All published articles of this journal are available on ScienceDirect.
Effect of Silver Nanoparticles Synthesized by ‘Green’ Methods on the Growth of in vitro Culture of Betula pendula L. whole Plants
Abstract
Background:
Metal nanoparticles, such as silver nanoparticles obtained by “green” nanosynthesis, have been increasingly used in research and practice in recent years due to their high biocompatibility and low toxicity. It is important to understand how green nanoparticles have regulatory effects on all groups of living systems, including plants. One of the key questions is how silver nanoparticles obtained by green methods modify plant growth in various cultivation and biotechnological systems, such as in vitro culture.
Objective:
The aim of this study was to establish how in vitro culture of birch plants (Betula pendula Roth) reacts to different levels of silver nanoparticles synthesized by green methods (based on plant extracts) and chemical approaches.
Methods:
The paper examined the nodal segments of silver birch Betula pendula Roth grown on Woody Plant Medium (WPM) with the addition of silver nanoparticles (0.3-300 mg L-1). After 30 days of cultivation in an in vitro environment, the growth of shoots and roots was measured. Silver nanoparticles were synthesized using L-ascorbic acid (reducing agent) and polyvinylpyrrolidone (PVP; stabilizer), as well as with needle extract (as a reducing agent and stabilizer).
Results:
Chemical nanosynthesis based on PVP and L-ascorbate, as well as green nanosynthesis using extract of spruce needles made it possible to obtain spherical nanoparticles with similar physical parameters. Low levels of AgNPs (0.3-10 mg L-1) synthesized by chemical techniques (PVP and L-ascorbate) stimulated the growth of birch shoots. In this case, the maximum stimulating effect on shoot growth was found at 10 mg of L-1 AgNPs (250-300% stimulation compared to the control). Under higher levels of nanoparticles (30-300 mg L-1), the stimulating effect decreased. Concentrations over 300 mg of L-1 inhibited the growth of birch plants. Very similar effects were observed in roots.
In experiments with nanoparticles synthesized using spruce needle extract, it was shown that low concentrations of AgNPs (0.3 and 1 mg L-1) did not cause a significant change in the size of birch shoots and roots. At the same time, higher levels of silver nanoparticles (3-300 mg L-1) significantly stimulated growth.
Conclusion:
The present study demonstrates the production of stable silver nanoparticles based on PVP and L-ascorbic acid, as well as an extract of Betula pendula needles. The resulting nanoparticles have a uniform shape and distribution. The presence of AgNP (1-300 mg L-1) in the nutrient media has a stimulating effect on Betula pendula shoot and root growth.
1. INTRODUCTION
Nanoparticles (NPs) are solid objects, one of the geometric dimensions of which does not exceed 100 nm. Nanosize gives NPs a number of unique physicochemical characteristics, such as a high area-to-volume ratio, the ability to interact with electromagnetic radiation in a special way, catalytic properties, and high mobility in biological systems [1]. Due to these characteristics, NPs are widely used in industry, agriculture, biotechnology, healthcare and scientific research. Among the wide variety of synthesized and natural nanoparticles, nanoparticles of metals and metal oxides are of particular importance, representing today the most actively used group of nanomaterials in practice [2].
Metal-containing NPs have a number of properties that can be applied in research and practice, including biotechnology. In particular, their high redox activity (as biocatalysts of redox reactions), the ability to show biocidal action against pathogenic fungi and bacteria, the ability to penetrate hard-to-reach places of the cell and biological systems and other qualities [3-5] are increasingly used.
Over the past 10-15 years, there has been a constant increase in the interest of researchers in the use of metal-containing NPs obtained by the so-called “green” nanosynthesis, i.e. using either biological objects for their synthesis, or using natural extracts, juices and homogenates [6-10]. Green nanosynthesis is particularly well developed for silver NPs (AgNPs) [11, 12]. Silver nanoparticles, i.e. the smallest solid particles of metallic Ag, can be relatively easily formed (synthesized) from solutions of silver salts, such as AgNO3, under normal “biological” conditions (room temperature and pressure; standard conditions) [13, 14]. They are formed by reducing Ag+ to Ag0 (metallic silver is formed), under the action of so-called reductants [15, 16].
For green nanosynthesis of AgNPs, natural organic reducing agents such as L-ascorbic acid, flavonoids, tannins, carboxylic acids and phenolic derivatives are often used as reducing agents [17-20]. In addition to the reduction of AgNPs, an important step also is the stabilization of NPs, i.e. prevention of fusion and formation of larger metal particles. Natural or biologically inert molecules such as various polysaccharides, polyvinylpyrrolidone (PVP), polyethylene glycol (PEG), starch, etc. can also be used as AgNPs stabilizers in nanobiotechnological applications [21-23]. The formation of silver nanoparticles can be detected visually or spectrophotometrically by changing the color of the solution and the appearance of light absorption in the region of 400-500 nm [24].
It has now been demonstrated that plant extracts can act simultaneously as reducing and stabilizing agents [25-27]. AgNPs obtained using plant extracts demonstrated high antimicrobial activity against gram-positive bacteria (Staphylococcus aureus, Bacillus subtilis) and gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa) [27], as well as pathogenic fungi [28-30]. It has been shown that AgNPs can penetrate the cell wall and outer membrane of bacteria, entering the cytoplasm, and can also form large agglomerates inside the cell wall near the cell membrane, gradually releasing ionic silver and causing a stable biocidal effect [31]. In this regard, nanosilver is increasingly seen in biotechnology as a kind of alternative to antibiotics and highly toxic biocides such as hypochlorides, alcohol or hydrogen peroxide [32, 33].
One of the newest applications of AgNPs in biotechnology is their use as regulators of plant growth and development in vitro, as well as agents that have both growth-modulating and disinfecting effects on plant cultures in vitro used in biotechnology [34-36]. However, to date, there is no data on testing the effect of AgNPs synthesized by green nanosynthesis on the growth of microclones of plants under in vitro conditions. Also, very little is known about the influence of AgNPs on the growth and development of the most important woody plants, such as silver birch (Betula pendula Roth), which has a leading position in terms of occurrence in mixed forests in Belarus and Northern Europe.
The aim of the present work was to investigate the effects of AgNPs obtained with biocompatible reducing agents and stabilizers (L-ascorbic acid and PVP) and extract of the needles of Norway spruce (Picea abies) on the growth and development of roots and shoots of silver birch (Betula pendula Roth) in vitro.
2. MATERIALS AND METHODS
2.1. Materials and Chemicals
Silver nitrate (>99.9%, AgNO3), polyvinylpyrrolidone (M.W.55000, PVP), and L-ascorbic acid (99%), necessary for the synthesis of AgNPs, were purchased from Sigma (Sigma-Aldrich, USA). Picea abies needles were obtained from the nursery of ornamental plants of the Unitary Enterprise “Shchemylitsa BSU”. For the preparation of solutions, deionized water of the First type of purification, with a resistance of 18.52 mOms, was used.
2.2. Green Synthesis of Silver Nanoparticles (AgNPs)
Silver nanoparticles were used immediately after their production. AgNPs were synthesized in two ways: (i) using L-ascorbic acid as a reducing agent, and PVP as a stabilizer and (ii) using spruce needle extract as a reducing and stabilizing system (“green synthesis”). The methods were adapted from previously published papers [17, 37].
All calculations are given in the Table 1. Suspensions were prepared in a volume of 150 ml with deionized water. Ascorbic acid was slowly added (dropwise) during the mixing of suspension on a magnetic stirrer. pH of the solution was brought to 6 using 10% KOH before adding ascorbic acid. The reaction of nanoparticle formation occurred at room temperature for 5 minutes.
During the reaction, the color of the solution changed from colorless to yellow or brown, which indicated the formation of metallic silver nanoparticles (Fig. 1). Solutions with concentrations of 0.3-10 mg L-1 of silver nanoparticles were prepared by dilution from solutions with higher concentrations.
| Concentration of nanoparticles, mg L-1 | AgNO3 | PVP | L-ascorbic acid |
|---|---|---|---|
| m, mg | m, mg | m, mg | |
| 300 | 70.8 | 5 | 9 |
| 100 | 26.6 | 1.7 | 3 |
| 30 | 7.1 | 0.5 | 0.9 |

In the case of nanosynthesis using spruce needle extract, an extract from freshly harvested Picea abies needles was prepared. Eight grams of spruce needles was added to 200 mL of deionized water. The extract was boiled for 10 minutes in highly purified water. The extract was then settled and cooled to room temperature and filtered through Whatman Grade 1 filter paper (Whatman Inc.; USA). A suspension of silver nanoparticles with a concentration of 300 mg L-1 was obtained by adding freshly prepared spruce needle extract (20 mL) to 180 mL of solution containing 94.4 mg AgNO3 at room temperature with continuous stirring for 5 minutes. In parallel, the pH value of the solution was brought up to 6.0 (standard pH of plant apoplastic space, which is usually used in experiments with plants in vivo). After adding spruce needle extract, brown staining of the solution was observed, indicating the formation of AgNPs (Fig. 2) [17, 38].

Microscopic analysis of the nanoparticles was performed using a transmission electron microscope Jeol JEM (JEOL Ltd.; Japan) (Fig. 3 and Fig. 4). The ascorbate-PVP-based nanoparticles described by TEM were spherical in shape with a diameter of 38±3 nm (n = 100). In some cases, they were presented in the form of agglomerates.
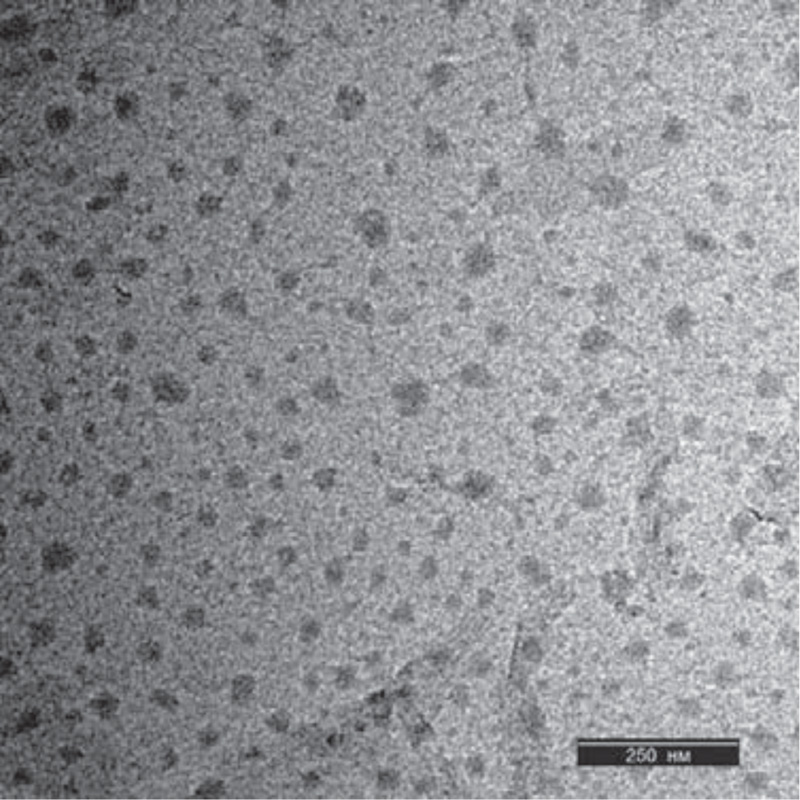
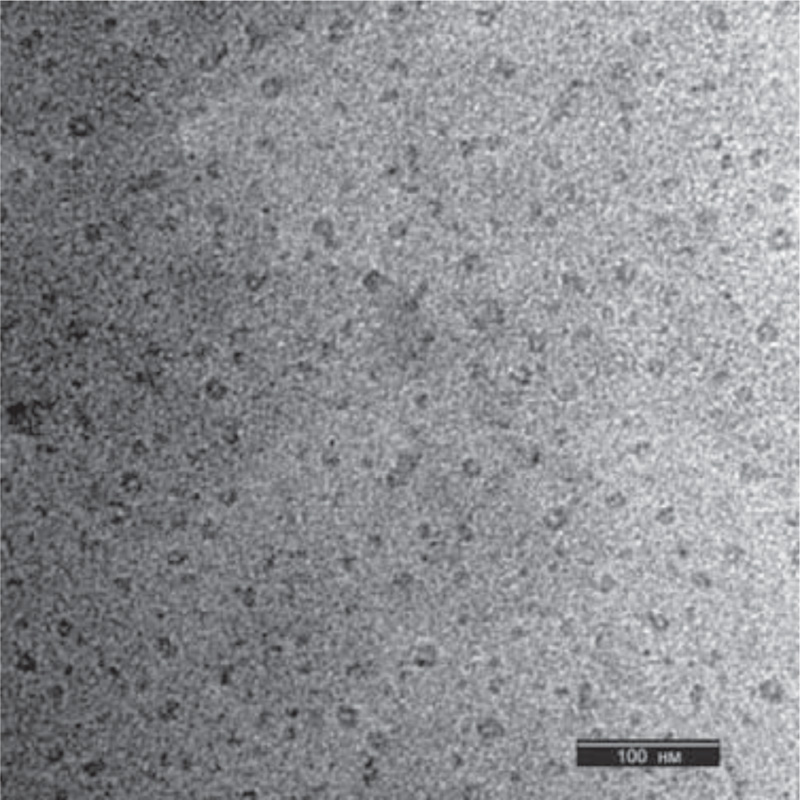
The spruce needle-based nanoparticles analyzed by TEM were spherical and smaller than ascorbic-PVP-based nanoparticles, 23±3 nm (n = 100). For nanoparticles synthesized on the basis of spruce needle extract, the presence of a large amount of organic residues in the initial suspension was characteristic. These nanoparticles had more elongated or cylindrical shape. In terms of the volume of solid material, they were close to nanoparticles synthesized using ascorbate and PVP.
2.2.1. UV-Vis Spectroscopy
The obtained nanoparticles were initially tested using the UV-Vis Cary 50 scanning spectrophotometer (Agilent, USA). The appearance of an absorbance peak at 410 nm in the case of nanoparticles, synthesized with PVP and L-ascorbic acid, and 411-475 nm, in the case of green nanoparticles synthesized with spruce extract was recorded. According to literature data [24, 26], these peaks correspond to the formation of silver nanoparticles.
2.3. Betula Pendula Roth in vitro Culture and its Treatment by AgNPs
The sterile culture of Betula pendula Roth whole plants was used. The culture was a kind donation from the Forest Institute of the National Academy of Sciences of Belarus. Cultivation of the culture was carried out in an incubator at a constant temperature of 23° C and a relative humidity of 60% with a light period of 16 h (light) and 8 h (darkness) for 30 days (luminous flux: 200 μmol photons m-2).
As a nutrient medium for growing explants, WPM (Duchefa, Netherlands) was used in the composition of 100% original salts. 3% sucrose and 0.9% phytoagar (Duchefa, Netherlands) were added to WPM. The control plants were grown on 100% WPM media. During tests with NPs, plants were grown on WPM media containing 0.3 to 300 mg L-1 AgNPs. About 35 plants were tested for each concentration of NPs.
3. RESULTS
3.1. Effect of Silver Nanoparticles Synthesized using L-ascorbic Acid and PVP on the Growth of Birch in vitro Whole-plant Culture
The use of PVP and L-ascorbate made it possible to obtain nanoparticles with similar physical parameters (Figs. 3 and 4). These nanoparticles were introduced into gel media at various concentrations; birch plants were grown on these media. The data presented in Figs. (5 and 6) suggest the dose-dependent nature of the effects of silver nanoparticles (0.3-300 mg L-1) on the growth and development of shoots and roots of birch plants. AgNPs stimulated the growth of birch shoots, when applied at all the concentrations. The maximum stimulating effect on shoot growth was observed for 10 mg of L-1 AgNPs (250-300% increase compared to control; Fig. 5). AgNPs in concentrations higher than 10 mg of L-1 also increased growth rate considerably, but this effect is much less. Concentrations greater than 300 mg of L-1 inhibited the growth of birch plants, and independently administered L-ascorbic acid and PVP (without silver) did not alter the growth of the birch plant (data not shown).
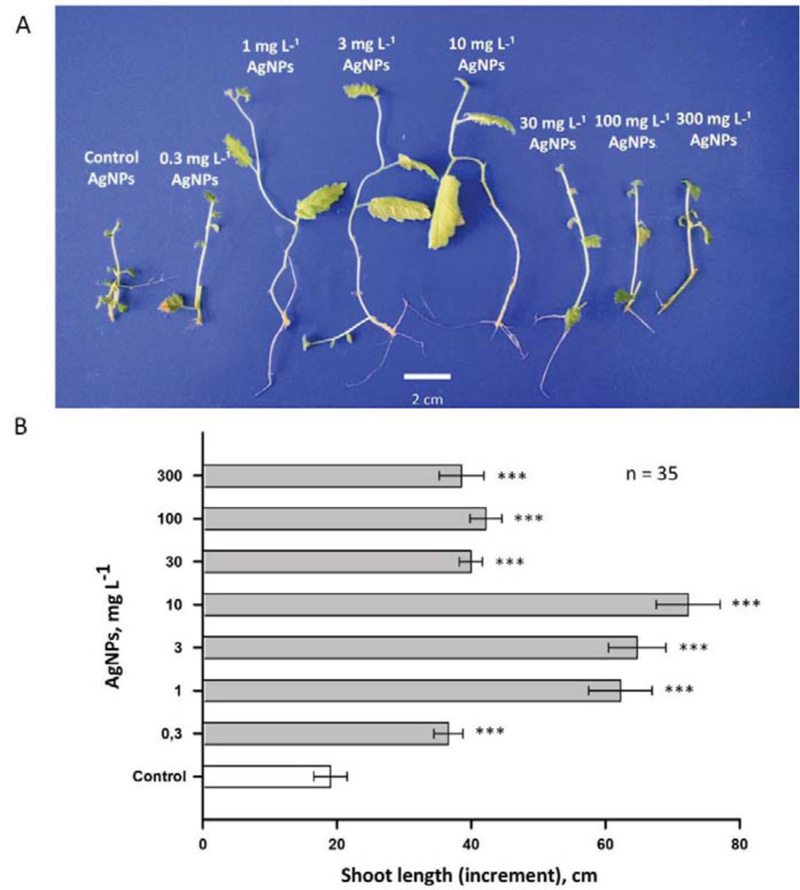
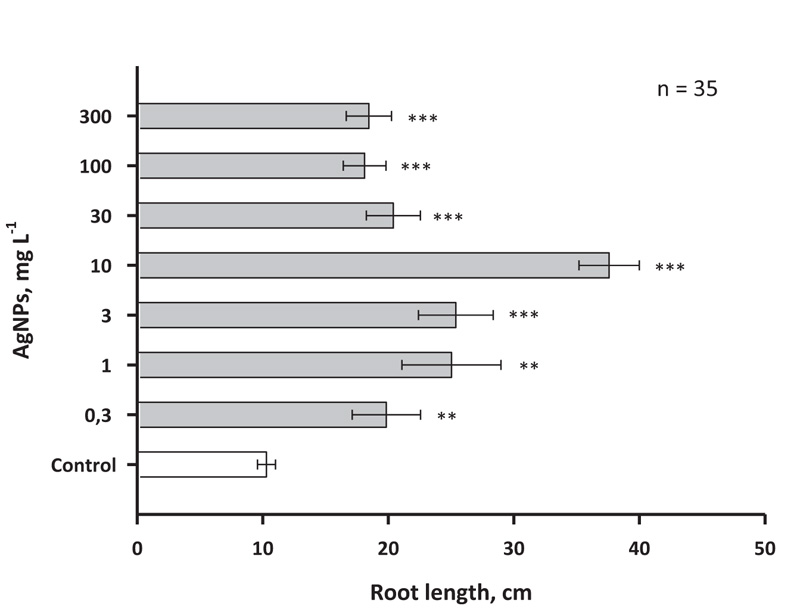
Effects of AgNPs were also measured in the roots of birch plants (Fig .6). In this case, the concentrations of AgNPs 1 and 3 mg L-1 had a smaller stimulating effect than was observed for the shoots. At the same time, 10 mg of L-1 AgNPs also, as in the case of shoots, induced a threefold increase in the growth rate (at least in the interval of 30 days).
As with the shoot growth effect, concentrations of AgNPs of 30, 100 and 300 mg L-1 caused only a small stimulating effect. It is noteworthy that relatively large concentrations of nanosilver, such as 100 and 300 mg L-1, did not cause serious inhibition of growth in birch, which may indicate a significant difference in the response to AgNPs in woody and herbaceous plants, since the latter earlier inhibition of growth processes under the action of nanosilver was previously reported a number of times [39].
3.2. Effect of Silver Nanoparticles Synthesized using Spruce Needle Extract on the Growth of Birch Plants under In vitro Conditions
Addition of nanoparticles synthesized using spruce needle extract to the nutrient medium did not result, at low concentrations of nanoparticles (0.3 and 1 mg L-1), in a significant change in the size of birch plants (Fig .7). At the same time, higher levels of silver nanoparticles (3-300 mg L-1) induced a statistically significant (p < 0.001) growth acceleration of 25-35%. Notably, the effect was similar for five highest nanoparticle concentrations tested. This may indicate a saturation of the effect during the transition from 1 to 3 mg of L-1, i.e. an extremely narrow range of active concentrations of nanoparticles.
Silver nanoparticles synthesized using the spruce needle extract had no effect or caused a slight (up to 20%) increase in the growth rate of birch roots when they were introduced into the medium at a concentration of 0.3-1 mg L-1 (Fig .8). At a transition from 3 to 30 mg L-1, there was a significant increase in the growth rate of birch roots. This effect was manifested in a twofold increase in the size of birch microgrowths on the 30th day of observations. Then, when switching to 100 mg L-1 (p < 0.001), the effect weakened, presumably due to an increase in the toxic effect of silver and reaching approximately 50-60% increase in the size of microgrowths compared to the control. Against the background of 300 mg L-1 AgNPs synthesized on needle extract, the growth rate was the same as in the case of the control group of plants.
4. DISCUSSION
To date, studies showing the effects of silver nanoparticles on various plants are based only on nanoparticles obtained using chemical and physical methods. Nothing is known about the influence of “green” silver nanoparticles on woody plant growth. “Green” nanosynthesis is a good alternative to chemical synthesis due to its greater environmental friendliness, cheapness and ease of production [40].
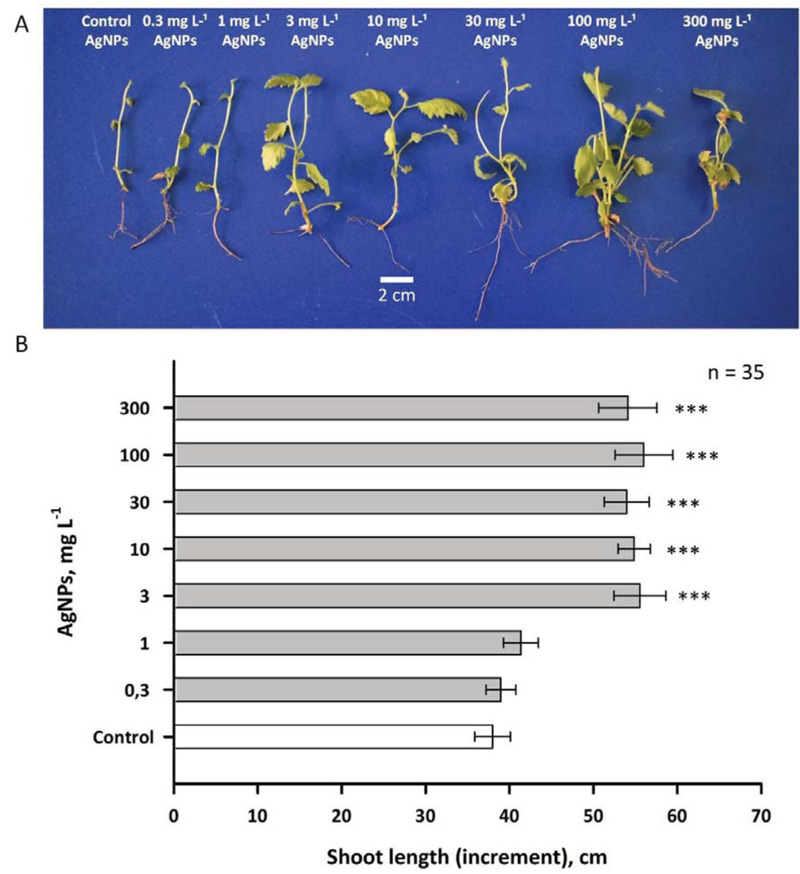
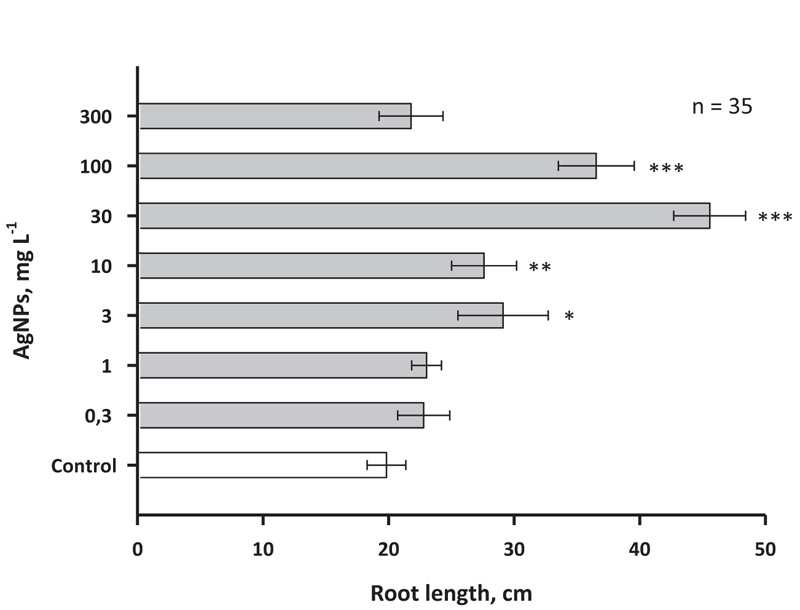
In the present work, it was shown that the addition of nanoparticles obtained by chemical synthesis methods based on PVP and ascorbate to the medium modified the growth and development of both shoots and roots of young birch trees, starting with very low concentrations (0.3-1 mg L-1). Similar results for AgNPs synthesized on the basis of PVP and ascorbate were obtained in experiments with microclones of stevia grown on a medium with the addition of different concentrations of AgNPs [34]. It is known that nanoparticles cause regulatory effects on plant cells because they pass through biological membranes and can also be transported via plasmodesmata (channels of approximately 40 nm in diameter) [34]. Low nanosilver concentrations (5-50 mg L-1) have also been shown to cause an increase in the length of vanilla and sugarcane microclones [40, 41]. It is worth noting that along with growth stimulation, nanosilver caused the formation of reactive oxygen species (ROS), which can have the growth-stimulating activity [42-46]. Alternatively, it was suggested that growth stimulation may also be due to silver nanoparticle-induced antioxidant response that has a positive effect on callus formation and accelerates growth under in vitro conditions [42]. An increase in ROS synthesis under the action of AgNPs against the background of increased growth rate was also noted in Oryza sativa culture; the effect was manifested both for shoots and roots of this species [43]. In an experiment conducted with Arabidopsis plants, it was shown that AgNPs close to spherical had higher growth-stimulating activity against shoots and roots than AgNPs of other forms [44]. At the same time, an effect on the level of anthocyanin accumulation and the total protein content was also noted. Among the possible mechanisms of stimulation of plant growth with nanosilver, it is important to note the possible blocking by silver ions of ethylene signalling, as has been shown for the roots of Crocus sativus [45].
There is evidence indicating the ability of nanoparticles to cause a moderate (non-lethal) increase in the level of reactive oxygen species in plant cells, which leads to the activation of a protective pathway to combat oxidative stress [34, 38]. Nanoparticles can initiate mechanical damage of membrane lipids and their peroxidation, destabilizing the membrane and promoting penetration of nanoparticles into the cell [34]. This can result in physiological and biochemical changes in the cellular environment, such as a decrease in the photosynthetic ability of the cell [34, 38]. Аmong the mechanisms of action of silver nanoparticles on plant cells, it is also important to note the ability to induce calcium-permeable pores, manifested in an increase in the calcium conductance of the plasma membrane and elevation of cytosolic free Ca2+ (observed in Arabidopsis thaliana root protoplasts) [38]. Cytosolic Ca2+ is a critical growth-promoting factor stimulating plant cell polar elongation [46]. At the same time, Ca2+ is a signalling agent and a stimulus for growth arrest with its too strong increase in the cytoplasm. This may explain the stimulating effect of silver nanoparticles in low and moderate concentrations and their inhibitory effect at high concentrations.
The literature describes only a few studies on the effects of AgNPs obtained by the method of “green” synthesis on the growth characteristics of plants [47]. For example, adding 25-100 mg L-1 of green-derived AgNPs to the medium has been shown to cause an increase in chlorophyll content [34, 45, 47]. To our knowledge, the effective use of spruce needle extract as a medium for the synthesis of AgNPs has been demonstrated here for the first time. Our findings point to the possibility of using AgNPs derived from spruce needle extract as a possible biotechnological technique to stimulate the growth of in vitro crops.
The spruce needles and bark have organic compounds that are responsible for the reduction and capping of Ag+ into AgNPs, thereby providing stability to the silver nanoparticles [26]. Flavonoids can chelate and actively reduce metal ions in nanoparticles, due to numerous hydroxyl and carbon groups [26]. Stimulation of growth processes of shoots and roots with nanosilver obtained using spruce needle extract occurred at higher levels of AgNPs than for nanoparticles obtained by the method based on PVP and ascorbate (Figs .2-5). This may be due to the presence of a large number of Ag-binding organic compounds in spruce needle extract. It can be assumed in this case a longer period of release of silver from metal-organic compounds.
Despite the important advantages in the use of nanosilver in plant biotechnology, there are serious risks that limit the use of this material. The high concentrations of silver nanoparticles can have a toxic effect on the root system of plants due to the release of a large number of free radicals [48]. There are data on the penetration of silver nanoparticles when spraying plants through the stomata (Lactuca sativa leaves), which can adversely affect photosynthetic processes [49, 50].
Soil microflora can be affected by silver nanosilver particles [51]. Silver nanoparticles have the property to accumulate in various parts of plants, especially inside vascular bundles and adjacent cells, which can undoubtedly affect the quality of edible plant biomass [34]. According to studies in recent years, the concentration in some soils and silty deposits of water bodies of water can reach 3 mg L-1 and it has a clear tendency to increase [52, 53].
There is a lot of evidence indicating a negative effect of silver nanoparticles in high concentrations (50-1000 mg L-1). In this case, there is a suppression of seed germination, root growth and shoots of Oryza sativa, Vigna radiata, Brassica campestris [54-58], a decrease in transpiration and biomass gain in Cucurbita pepo [59], cytotoxic and genotoxic effects on the roots of Allium cepa [60]. Several studies have documented a slight decrease in nanosilver toxicity when gel media are used (compared to water culture) [61-63]. In experiments with Polyboroides radiatus and Sorghum bicolor, approximately equal toxicity and bioavailability of silver nanoparticles have been shown both in gel environment and in soil [64]. Thus, the data available in the literature are qualitatively comparable to those obtained by us in the present study. Undoubtedly, silver nanoparticles need to be studied in more detail for environmental impact and biocompatibility, if they enter the environment on a large scale in vivo. The introduction of this material, even in a biocompatible form, should, if possible, be carried out in closed laboratory systems, and not in open fields, where nanosilver can accumulate and have a cumulative toxic effect on human health and biota.
CONCLUSION
The present study demonstrates the production of stable silver nanoparticles based on PVP and L-ascorbic acid, as well as an extract of the needles of Norway spruce. The resulting nanoparticles have a uniform shape and distribution. The presence of AgNPs in concentrations from 1 to 300 mg L-1 in the nutrient media used to grow the sterile microclones of Betula pendula has a stimulating effect on shoot growth and root system development.
LIST OF ABBREVIATIONS
| WPM | = Woody Plant Medium |
| NPs | = Nanoparticles |
| PVP | = Polyvinylpyrrolidone |
| PEG | = Polyethylene Glycol |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used in this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Data is available at https://drive.google.com/drive/folders /1P-aQr8cCRvhf4wzrvkZmIhSYJtb32-Mw?usp=sharing.
FUNDING
This work was supported by the State Research Programs of Belarus to V.D. (State Registration Grant Numbers: 20211705 and 20213577).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


