RESEARCH ARTICLE
The Effect of Phytohormonal Steroids in Combination with Succinic Acid on the Resistance of Hordeum Vulgare L. to Helminthosporium teres Sacc.
Neli Manzhаlesаva1, Raisa P. Litvinovskaya1, *, Svetlana N. Poljanskaja2, Vladimir A. Khripach1
Article Information
Identifiers and Pagination:
Year: 2022Volume: 16
Issue: Suppl-1, M9
E-location ID: e187433152207130
Publisher ID: e187433152207130
DOI: 10.2174/18743315-v16-e2207130
Article History:
Received Date: 3/11/2021Revision Received Date: 20/1/2022
Acceptance Date: 8/4/2022
Electronic publication date: 31/10/2022
Collection year: 2022

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim:
The aim of this investigation is study the BS effect on the growth of Helminthosporium teres Sacc. and to elucidate the protective effect of brassinosteroids and their tetrahemisuccinates on spring barley (Hordeum Vulgare L.) exposed to biotic stress caused by this phytopathogenic fungus.
Background:
Brassinosteroids and their tetrahemisuccinates are considered as protectors of the fungal infection caused by H.teres.
Objective:
Evaluation of the effect of brassinosteroids, their mixtures and conjugates with succinic acid against the pathogen H.teres, as well as in spring barley crops against a natural infectious background.
Methods:
The fungistatic activity of brassinosteroids and their tetrahemisuccinates was evaluated in relation to the phytopathogenic fungus H.teres. The effect of BS and their derivatives on the processes of adaptation of spring barley plants to the pathogen of net spotting of the phytopathogenic fungus H.teres was studied on a model pathosystem and in the agrocenosis.
Results:
A fungistatic activity of the synthesized substances was revealed, which consists in inhibiting the growth of the phytopathogenic fungus H.teres on a solid nutrient medium by 35-40%. Succinic acid can enhance the fungistatic effect of brassinosteroids, which is more noticeable when using conjugates with succinic acid than in mechanical mixtures. In field experiments, it was shown that the studied compounds and their mixtures applied as a single spraying in the beginning of tubing phase reduced the development of leaf spots caused by phytopathogenic fungi.
Conclusion:
The results obtained indicate that brassinosteroids with succinic acid both in the form of conjugates and mechanical mixtures show fungistatic activity.
1. INTRODUCTION
The modern concept of plant protection is based on the use of ecofriendly non-pesticidal chemical agents-inducers of disease resistance, acting through the strengthening of natural immune response of plants. These substances should enhance the plant's defense against pathogens. It is well known that along with the classical groups of plant hormones such as auxins, ethylene, gibberellins, abscisines and cytokinins, brassinosteroids also perform hormonal functions [1-3]. They are steroid compounds that can penetrate the cell membrane and selectively interact with specific nuclear receptors, thereby causing changes in the genetic apparatus of the cell, that is, carrying out hormonal regulation. BS is found in every plant cell and support the normal functioning of the plant's immune system. Especially important is the effect of BS in unfavorable conditions, for example, at high, low temperatures and frosts [4-8], flooding and drought [9-11], soil pollution with heavy metals [12-15], soil salinization [16, 17], diseases and pesticide [18, 19].
The studies carried out to date indicate that phytohormonal steroids play important roles in regulating growth and developmental processes. In addition to acting as a regulator of plant growth, they also play a role in defense responses to pathogens [20]. Many research have been done on their role in plant resistance to fungi, bacteria and viruses. The protective role of BS has been well documented. For example, exogenous application of EB in 0.1 mM to 10 ml per plant of Cucumis sativus at the root and leaves level helps to reduce fusarium pathogen-induced accumulation of ROS along with protective substances such as flavonoids and total phenolic compounds. In the same study, it was further added that brassinolide treatment ameliorates the activities of various defense-related enzymes and ROS-scavenging enzymes and is thus responsible for reducing disease severity [21, 22]. Effect of root and foliar applications of EB on fusarium wilt and antioxidant metabolism in cucumber roots has been noted. Exogenously applied brassinolide induced resistance in barley plants to several fungal pathogens exhibiting different trophic lifestyles [23]. In particular, application of the EB to heads of «Lux» barley reduced the severity of Fusarium head blight caused by Fusarium culmorum by 86% and reduced the FHB-caused loss of grain yield by 33%. In addition, the growth of plants in soil amended with EB resulted in 28% and 35% reductions in Fusarium seedling blight symptoms on the «Lux» and «Akashinriki» barley, respectively. EB at 0.1-mM concentration, when sprayed at the whole seedling of Cucumis sativus promoted the expression of genes involved in defense responses against cucumber mosaic virus (CMV). Untreated controlled distilled water seedlings developed typical CMV symptoms and increased MDA content, which lowered to significant levels in seedlings primed with EB [24].
It has been demonstrated that treatment with brassinolide induces resistance in tobacco plants against tobacco mosaic virus, the bacterial pathogen Pseudomonas syringae, and the fungal pathogen Oidium sp., and in rice against Magnaporthe grisea and Xanthomonas oryzae which cause rice blast and bacterial blight, respectively [25]. Field studies in crop plants suggest that exogenously applied BS could confer tolerance to plants from a wide spectrum of pathogen infections. In potatoes, brassinolide treatment reduced the damage to plants caused by phytophthora, a genus of oomycetes (water molds) [26], and a similar effect of BS was also observed in tomato, cucumber, sugar beet, and some other plants [27]. In rice, instead of enhancing the plant’s resistance, BS were found to increase the susceptibility to the hemibiotrophic pathogens Pythium graminicola and Meloidogyne graminicola [28]. BS have been reported to suppress the protection of rice against root-knot nematodes [29]. BS also induced the susceptibility of potato tuber tissues by stimulating the mycelial growth, intensifying the spore formation of Phytophthora infenstans, and weakening the immune status of plant tissues [30].
To study how BS induce stress tolerance, the authors [24] manipulated the BS levels in cucumbers through a chemical genetics approach and found that BS levels were positively correlated with the tolerance to photo-oxidative and cold stresses and resistance to CMV.
An interesting experiment was conducted on tobacco and rice plants treated with brassinolide. Plants were infected with the pathogen 5 days after treatment with BS. Interestingly, BS-induced tobacco resistance does not correlate with an elevation of salicylic acid levels or induction of pathogenesis-related gene expression, suggesting that the mechanism by which BS induces resistance is different from systemic acquired resistance (SAR) and tobacco-induced resistance [25].
However, there is evidence that exogenously applied BS showed no effect on inducing the resistance of wild-type Arabidopsis plants infected with the hemibiotrophic bacteria Pseudomonas syringae pv. tomato (Pto) DC3000 or the necrotrophic fungus Alternaria brassicicola [31].
It was shown that BS act not only by themselves but also in close synergism with other hormones, which is demonstrated by their interaction with auxins when stimulating the production of another plant hormone, ethylene involved in the regulation of various processes of plant development [32]. Good results on the combined action of BS and auxins were also obtained on green algae Chlorella vulgaris. Studies have shown that under their mutual influence, the stimulation of the proliferation of algal cells and the accumulation of proteins, chlorophyll and monosaccharides in the cells significantly exceeds the effect of the action of only one of the components of the mixture, and the greatest activity was shown by a mixture of brassinolide and indolyl-3-acetic acid (IAA) [33]. Similar synergistic effects also manifested changes in plant stress resistance to unfavorable environmental factors [34].
The above activity of mechanical mixtures of BS with other phytohormones made it possible to expect even greater activity from the ester conjugates of these compounds. Firstly, they have better solubility, and, secondly, they can act as a “depot” of active components that are gradually released due to enzymatic hydrolysis. Thus, we synthesized and tested derivatives with 5-aminolevulinic acid [35], which itself has a beneficial effect on plant growth and development [36], and also some mono- and di-derivatives of BS with IAA [37]. The activity of the latter ones was demonstrated in primary tests on wheat seedlings. In both cases, the effect of the conjugates exceeded the effect of the individual components and their mechanical mixtures. Moreover the positive effect of a synthetic brassinosteroid derivative modified with an indolylacetic acid on the growth and development of Arabidopsis thaliana and Triticum aestivum plants under salt stress was established [38]. This conjugate has an increased ability to stimulate the growth and development of plant cells under salinization conditions, which may be due to cross-hormonal interactions. Previously, we described the synthesis and evaluation of the protective effect of brassinosteroids and their conjugates with salicylic acid, which is considered one of the stress hormones involved in forming adaptive plant responses [39]. The synthesized conjugates increased the survival of millet sprouts under heat and salt stress and decreased the accumulation in them of lipid peroxidation products [40]. The studied conjugates had an inducing effect on the immunity of barley plants subjected to biotic stress. At the same time, the protective effect of each of the hormones, taken separately or in the form of a mixture, was inferior in magnitude to the action of the ester conjugate, in which both phytohormonal components are chemically bound [41].
In our present work on synthesizing and studying brassinosteroids and their derivatives with biologically important organic acids, we turned to studying tetrahemisuccinates of steroid phytohormones. Succinic acid (SA) is special among biostimulants due to its high efficiency and safety. It is the most important organic acid that has elicitory properties and induces nonspecific resistance to biotic stressors, exerting an activating effect on many metabolic processes of plants [42-44].
This investigation aims to study the BS effect on the growth of Helminthosporium teres Sacc. and elucidate the protective effect of brassinosteroids and their tetrahemisuccinates on spring barley (Hordeum Vulgare L.) exposed to biotic stress caused by this phytopathogenic fungus.
2. MATERIALS AND METHODS
2.1. Materials
Brassinosteroids and their 2,3,22,23-tetrahemisuccinates (conjugates) were synthesized in the Laboratory of steroid chemistry, Institute of Bioorganic Chemistry, National Academy of Sciences of Belarus. Spring barley seeds (variety Mustang) were obtained from the Republican Scientific Center for Agriculture, National Academу of Sciences of Belarus. The test object for studying the fungistatic activity of the compounds was the phytopathogenic fungus Helminthosporium teres Sacc. [Drechslera teres (Sacc) Shoem.] - the causative agent of reticular spotting of barley. Сulture of the fungus obtained at the Institute of Experimental Botany, National Academy of Sciences of Belarus. The reagents of Sigma company were used in the work.
2.2. Methods
2.2.1. Determination of the Fungistatic Activity
The fungistatic activity of the compounds was evaluated in relation to the phytopathogenic fungus H.teres. In sterile Petri dishes with a diameter of 15 cm, 15 ml of pre-sterilized molten Chapek medium containing 0,5 g KH2PO4, 0,5 g MgSO4, 0,5 g KCl, 1,2 g urea, 20 g lactose and 20 g agar L−1, the investigated substances were poured. After solidification of the medium, spores were introduced to the agar surface in the center of the cup. Cultures were grown under fluorescent lamps with an intensity of 6000 lux with a photoperiod of 16 h at 20°C. After 10 days of cultivation, the diameter of the growth zones was measured and the intensity of spore formation was evaluated under a microscope.
2.2.2. Study of the Effect of Phytohormonal Steroids on the Formation of Plant Adaptation to Biotic Stress in Laboratory Experiment
In laboratory experiment spring barley plants of the Mustang variety were grown under light culture conditions (3-6 thousand lux) until the age of the 2nd leaf (14 days). Treatment of seedlings was carried out by spraying with aqueous solutions of substances after a day of inoculation with an aqueous suspension of pathogen conidia with 1% Twin-80 (3-5 thousand/ml) [45]. Plants treated with water served as a control. Lights were switched off, and high humidity was maintained for 24 h after inoculation. Samples for analysis were taken on the 2nd day after infection (no signs of damage, biotrophic stage) and the 4th day (manifestation of infection in the form of brown spots and streaks, necrotrophic stage).
2.2.3. Determination of Lipid Peroxidation Products (POL)
For the quantitative determination of POL products (calculated as malondialdehyde, MDA), a test with 2-thiobarbituric acid was used which is based on the binding of the latter to lipid peroxides and the formation of colored products [46]. A sample of fresh mass was ground to a homogenate of 0.25% TBA in 10% trichloroacetic acid. The homogenate was transferred to centrifuge tubes covered with aluminum foil, and the level was marked. The samples were heated for 30 minutes at 95 °C, then cooled in running water. Then it was centrifuged for 15 minutes at 8000 g. The supernatant was spectrophotometered at 532 nm. The quantity of MDA was calculated using a molar extinction coefficient of 1.55x105 M-1 cm-1. The experiments were performed in triplicate with 3 repetitions in each series. Each repetition consisted of at least 10 plants.
2.2.4. Determination of the Yield of Water-soluble Substances
To determine the yield of water-soluble substances from leaf tissues, a conductometric method was used [47] with minimal modifications. The leaves of the control and experimental plants were washed with distilled water, dried, cut into equal segments and placed in distilled water in a ratio of 50 ml of water per 1 g of the raw sample weight . The leaves were incubated for 3 hours, after which the leaves were removed, and measurements were carried out using a conductometer “Hanna, HJ 9932” while taking into account the readings of distilled water. The number of dissolved substances was expressed in mcg/g of raw mass.
2.2.5. Determination of Biological Efficiency
The influence of phytohormonal steroids, their compositions with SA and conjugates on the degree of plant damage by phytopathogenic fungi as well as grain productivity of the crop were determined in the agrocenosis of spring barley of the Raider variety. The experiments were laid on the experimental base of the Republican Scientific Center for Agriculture, National Academy of Sciences of Belarus (Zhodino), using the accepted technology of growing this crop. Plots with an area of 1 m2 were laid in 4-fold repetition in accordance with the methodology of field experience [48].
2.2.6. Accounting for Fungal Diseases
The accounting of leaf diseases of barley in the agrocenosis was carried out according to their external characteristics on a natural infection background. Accounting elements - prevalence (frequency of occurrence) and intensity of development (degree of damage), expressed as a percentage [49]. The total number of plants in the sample was 50 in four replicates for each variant.
The intensity of development of leaf diseases (degree of damage) was determined by the area of the affected leaf surface using a counting scale to determine the percentage of infected leaf area [50].
2.2.7. Determination of the Biological Effectiveness of Phytohormonal Steroids
The biological effectiveness of the compounds against a complex of pathogens causing leaf diseases in spring barley was determined by comparing the percentage of affected plants and the intensity (degree of damage) in the control and experimental variants. The biological efficiency of the treatment was calculated by comparison with the control according to the Abbot formula [51].
2.2.8. The Structure of the Crop
The structure of the crop was determined by the individual analysis of plants in a sheaf (25 plants) in four-fold repetition for each variant.
2.2.9. Statistical Analysis
All experimental data were processed according to Rokitsky [52] or using a modified program for statistical processing Statium. The results in the tables are presented as average arithmetic mean with a standard error. The Student's t-test was used to compare independent samples. The differences that are significant at P < 0.05 are discussed.
3. RESULTS
3.1. The Fungistatic Activity of Brassinosteroids and their Derivatives
BS protect plants from diseases by changing the metabolism of the protected plant in a direction unfavorable for pathogens. However, under certain conditions, they can exhibit fungistatic, bactericidal and antiviral activity [18. 19, 26, 27]. Under laboratory conditions, in in vitro experiments, the fungistatic activity of brassinosteroids of the 24-epibrassinolide series, their tetrahemisuccinates and mixtures with succinic acid were studied. The test object was the pathogen of barley net blotch, the pathogenic fungus Helmintosporium teres Sacc. It turned out that the studied compounds and their compositions exhibited a pronounced direct fungistatic effect (Table 1). The growth of pathogen colonies was limited by 25-40% and was accompanied by the transition of the fungus from the usual concentric uniform growth of its colonies to chaotically uneven development with several growth centers. The structure of the mycelium also changed. Mechanical mixtures of compounds exhibited a relatively more active effect. The mechanical mixture of 24-epicastasterone and succinic acid most actively (almost 50%) suppressed the growth of the pathogen. Chemically modified brassinosteroids limited the growth of pathogen colonies at the level of action of individual phytohormones. Despite the limiting effect of mycelium growth, mechanical mixtures of compounds did not prevent sporulation, although they reduced its intensity, while brassinosteroids and their derivatives practically inhibited sporulation.
Thus, laboratory experiments established that not only BS of the 24-epibrassinolide series and their derivatives with SA and mechanical mixtures of brassinosteroids with SA have a fungistatic effect.
| S.No | Variant | Colony Diameter, cm (10 days) /% | Description of Colonies (10th day) |
|---|---|---|---|
| of control | |||
| 1 | control | 6,6±0,25/100 | The colony has developed from the center; the mycelium in the center of the colony is light, loose, with dark spores at the edges. The spores are large, olive green with 3-5 septa. With the development of the colony, abundant sporulation is noted throughout the cup.. |
| 2 | EB | 4,5±0,15/68 | Colonies are uniform, concentric, growth retarded, and the colony does not reach the edges of the dish. Mycelium is white, dense, and grows along the edges. Sporulation is not observed. |
| 3 | THS of EB | 4,5±0,19/68 | Colony growth is uneven and limited. The mycelium is light, dense, and growing. Sporulation is not observed. |
| 4 | SA + EB | 4,1±0,09/68 | Colony growth is uneven and limited. Mycelium is light, dense, has numerous colonies, and sporulation is limited. Spores are large, olive-colored with 3-5 septa |
| 5 | SA | 5,3±0,18/85 | White dense mycelium, concentric, uniform growth. Mass sporulation. Spores are large, olive-colored with 3-5 septa |
| 6 | EC | 4,2±0,17/63 | Colony growth is uneven and limited. The mycelium is light, dense, and growing. Sporulation is not observed. |
| 7 | THS of EC | 4,3±0,18/64 | Colony growth is uneven and limited. The mycelium is light, dense and growing. There are isolated atypical (rounded spores). |
| 8 | SA + EC | 3,8±0,17/58 | Colony growth is uneven and limited. Mycelium is light, dense, has many colonies, grows, and sporulation is limited. The spores are large, olive green with 3-5 septa. |
3.2. Effect of Brassinosteroids and their Derivatives on the Plant Adaptation to the Net Blotch Pathogen
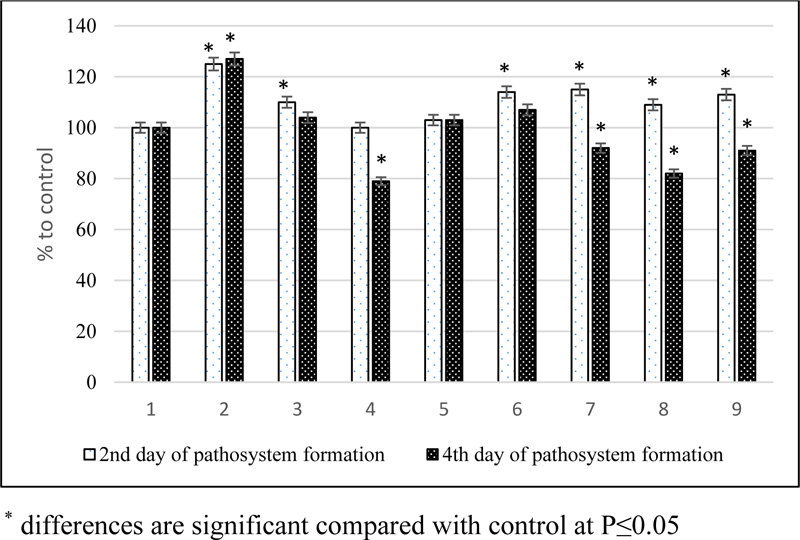
In laboratory experiments, the effect of natural brassinosteroids and their derivatives on the adaptation processes of spring barley plants to the pathogen of net blotch of the phytopathogenic fungus H.teres was studied. Plants treated with water served as control. Samples for analysis were taken on the 2nd day after infection (onset of the disease) and the 4th day (intensive development of the disease in the form of brownish-brown spots and streaks). Suppression of oxidative processes can be considered a protective reaction. He content of MDA - the end product of lipid peroxidation of the membranes and the excretion of water-soluble substances from plant tissues were determined (Fig. 1) to assess the nature of disease resistance and the possible regulatory effect of substances. In our experiments, the content of MDA in infected plant leaf tissues increased compared to the control at both stages of pathogenesis, indicating a developing pathological process.
Pretreatment of plants with BS, by their mixtures containing SA or by conjugates, followed by inoculation with phytopathogen spores, on the contrary, suppressed this activity in comparison with the infected control already at the first stage of pathogenesis. At the same time, in relation to the uninfected control, the level of peroxidation increased, except for variants with succinic acid and tetrahemisuccinate of 24-epibrassinolide. In the second stage, an even more intense inhibition of the lipid peroxidation process occurred compared to the infected control, which was most significantly manifested in the variants with the use of conjugates.
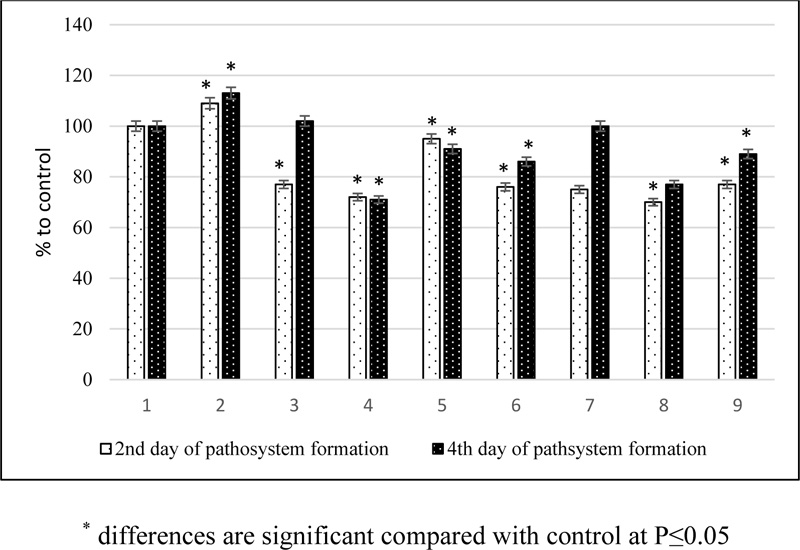
The structural state of plant membranes can also be judged by the level of excretion of water-soluble substances from plant cells. Plant infection increased the activity of the release of water-soluble compounds during the incubation development of the pathogen, which increased by the second stage of pathogenesis. Pretreatment of plants with phytoregulators followed by infection sharply reduced the intensity of exocytosis of water-soluble substances in all variants at the first stage of pathogenesis in comparison with the infected control and less intensively at the second (Fig. 2). The yield of water-soluble substances decreased most intensively under the influence of tetrahemisuccinates. It was shown that under biotic stress conditions, the studied compounds prevented the development of oxidative damage to plants, which also appeared with inhibition of the release of water-soluble substances from leaf tissues.
Thus, during the formation of the pathosystem barley-pathogen of net spotting tetrahemisuccinates of BS prevented the development of oxidative damage of plants, which resulted in a decrease in the content of lipid peroxidation product MDA and inhibition of the release of water-soluble substances from leaf tissues. Under the conditions of the formation of facultative parasitic relations in the phytopatosystem, as is the case with the defeat of barley by netted spot, necrosis is inhibited, which forms an environment incompatible with the development of necrotroph. Phenotypically, this manifested in the almost complete absence of signs of plant damage.
3.3. Protective and Stimulating Action of Brassinosteroids Against Leaf Diseases in Agrocenosis
In the modern strategy for the use of chemical plant protection agents, the most promising seems to be the use of protective and stimulating substances of natural origin, capable of regulating plant growth and inducing the formation of resistance to diseases. Steroid phytohormones and their derivatives can take a special place in this. In the present study, we found the expressive influence of phytohormonal steroids and their derivatives on the formation of disease resistance and the structure of the yield of spring barley of the Raider variety.
In the field, solutions (at a concentration of 10-6M) of 24-epibrassinolide, 24-epicastasterone and their tetrahemisuccinates, as well as mixtures of BS with SA were studied. Under the conditions of a field experiment in 2020, during the growing season in the agrocenosis by the stage of milk ripeness, the damage to plants reached an average of 1 point (sub flag and flag leaves). Observations showed that the test substances and their compositions applied as a single spraying at the beginning of the tubing phase reduced the development of leaf spots caused by phytopathogenic fungi (Table 2). Thus, by the stage of milk ripeness, the prevalence of the disease and the degree of damage to the flag leaf decreased by 2-6 times compared to the control, and on the second top leaf from 10 to 70%. The most active inhibition of diseases gave 24-epicastasterone in combination with succinic acid both in the form of a mechanical mixture and in the form of a conjugate, exceeding the reference fungicide Echion in biological efficiency by 10%. The biological effectiveness of 24-epibrassinolide tetrahemisuccinate and fungicide was 71,4%, and a mixture of 24-epibrassinolide and succinic acid was - 42.8%.
| Variant | 2nd Top Leaf | Flag Leaf | |||
|---|---|---|---|---|---|
| Prevalence of Diseases | The Extent of the Disease | Prevalence of Diseases | The Extent of the Disease | Biological Effectivenes | |
| Control | 100 | 70,0 | 70 | 17,5 | - |
| Fungicide Echion | 80 | 18,8 | 20 | 5,0 | 71,4 |
| 24-epibrassinolide | 90 | 50,0 | 30 | 7,5 | 57,1 |
| 24-epibrassinolide + succinic acid | 60 | 15,0 | 20 | 5,0 | 71,4 |
| Succinic acid | 90 | 22,5 | 40 | 10,0 | 42,8 |
| Tetrahemisuccinate of | 70 | 15,0 | 20 | 5,0 | 71,4 |
| 24-epibrassinolide | |||||
| 24-epicastasterone | 30 | 22,5 | 20 | 2,5 | 71,4 |
| 24-epicastasterone+ succinic acid | 70 | 17,5 | 10 | 2,5 | 85,7 |
| Tetrahemisuccinate of | 100 | 20,0 | 10 | 2,5 | 85,7 |
| 24-epicastasterone | |||||
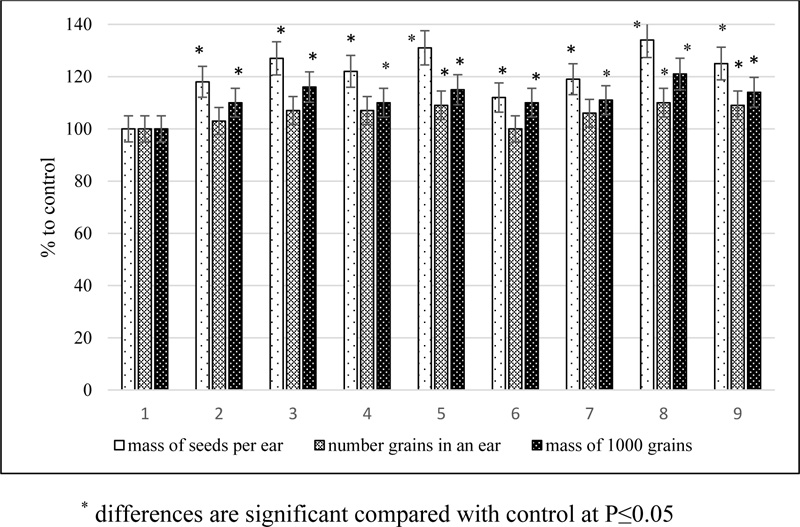
Phytoregulators in a dose of 25-50 mg per hectare also had a positive effect on the structure of the barley yield, improving it qualitatively by increasing the mass of 1000 grains to 21% and quantitatively by increasing the number of grains in an ear to 10% (Fig. 3).
As a result of the application of a mechanical mixture of 24-epibrassinolide with succinic acid, the mass of seeds per ear increased by 31%, and the mixture of 24-epicastasterone with succinic acid increased by 34%. The conjugates had a weaker stimulating effect on plant productivity, increasing the seed weight from 1 ear by 10 and 14% 24-epibrassinolide tetrahemisuccinate and 24-epicastasterone tetrahemisuccinate, respectively.
Thus, the combined use of phytohormonal steroids with SA, both in the form of conjugates and mechanical mixtures, can be used to increase the resistance and productivity of spring barley plants in agrocenosis.
4. DISCUSSION
One of the aims of this work was to study the effect of phytohormonal steroids and their mixtures and derivatives with succinic acid on the growth of the phytopathogenic fungus Helminthosporium teres Sacc. and sporulation, i.e., their influence on the vegetative and generative spheres of the pathogen (Table 1). There are data in the literature that if the inductor negatively effects the growth of the fungus, then it negatively affects toxin production [53]. This is true for necrotrophic pathogens like H.teres. Such pathogens act on plant tissues with toxins and enzymes - first, they kill the cells and then colonize them. It can be assumed that the toxin produced by the pathogen in the presence of phytohormonal steroids, their tetrahemisuccinates, and mixtures with succinic acid destroys plant tissue less effectively. For practice, it is also important that the disease resistance inducers like BS, which control and change the metabolism of fungi to reduce their aggressiveness, reduce the risk of pathogen resistance to the action of such substances.
Another task of our work was studying the influence of brassinosteroids and their derivatives on the plant adaptation to the net blotch of barley. Understanding the mechanism of the disease resistance inductor action in the pathogen-host system can be achieved by studying the metabolism changes caused by BS. The change in disease resistance is essentially a change in the metabolic processes in the host that occurs during the infectious process. The resistance of plants to diseases will be correspondingly weakened or strengthened, depending on how the conditions for the parasite survival in plant tissues have changed. Oxidative stress is a consequence of the action on living organisms of various adverse factors. Therefore, redox processes are considered criteria for adapting a plant organism to infection. The causative agent of barley net blotch belongs to the hemibiotrophic biological group. The mycelium of the fungus grows in the living tissues of the plant, but the formation of spores occurs in the dead tissues. Representatives of this group of pathogenic fungi first direct the pathogenesis they induced along the biotrophic pathway and after a while, switch the development of the pathological process to the necrotrophic one. The biotrophic stage proceeds for several days, after which the development of the fungus enters the toxin-forming pathogenic stage, triggering intense necrosis.
The so-called oxidative and lysosomal explosion occurs in plants' affected tissue. Plasmolysis begins in the cells, and necrosis appears on the leaves. Thus, the development of necrosis due to the vital activity of the pathogen is not a protective plant reaction. Necrotic tissue is the ecological habitat necessary for the existence of the causative agent of net spotting. On the contrary, the suppression of oxidative processes can be considered a protective reaction. A convenient and widely used method for assessing the intensity of oxidative stress in plants is measuring the MDA level. In our experiments, the content of MDA in leaf tissues of infected plants increased compared to the control at both stages of pathogenesis, which indicates the development of a pathological process (Fig. 1). Under the action of steroid phytohormones and their derivatives, the formation of MDA is inhibited, especially at the second stage of pathogenesis. This is about the realization of the adaptive-protective potential of the plant. At the same time, in relation to the uninfected control plants, the level of peroxidation under the influence of the studied compounds was slightly increased (by 3-10%). It is known that lipid peroxidation products can act as primary mediators of stress, including protective mechanisms. Therefore, such a change in the LPO level in our experiments may indicate that pretreatment with brassinosteroids and their derivatives initiates signaling pathways and triggers a cascade of local biochemical processes involved in the formation of the plant phytoimmune response.
The permeability of plant cell membranes by determining the intensity of release of water-soluble substances from tissues can also indicate plant resistance to stress. An increase in the release of water-soluble substances from the leaves indicates an intensification of metabolism (healthy plants) or severe tissue damage (infected plants). The suppression of the release of water-soluble substances from the leaves in the affected plants shows that the protective effect of the compounds is successful (Fig. 2).
Thus, during the formation of the pathosystem tetrahemisuccinates of BS prevented the development of oxidative damage of plants, which resulted in a decrease in the content of lipid peroxidation product MDA and inhibition of the release of water-soluble substances from leaf tissues. The effect of mechanical mixtures in terms of the direction of the effect induced by them was similar to the effect of tetrahemisuccinates, but the level turned out to be lower than the effect of conjugates. Phenotypically, we observed the following picture. In the infected leaves of untreated plants, the symptoms of the disease were intensely manifested by the fourth day of pathogenesis in the form of bordered necrosis, occupying a large surface of the leaf. Pretreatment of plants with brassinosteroids and their conjugates with succinic acid almost completely suppressed the development of net helminthosporiasis, as evidenced by the appearance of only small dark brown dots on the lower primary leaves. At the same time, pretreatment with tetrahemisuccinates suppressed the development of the disease to a greater extent than pretreatment with brassinosteroids. Treatment of plants with succinic acid turned out to be the least effective; mechanical mixtures occupied an intermediate position.
In the modern strategy for using chemical plant protection agents, the most promising seems to be the use of protective and stimulating substances of natural origin, capable of regulating plant growth and inducing the formation of resistance to diseases. Steroid phytohormones and their derivatives can take a special place in this. In the present study, we found an expressive influence of phytohormonal steroids and their tetrahemisuccinates on the formation of disease resistance and the structure of the crop of spring barley of the Raider variety. The combined use of phytohormonal steroids with succinic acid, both in the form of conjugates and mechanical mixtures, can be used to increase the resistance and productivity of spring barley plants in agrocenosis (Table 2, Fig. 3). The most active inhibition of diseases gave 24-epicastasterone in combination with succinic acid both in the form of a mechanical mixture and in the form of a conjugate, exceeding the reference fungicide Echion in biological efficiency by 10%. The biological effectiveness of 24-epibrassinolide tetrahemisuccinate and fungicide was 71,4%, and a mixture of 24-epibrassinolide and succinic acid was - 42.8%. However, if we evaluate the effect of brassinosteroids, their mixtures and conjugates with succinic acid in a complex, then preference for further development should be given to a mixture of phytohormonal steroids with succinic acid. It was not inferior to the corresponding conjugates in terms of biological effectiveness against pathogens and was more effective in terms of their effect on plant productivity.
CONCLUSION
Succinic acid enhances the plant-protective effect of brassinosteroids against fungi infection (H.teres) both when applied as derivatives and in mechanical mixtures. The effect results from combined cell-stimulating and fungistatic activities of SA and BS, which were found to be characteristic of the individual compounds, their mixtures and chemical conjugates in studied conditions. The obtained results can increase the resistance and productivity of spring barley plants in agro production.
LIST OF ABBREVIATIONS
| BS | = Brassinosteroids |
| IAA | = Indolyl-3-Acetic Acid |
| SA | = Succinic Acid |
| POL | = lipid Peroxidation Products |
| MDA | = Malondialdehyde |
| TBA | = Thiobarbituric Acid |
| EB | = 24-epibrassinolide |
| EC | = 24-epicastasterone |
| THS | = Tetrahemisuccinate |
| H.teres | = Helminthosporium teres Sacc |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies based on this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This study was funded by National Academy of Sciences of Belarus.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.