]. It was predicted as an interfacial membrane protein with a four-helix bundle located on the stromal side of the thylakoid membrane and encapsulating a di-iron center in its active site facing stroma [40, 93, 94]. The two iron atoms are ligated by four histidines and two glutamate residues [85, 95]. Site-directed mutagenesis in vitro and in planta has shown that the six Fe-binding residues are indispensable for the activity of the protein [91].
gh encodes a 366 amino acids residue long protein with a predicted molecular mass of 42.1 kDa [84, 95]. Its amino acid sequence is identical to im by 67%, with most of the variability in the putative N-terminal plastid targeting peptide. im homolog cDNAs have also been isolated in pepper (Capsicum annuum) [95] and rice (Oryza sativa, OsIM1) [96]. The amino acid sequence of OsIM1 showed 66% and 62% identity with GH and IM, respectively [96]. IM homologs are also found in algae [97] and some cyanobacteria [98, 99]. According to genomic analysis, these organisms have single-copy genes [29, 73, 84, 96]. Although IM is generally present as a single copy, two copies are found in some cyanobacteria, red algae and green algae [100, 101]. Sun et al. [102, 103] also reported the identification of two putative genes, PTOX1 and PTOX2, in Glycine max. A recent phylogenetic analysis showed that the duplication of this gene apparently occurred during speciation involving an ancestor of the genus Glycine [104]. Both genes are ubiquitously expressed in G. max tissues, but their mRNA levels change during development and under stress conditions. PTOX1 was more strongly expressed in young, and PTOX2 in old tissues [104]. Despite high protein identity (97%), molecular docking showed that PTOX1 has a higher affinity to PQH2 than PTOX2. Thus, the functional significance of PTOX gene duplication for G. max metabolism was confirmed, suggesting that PTOX1 may be related to chloroplast efficiency and PTOX2, to senescence and/or apoptosis [104].
IM is homologous to mitochondrial alternative oxidases (AOX), the terminal oxidases in the alternative mitochondrial respiration pathway that redirect electron flow from ubiquinol to water, using molecular oxygen as a terminal acceptor [37]. Alternative oxidases are mitochondrial integral membrane proteins [105] that keep the balance between mitochondrial carbon and energy metabolism [83, 106]. Like PTOX, AOX plays a pivotal role in response to stress conditions. AOX is an important sensor of cellular redox balance, which is its central function [107]. Recently, evidence has been obtained confirming the focal point of AOX in the coordination of signaling pathways between mitochondria and chloroplasts [108].
Due to its involvement as a cofactor in carotenoid [88, 89] and chlorophyll [109] biosynthesis, PTOX plays a role in chloroplast biogenesis. The activity of PTOX is probably most relevant at the beginning of chloroplast biogenesis when the components of the electron transport chain are just starting to be synthesized, and linear ET is not yet fully active [110]. At this stage, in im plants, the carotenoid biosynthesis may get suppressed by the lack of electron acceptors from phytoene desaturase, leading to photodamage and the variegated sectors [92]. However, once the chloroplasts maturate, PTOX gets dispensable due to a fully functioning linear ETC that can manage to keep the PQH2 at least partially oxidized at moderate light.
PTOX may function as a “safety valve” for excess electrons generated under environmental stresses [32, 39, 46, 58, 79, 111]. In this way, PTOX controls the stromal redox poise [111] and keeps the acceptor side of PSII oxidized, helping to protect PSII from photodamage [13, 58, 63, 112]. The role of PTOX in protecting against photoinhibition was confirmed when gh leaves were examined for their resistance to high light. Although the green leaves phenotypically were almost the same as those of wild-type plants, significantly more photodamage was detected in the PTOX-deficient gh mutant [112].
Thus, there is increasing evidence that PTOX is a versatile terminal oxidase in chloroplast metabolism and that its major physiological role varies depending on development and/or physiological context. It probably plays a greater regulatory role in the CET of C4, in contrast to C3 plants, because CET is a key regulator of ATP synthesis [39, 113]. The variety of PTOX functions includes stress tolerance, early chloroplast development, pigment biosynthesis, regulation of the ATP/NADPH ratio, and control of photosynthesis [92, 113, 114]. Also, given the ubiquitous expression of PTOX, new functions presumably will be found in other plastids than chloroplasts [115].
Chlororespiration’s control over the redox state of the PQP in darkness probably makes PTOX a key enzyme in the bioenergetics of the photosynthetic cell because its activity sets light-harvesting balance. Moreover, PTOX, by acting as a sensor of the redox and ATP status in the chloroplast stroma, helps trigger the adaptive ultrastructural changes in thylakoids [80].
6. STRESS-INDUCED REGULATION OF CHLORORESPIRATION BY DYNAMIC CHANGES IN PTOX LOCALIZATION
In plants exposed to adverse environmental factors, increased chlororespiration activity plays a protective role against photoinhibition of photosynthesis. Under conditions favorable for CO2 assimilation, however, the rate of chlororespiration should be strictly controlled, because it competes with linear and cyclic electron flux and negatively affects the production of ATP and NADPH [112, 116, 117]. Recent studies have proved that chlororespiration is regulated by changing the PTOX localization relative to the thylakoid membrane [48, 53, 112, 116-120].
According to the results of the first PTOX localization experiments, this protein was found in the stromal fraction of spinach thylakoid membranes, as well as within etiolated leaf tissue lacking PS II complex, suggesting roles outside photosynthetic electron transport [94]. Later, Feilke [116] showed that PTOX activity depends on the stromal pH. PTOX was tightly bound to thylakoid membranes in Arabidopsis leaves under high light intensity and weakly bound in the dark [112]. In dark-adapted tobacco leaves, PTOX was detected mainly in the soluble protein fraction isolated at pH 6.7. After exposure to strong light at pH 8.0, it was only bound to the membrane. On this basis, a model of the regulation of PTOX activity was proposed postulating the existence of two forms of the enzyme, a stromal soluble form and a membrane-bound one [112, 116]. Because the contact of the soluble PTOX with its lipophilic substrate PQH2 is difficult, at neutral pH the stromal form is enzymatically inactive, excluding the competition between PTOX and LET. When PTOX binds to the thylakoid membrane, its enzymatic activity increases as a result of gaining access to its substrate, protecting PQP from overreduction under stress conditions such as high light (Fig. 2A). The dependence of PTOX association with the thylakoid membrane on pH and ionic strength in vitro was studied using recombinant purified PTOX from O. sativa fused with the maltose-binding protein, MBP-OsPTOX. PTOX attachment to liposomes produced from thylakoid lipids was stronger at pH 8.0 than at pH 6.5, indicating that PTOX association with the membrane is facilitated under slightly alkaline conditions [117].
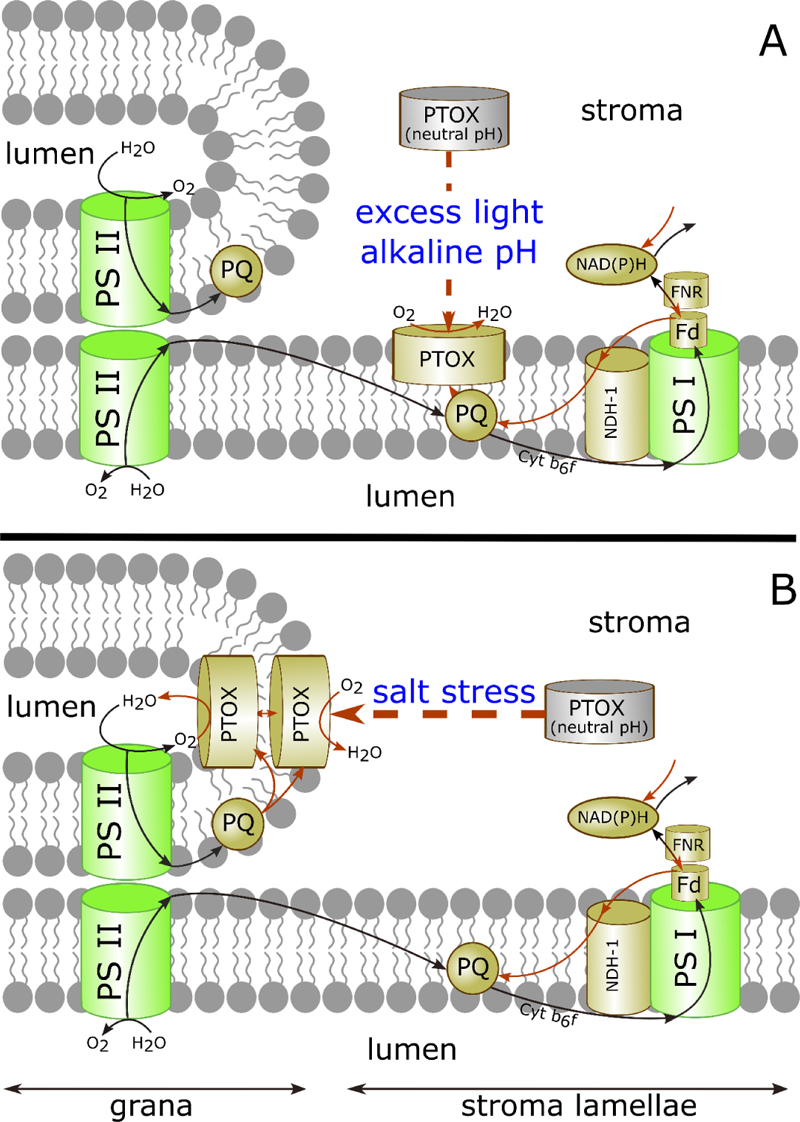
Reversible changes in the stromal pH occur during light energization and dark de-energization of thylakoids and are associated with transmembrane proton transport and proton driving force (pmf) formation [121, 122]. The possible functional relationship between pmf and localization of PTOX was investigated using uncouplers [117]. Since pmf consists of the electrical potential (Δψ) and proton concentration gradient (ΔpH)-dependent components, the effects of two ionophores have been studied: potassium-specific transporter valinomycin, eliminating the electric potential [123]; and an H+/K+ exchanger nigericin dissipating the proton gradient [124]. More PTOX protein was detected in the soluble protein fraction when leaves were treated with uncouplers before subjection to high light, irrespectively of the nature of the uncoupler. Thus, pmf is important for the attachment of PTOX to the thylakoid membrane.
Bolte et al. [117] investigated with confocal fluorescence microscopy the localization of PTOX in chloroplasts of high light-treated and dark-adapted guard cells of A. thaliana overexpressing PTOX fused with the green fluorescent protein. After the plants were illuminated with strong light, the GFP fluorescence was evenly spread over the chloroplast in a network-like structure. In dark-adapted plants, fluorescent labels were localized in spots. Similar spots were observed in chloroplasts of illuminated leaves infiltrated with nigericin. The authors noted a significant variation in the size of PTOX spots in chloroplasts, which was probably caused by differences in the ratio between membrane-bound and soluble forms of the enzyme, and by different levels of its oligomerization. It has been hypothesized that PTOX highly accumulates in the soluble form with spot formation and dissociates into smaller aggregates when the pH increases and PTOX binds to the membrane [117, 118].
Johnson and Stepien [58] suggested that the regulation of PTOX activity occurs not only via the reversible binding of the protein to the thylakoid membrane, but also through its migration within the membrane, mediated by unknown co-factors or post-translational modifications. Although in most plant species PTOX has low activity being 30-500 times inferior to the photosynthetic electron flux [38], they found that PTOX-dependent electron transport in Eutrema salsugineum species under salt stress can account for 30% of the total linear ET, representing an important flux capable of preventing over-reduction of PS II [48]. E. salsugineum is a halophytic species of the Brassicaceae family that can naturally tolerate several types of abiotic stress, including extreme salinity and cold, which significantly reduce crop productivity. It is widely used as a laboratory model for research on the biology of plant stress [125]. Although salt stress induced a strong up-regulation of PTOX protein in E. salsugineum, mere PTOX overexpression did not result in high catalytic activity suggesting that other factors or proteins are necessary. Stepien and Johnson [126] showed that PTOX activation involves the translocation of the protein from the unstacked stromal lamellae to the thylakoid grana and a protection of the protein from trypsin digestion (Fig. 2B). The protein migrates to the grana and may be translocated to the lumen on salt treatment in E. salsugineum, and this process may be associated with the activation. The mechanisms underlying the migration of PTOX between grana and stroma are not known. It was assumed that the expression of a second protein is required for the movement of PTOX from stroma lamellae to the grana in E. salsugineum [126]. Apparently, PTOX diffusion is restricted between grana stacks where the membranes are tightly contiguous [127, 128]. Considering the strict limitations of lateral protein movement due to the domain organization and high intramembrane concentration of proteins, Stepien and Johnson [126] suggested that PTOX is translocated from the stroma-facing side to the luminal side of the membrane and that it is active in the thylakoid lumen. Thus, dynamic changes in PTOX attachment to thylakoids allow regulation of its enzymatic activity modulating the access to the lipophilic substrate.
7. PTOX AS A CANDIDATE FOR ENGINEERING STRESS TOLERANCE IN CROP PLANTS
Environmental stress tolerance is one of the key factors determining crop productivity in changing climate conditions, therefore extensive studies are being undertaken to create transgenic plants with elevated abiotic stress resistance [129]. In this regard, the putative function of PTOX as a safety valve maintaining the oxidized state of the intersystem quinone pool and ensuring the protection of PSII from photodamage [13, 63, 64, 112] draws attention to it as a potential candidate for stress tolerance engineering in crop plants. PTOX was overexpressed in various systems [40, 130-134]. Contrary to expectations, Rosso et al. [131] showed that up to 16-fold PTOX overexpression in A. thaliana did not increase the ability to maintain the plastoquinone pool oxidized and did not provide significant photoprotection. In tobacco, overexpression of PTOX resulted in increased susceptibility to photoinhibition [132]. In addition, the oxygen-evolving activity in isolated thylakoid membranes of these plants was more suppressed in strong light than in the thylakoids of the wild-type line. In contrast, in low light, the thylakoids of the PTOX overexpressors were more protected from photoinhibition, whereas in WT plants they were significantly damaged. When the light was excessive, PTOX in overexpressors generated superoxide as a byproduct of its catalytic activity, acting as a pro-oxidant. Feilke et al. [135] also found that PTOX generates ROS in a side reaction when the availability of the substrate is limited. Additional ROS may be produced either due to peroxide intermediates in the reduction of O2 to H2O or the production of protein-derived radicals as a result of the modification of iron species in the active center of the enzyme [58, 132].
Thus, mere PTOX overexpression cannot increase plant resistance to photoinhibition, and PTOX can act as a safety valve under stress only when the superoxide produced is deactivated by an effective antioxidant system. When comparing defense mechanisms and regulation of salinity tolerance in the chloroplasts of halophyte Thellungiella salsuginea with the glycophyte A. thaliana, it was shown that high PTOX activity was accompanied by intense H2O2 generation. Intense H2O2 generation was suggested as an important component of stress resistance in Thellungiella, keeping the antioxidant system activated and serving as a signal for gene expression [136].
Using chloroplast transformation technology, Ahmad et al. [133, 134] obtained tobacco plants overexpressing PTOX1 from green alga Chlamydomonas reinhardtii. The transformed plants were more sensitive to high light while were more resistant to salt stress and showed better recovery and less chlorophyll bleaching after exposure to high levels of NaCl. These results are the first report linking PTOX overexpression to salt tolerance at the level of germination and root development.
8. A POSSIBLE ROLE OF MITOCHONDRIAL ALTERNATIVE OXIDASES IN CHLORORESPIRATION
In addition to the classical electron transport coupled to ATP synthesis, both plant chloroplasts and mitochondria have alternative pathways that involve NAD(P)H dehydrogenases (NDH or ND) and alternative oxidases (PTOX or AOX) [106]. These alternative pathways are thought to help prevent cellular damage during exposure to various environmental stresses. Both PTOX and AOX belong to the di-iron carboxylate group of protein oxidases [93, 137, 138]. They are located on interfacial surfaces of thylakoidal and mitochondrial membranes, respectively. The active sites of both oxidases include a four-helix bundle coordinating a binuclear di-iron center that binds and activates Fe atoms coordinated by several highly conserved glutamate and histidine residues [30, 139]. The enzymes share cyanide (CN) resistance and inhibition by n-PG or n-octyl gallate [106, 130]. Plants have several isoforms of AOX, usually one of them is constitutive and the others are stress-induced [140]. In all species examined to date, PTOX is encoded by a single gene, while mitochondria have five its distantly related homologs (AOX1a, 1b, 1c, 1d, and AOX2) [106]. PTOX and AOX originated from an ancient di-iron oxidase, and diverged early, before the origin of mitochondria and chloroplasts [141].
According to existing conceptions, AOX plays a central role in coordinating signaling pathways between mitochondria and chloroplasts [108] and can protect photosynthesis from photodamage by dissipating excess reducing energy in chloroplasts [142, 143]. Moreover, AOX can directly enter chloroplasts and regulate electron transport in the thylakoid membrane [144]. All five AOXs can use PQH2 as a substrate, and AOX1a, AOX1b, and AOX2 may be targeted to chloroplasts [106]. Based on findings obtained and literature data [144, 145], Wang et al. [106] hypothesized that AOX1a, AOX1b, and AOX2 might be dually targeted to mitochondria and chloroplasts, while AOX1c and AOX1d are specifically localized in mitochondria. It should be noted, however, that all five Arabidopsis AOXs were predicted to be localized in mitochondria, according to the analysis of targeting peptides [146, 147].
Overexpression of AOX2 that was assumed to target dually to mitochondria and chloroplasts [106], rescued the variegation phenotype of the Arabidopsis im mutant. When AOX1a was introduced into chloroplasts, the growth defect of im was partially corrected. Thus, AOX2, and AOX1a may act as a PQH2 oxidase in chloroplasts, suggesting that the substrate specificity of PTOX and AOX in plants may not be as strict as in vitro experiments [144].
AOX1b and AOX2 are weakly expressed under normal conditions. When their expression is upregulated, especially under stress conditions with impairment of chloroplast function, AOX1b and AOX2 can enter chloroplasts. Although mitochondrial AOX can buffer redox poise fluctuations in chloroplasts via the malate/oxaloacetate shuttle [138], only chloroplast-localized AOX could correct the im phenotype [106]. Therefore, it can be speculated that AOX1b and AOX2 are not required for the maintenance of general mitochondrial functions but play an important role under stress conditions, including involvement in chlororespiration.
CONCLUSION
Initially, the existence of oxygen uptake as a valid respiratory process in chloroplasts was regarded with great skepticism since, under conditions close to optimal, this reaction in healthy plants is barely noticeable. Decades after its discovery, compelling evidence was accumulated on activation of chlororespiration under various stresses and its involvement in plant defense and adaptation to extreme environmental factors such as high light, heat, cooling, and drought.
The discovery of the key actors, PTOX and NDH enzymatic complexes, greatly improved the understanding of the chlororespiration mechanism. In contrast to other alternative electron transfer pathways, PS I-mediated CET and pseudo-cyclic electron transport, chlororespiration does not produce toxic ROS, since water is the direct product of PTOX-catalyzed electron transfer to oxygen. Thus, to some extent, PTOX operation may functionally substitute for H2O2-scavenging activity. Together with other alternative electron sinks from photosynthetic carriers under their over-reduction, chlororespiration may participate in a flexible and efficient system of regulation of light energy conversion under extreme and variable environmental conditions.
LIST OF ABBREVIATIONS
| ETC | = Electron Transport Chain |
| PQP | = Plastoquinone Pool |
| PTOX | = Plastoquinol Oxidase |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was financially supported by the National academy of sciences of Ukraine (Grant number 0112U002315).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


