All published articles of this journal are available on ScienceDirect.
Rapid Accumulation of Cadmium and Antioxidative Response in Tobacco Leaves
Abstract
Background:
Cadmium (Cd) is one of the most dangerous environmental pollutants. Plant damage caused by oxidative stress during long-term Cd accumulation is well documented, while the primary response to Cd uptake is poorly understood.
Objective:
We assess the short-term injury and the primary reaction of the antioxidant system to the rapid accumulation of Cd in tobacco leaves.
Methods:
Leaf rosettes of the 5-week-old plants without roots were exposed to 100, 500 and 5000 μM Cd chloride for 2 and 12 hours. Cd accumulation, oxidation levels of proteins and lipids, content of reduced (Asc) and oxidized (DHA) ascorbate and activity of CAT, APX and POD were determined.
Results:
An accumulation of Cd in high concentration, but only a relatively small increase in the oxidation of proteins and lipids was found in the leaves after 2 hours of treatment. These effects were transient and disappeared after 12 hours. No visible damage to plants was observed. After 12 hours, the total ascorbate content (Asc + DHA) decreased by 18%, remained unchanged or increased by 85% after the application of 100, 500 and 5000 µM Cd chloride. The increase in the ascorbate pool, which should be considered as a component of the protective response, was caused by the accumulation of DHA. The activity of APX and POD remained unchanged, while that of CAT decreased, indicating that antioxidant enzymes activation is not involved in the primary response to Cd.
Conclusions:
The primary generation of ROS induced by Cd does not appear to be a deleterious manifestation of Cd toxicity, but rather a component of stress signaling that causes activation of the protective response. Uptake of Cd caused severe damage to the plant after long-term, but not after short-term treatment, suggesting that the damage is the result of secondary effects of Cd toxicity.
1. INTRODUCTION
Cadmium (Cd) belongs to the group of non-essential elements and is one of the most dangerous environmental pollutants, toxic to plants and animals [1-3]. Cd is highly available in the soil and its presence in ecosystems is increasing due to human activities such as metalworking, the use of some phosphate-based fertilizers, etc. Due to the high solubility in water, Cd salts are easily absorbed by the roots and transported via the xylem to the shoots [1, 4, 5]. Similar to other heavy metals, most of the Cd entering the plant accumulates in the roots, and only a small portion is translocated to the aerial part [6-16]. Thus, the root system represents a kind of barrier that protects photosynthetically active leaves from toxic Cd ions [5, 17].
In leaves, Cd is absorbed by mesophyll cells. At low concentrations (up to 90 nM) the transport of Cd across cell membranes requires energy, whereas at higher concentrations, it is a passive process. In the cells, Cd accumulates in the cytosol, where at low concentration, it forms complexes with thiol groups of reduced glutathione (GSH). However, at high concentrations, it is transported and sequestered in vacuoles, forming complexes with organic acids and phytochelatins (PC), i.e. ligands that bind heavy metals. PC are GSH oligomers found in monocots, dicots, gymnosperms and algae, the synthesis of which is drastically induced by the accumulation of heavy metal ions. Specific proteins such as metallothioneins are also involved in Cd sequestering. Nevertheless, Cd not only reaches the cytosol and vacuoles but also other compartments such as the cell nucleus, chloroplasts and mitochondria and damages these organelles [1, 3, 5, 18-24].
The accumulation of Cd in plants results in growth retardation, leaf chlorosis, induction of senescence and even death [1, 4-7, 14, 15, 25, 26]. The visible symptoms of Cd toxicity reflect numerous changes at the biochemical level, such as damage to chloroplasts ultrastructure and electron transport chain (ETC), lower expression/activity of Calvin cycle enzymes, and changes in carbohydrate metabolism [10, 15, 16, 27-29], inhibition of growth and development due to cytoskeleton disruption [30, 31], disturbance in water balance and uptake of nutrients [4, 32] as well as activation of sulphur assimilation and synthesis of amino acids cysteine, glycine and glutamate, which are required for GSH and PC synthesis [5, 15, 20, 23].
Most of the disorders observed in plants after Cd uptake are caused by oxidative stress as a result imbalance between the production of reactive oxygen species (ROS) and cellular antioxidant defense [5, 10, 12, 13, 15, 20]. ROS such as H2O2, singlet oxygen, superoxide and hydroxyl radicals are permanently produced in the cell as an unavoidable consequence of aerobic metabolism. However, an excessive generation of ROS leads to the damage of proteins, lipids, and DNA [33-36].
Plants possess several possible sources of ROS, such as ETC in chloroplasts and mitochondria, photorespiration in peroxisomes [37, 38], or enzymatic sources, including plasma membrane-located NAD(P)H oxidases [39] as well as cell wall-bound peroxidases/amineoxidases [40]. The generation of ROS can be activated by abiotic and biotic stress-causing oxidative damage [15, 33, 35, 36, 41].
ROS levels in the plant cell are under the strict control of a complex system of antioxidant protection, which includes both enzymes (superoxide dismutase – SOD; dehydroascorbate reductase – DHAR, catalase - CAT, ascorbate peroxidase – APX, glutathione reductase – GR, peroxidase – POD) and low molecular weight protective compounds (ascorbate, glutathione, polyphenols, tocopherols, etc.). Accordingly, the decrease in the activities of ROS-removing enzymes as well as the depletion of cellular antioxidant pools can contribute to increased ROS levels under stressful conditions [5, 20, 37, 38, 41-45].
In contrast to metals such as copper, iron or manganese, Cd is a non-transition metal, i.e., it cannot directly catalyze the formation of ROS in the cell. However, the oxidative stress caused by Cd uptake is well documented, although its mechanism is still not fully understood [5, 20, 36, 44]. It has been found that Cd ions interact with thiol groups of cysteine in protein molecules, especially in the active centers of enzymes, which leads to their inactivation [1, 2, 5, 20]. Especially, Cd disturbs electron transfer in photosystem II, which leads to enhanced formation of ROS in chloroplasts. Another source of ROS induced by Cd is the generation of H2O2 in mitochondria and by the plasma membrane-located NAD(P)H oxidases [20, 21, 46-48].
Heavy metal stress is chronic in nature. Accordingly, when studying the effects of heavy metals, most often, plant treatments over several days or even weeks are used [6, 8, 9, 11-13, 16, 20, 26]. This approach makes it possible to analyze the long-term effects of the gradual accumulation of metal ions in plant tissues. Alternatively, cell culture treatments are used to characterize the short-term primary response to heavy metal ions [20, 47, 49, 50], but in this case, the tissue-specific effects in planta remain unexplored. Accordingly, we used here an experimental design that provides a rapid uptake of Cd ions by leaves of tobacco (Nicotiana tabacum L.), a Cd-tolerant plant species supposed to be a promising crop for phytoremediation of soils polluted with heavy metals [11, 51]. The applied experimental design made it possible to assess the short-term cell damage and the response of the antioxidant system caused by the accumulation of these ions.
2. MATERIALS AND METHODS
2.1. Plant Material, Cultivation and Cadmium Treatment
Nicotiana tabacum (line W38) plants were grown for 5 weeks on the soil in a climatic chamber at 28°С in a light/dark cycle of 16/8 h, illumination of 35-40 µM / m2·s–1 and relative humidity of 60-70%. For stress treatment, plant rosettes were cut and placed on 0.5х MS [52] cultivation medium with the addition of CdCl2 at concentrations of 100, 500 and 5000 µM. Plants incubated on 0.5x MS without the addition of CdCl2 served as controls. Stress treatment was performed in the dark at room temperature for 2 and 12 hours. The leaves were then frozen in liquid nitrogen and used for experiments. For each sample, the leaves from 12 plants were pooled. All measurements were performed for five batches of plants in four replicates each.
2.2. Determination of Cadmium Content
The plant material was dried at 105°C for 24 hours until constant weight was achieved and ground to a fine powder before wet ashing in an HNO3-HClO4 (3:1) mixture. Cd content was determined by atomic absorption spectrophotometry (C-115M1, Russia).
2.3. Biochemical Measurements
The content of protein carbonylic groups (PCG) was determined according to the dinitrophenyl hydrazine methods [53, 54].
The lipid peroxidation was assayed by measuring thiobarbituric acid reactive substances (TBARS) as described by [55].
The reduced ascorbate (Asc) content was determined by measuring the decrease in A265 absorption upon the addition of 3 units of ascorbate oxidase from Cucurbita sp. (Sigma, St. Louis). Dehydroascorbate (DHA) content was determined indirectly by measuring the formation of Asc after nonenzymatic reduction by dithiothreitol, as described earlier [45, 56].
2.4. Enzymes Activity Measurement
To measure the activity of antioxidant enzymes, the plant material was homogenized in liquid nitrogen and the proteins were extracted using appropriate buffers [42, 57]. Extraction buffer containing 0.1 M tris-HCl (pH 6.8), 20% glycerol, 30 mM dithiothreitol and 0.1% insoluble polyvinylpolypyrrolidone was used to determine the activity of CAT. To assay the activity of APX and POD, extraction buffer containing 50 mM sodium phosphate (pH = 7.0), 0.25 mM EDTA, 10% glycerol, 2% polyvinylpyrrolidone-25, and 0.5 mM sodium ascorbate was used. The protein concentration in the samples was determined by the Bradford method [58].
CAT activity was evaluated as described previously [57, 59] by measuring the decrease in H2O2 content in the sample. To stop the reaction, a solution of ammonium molybdate was added to the reaction mixture. The concentration of the colored complex between H2O2 and ammonium molybdate was determined spectrophotometrically at 410 nm.
The total activity of APX and POD was measured according to the methods described previously using ascorbate and guaiacol as substrates [42, 57, 60].
3. RESULTS
3.1. Accumulation of Cd ions in Tobacco Leaves
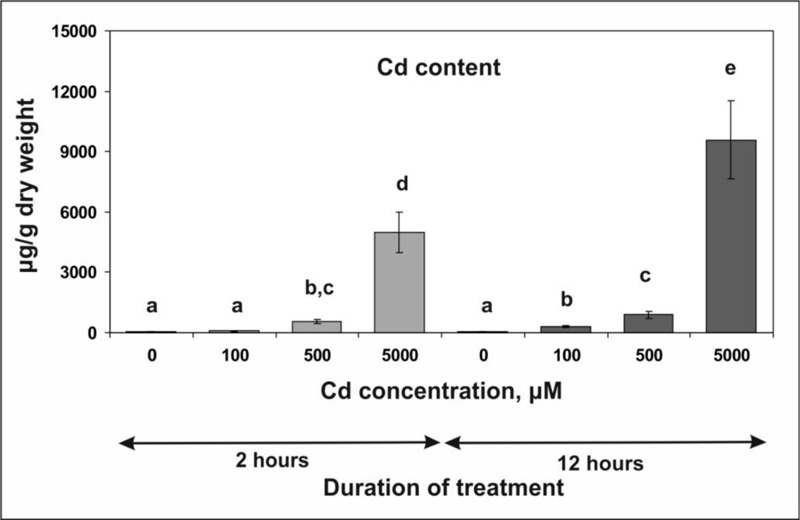
To elucidate the mechanisms of early plant cell response to Cd stress, an experimental design was developed that provides a rapid accumulation of Cd ions in tobacco leaves. Taking into account that the root system represents a barrier that prevents the rapid transport of heavy metal ions (including Cd) in the leaves in both monocots and dicots [6-9, 14, 61, 62], including tobacco [13], plant rosettes without roots were used in our experiments. Since tobacco is tolerant to Cd [13, 51], higher concentrations of Cd chloride, i.e., 100, 500 and 5000 µM, were used in our experiments.
The incubation of the rosettes on the solutions of CdCl 2 led to its rapid accumulation ( Fig. 1 ): 2 hours after the beginning of the treatment, the Cd concentration in the samples (calculated per fresh weight) reached about 40 to 50% of its concentration in the medium. Increasing the incubation time to 12 hours resulted in further accumulation of Cd.
3.2. Stress-related Metabolite Measurements
To assess the cell damage caused by Cd, two stress-related parameters, thiobarbituric acid reactive substances (TBARS) and protein carbonylic groups (PCG) levels, were measured, which reflect membrane lipid peroxidation and the oxidation of cellular proteins due to excessive production of ROS, respectively [54, 63].
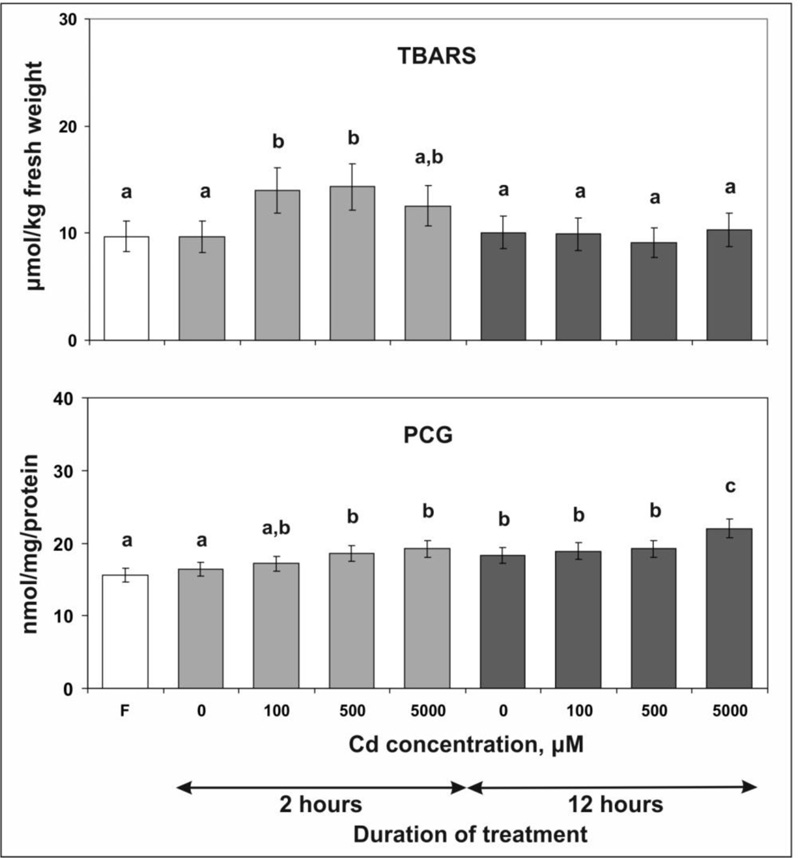
The data obtained show that incubation of tobacco leaves for 2 hours on MS medium with 100 or 500 µM Cd chloride increased the TBARS content by 44 and 47% (Fig. 2). However, no further accumulation of TBARS was observed when the Cd chloride concentration was increased to 5000 µM: in this case, the value was only 30% higher than in the control sample. Moreover, after prolonging the stress treatment to 12 hours, a decrease in the level of TBARS to the control value was found for all applied concentrations of Cd chloride, which indicates the activation of protective mechanisms.
It was also shown that 2 hours of stress led to a concentration-dependent accumulation of PCG in tobacco leaves: the most significant increases of 13 and 17% compared to the control were observed with the application of 500 and 5000 µM Cd chloride. After 12 hours of exposure, an increase in the PCG content of 21% was observed only after the application of the highest concentration of Cd chloride.
3.3. Asc/DHA Content
To evaluate the effect of Cd ions accumulation on the cellular redox status the contents of reduced and oxidized forms of ascorbate, Asc and DHA, were determined (Fig. 3). The data showed that the treatment of tobacco leaves with Cd chloride in concentrations of 100 and 500 µM for 2 hours did not cause changes in the content of Asc and DHA. However, the application of 5000 µM Cd chloride resulted in a decline of Asc content by 17%, whereas the content of DHA increased by 47%. After 12 hours of treatment with 500 and 5000 µM Cd chloride, more pronounced changes were observed: the Asc content decreased by 41 to 42%. At the same time, the DHA content increased by 61 and 232%, respectively.
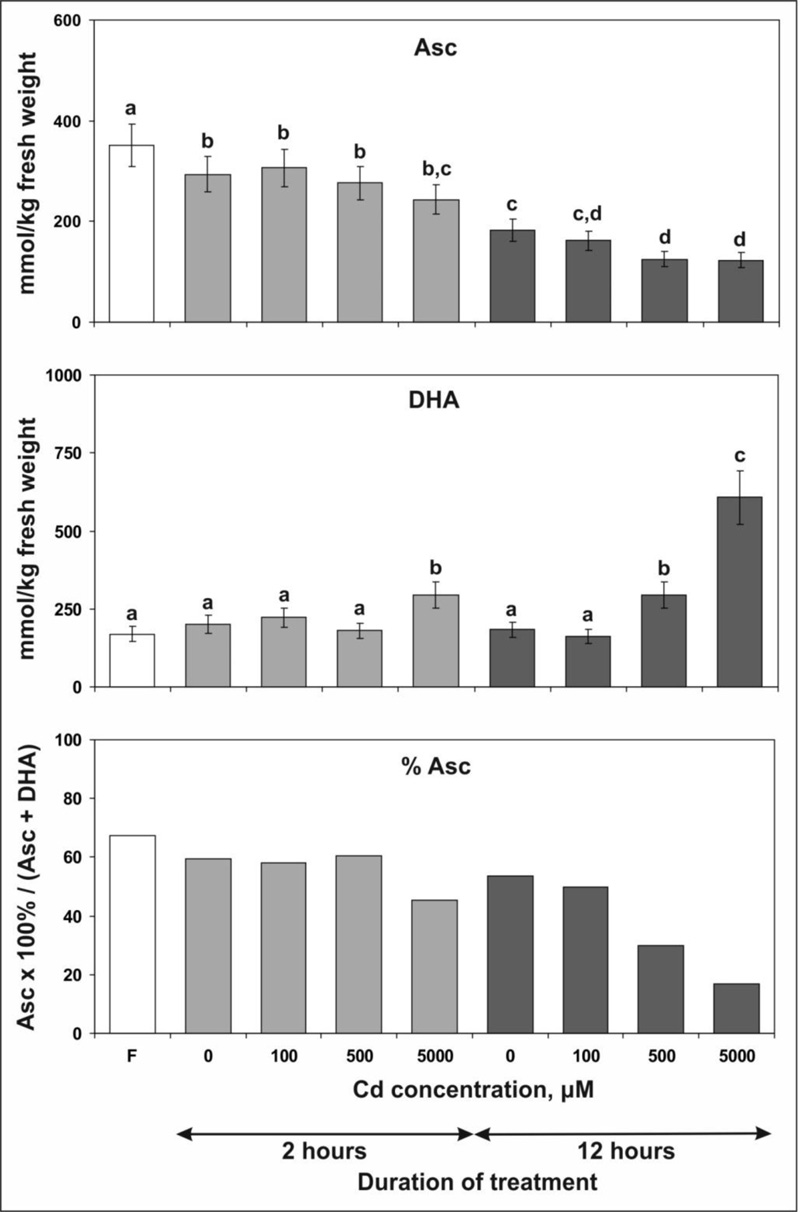
It should also be noted that incubation of plant rosettes for 2 and 12 hours on MS medium without Cd chloride (control samples) resulted in a decrease of Asc content by 16 and 40%, respectively, compared with intact plants. A plausible explanation for this effect is that the incubation of leaves on MS medium was performed in the dark, whereas before the experiment, the plants were exposed to light. The displacement of leaves in the dark has a negative effect on the synthesis of Asc in chloroplasts [64], which, in turn, should lead to the gradual depletion of the Asc pool.
3.4. The Activity of Enzymes Splitting H2O2
At the final stage of our experiments, we measured the activity of three enzymes, CAT, АРХ and POD, involved in the splitting of H2O2. It was shown that incubation of plant rosettes for 2 hours in a medium with Cd led to a concentration-dependent decline in catalase activity compared with the control (Fig. 4). The most significant decrease of 44 and 56% was found after the application of 500 and 5000 µM Cd chloride, respectively.
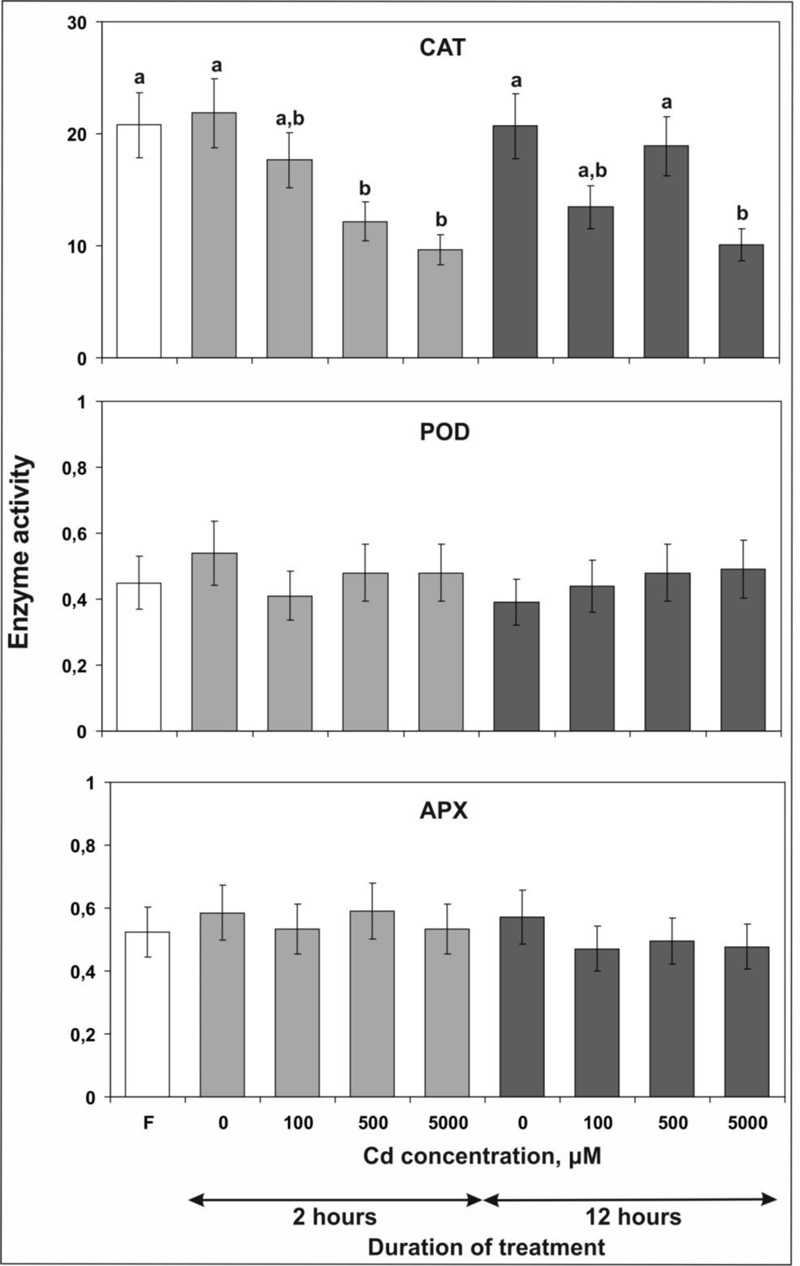
After 12 hours of stress treatment, a statistically significant decrease in CAT activity by 51% was observed only at the highest concentration tested. Remarkably, after using lower concentrations, CAT activity appeared almost the same (100 µM CdCl2) or even higher (500 µM CdCl2) than after 2 hours of treatment indicating activation of the protective reaction of the plant cell.
In contrast to CAT, no significant changes in the activity of APX and POD were detected in samples treated with Cd chloride (Fig. 4).
4. DISCUSSION
4.1. Accumulation of Cd and Plant Damage
To characterize the primary response of tobacco plants to the uptake of Cd, we examined the changes that occur in the leaves upon short-term exposure to high concentrations of Cd chloride. In order to achieve a rapid accumulation of Cd in the leaves, we subjected the plant rosettes with the pruned root system to 2 and 12 hours of treatment.
In contrast to our experiments, when studying the effect of heavy metal ions on plants, lower concentrations and longer exposure times are typically used, which leads to a gradual accumulation of the ions in plant tissues. For instance, potato (Solanum tuberosum) and plum (Prunus domestica) plants were cultivated in vitro on the MS medium supplemented with 21, 44, 130, 260 and 390 µM Cd nitrate. After one month, the Cd concentration in the leaves was approximately the same or 1.5 times higher than the concentration in the nutrient medium. The accumulation of Cd in roots and leaves and visible symptoms of plant damage correlated with the concentration of the Cd nitrate used for treatment. The greatest damage up to death was observed in the plants treated with 260 and 390 µM Cd nitrate, which accumulated in the leaves from 130 to 400 µg Cd / g DW [6].
In pea (Pisum sativum) plants, which were cultivated for several days on a liquid medium supplemented with 4 µM Cd nitrate, Cd content in leaves amounted to 20 µg / g DW and no visible effects were observed. However, treatment with 40 to 50 µM Cd nitrate or chloride resulted in a leaf concentration of Cd from 90 to 200 µg / g DW and a significantly reduced biomass accumulation, although no chlorosis was induced [8, 9]. The effect of higher Cd concentrations ranging between 75 and 150 µM was also studied, but plants where considerably damaged [9]. Tomato (Solanum lycopersicum) plants that were cultivated for 11 days on the liquid medium with 25 and 50 µM Cd chloride accumulated in leaves 25 and 49 µg Cd / g DW and showed clear symptoms of stress, e.g., loss of biomass and pigments [12].
In tobacco, which was grown on the nutrient medium supplemented with 100 µM Cd chloride for 11 days, the Cd concentration in the leaves was several times higher than in the medium, reaching 647 μg / g DW, but the accumulation of biomass was not significantly affected [13]. Cultivation of tobacco plants in the medium with 100, 300 and 500 μM Cd chloride for 5 days resulted in an accumulation of 200-900 μg Cd / g DW in leaves [11]. At 300 and 500 µM Cd chloride, visual symptoms of Cd toxicity were observed. Comparing these data shows that tobacco can tolerate Cd in higher concentrations than many other plants, i.e., it belongs to the species that can over-accumulate Cd [8].
In our experiments, a high Cd content in tobacco leaves (approx. 900 µg / g DW) was achieved through the treatment with 500 µM Cd chloride for 12 hours (Fig. 1). Thus, our experimental design really makes it possible to obtain high concentrations of Cd in tobacco leaves in a relatively short treatment time, which is useful for assessing the short-term effects of Cd toxicity. Furthermore, the exposition of leaf rosettes to 5000 μM Cd chloride enabled us to achieve extremely high Cd levels in plant tissues that, to our knowledge, have never been detected in nature or in experiments. Nevertheless, no visible damage to plants was observed.
Hence, the accumulation of high concentrations of Cd in the leaves caused serious damage to the plant after long-term treatment, but not after short-term treatment suggesting that the damage to plants is the result of secondary effects of Cd toxicity.
4.2. Production of ROS and Oxidative Damage
Cd is a non-transition metal and, therefore cannot directly catalyze the formation of ROS in Fenton or Haber-Weiss reactions [36]. However, the accumulation of Cd in the plant cell elevates ROS levels by diminishing the antioxidant defense and inhibiting the transfer of electrons in photosystem II [20, 44, 48], which leads to oxidative damage such as peroxidation of unsaturated lipids in cell membranes and oxidation of proteins [5, 10, 12, 20, 63, 65-67].
Previously, it was found that incubation of sunflower (Helianthus annuus) leaf segments in the water solution of 500 µM Cd chloride for 12 or 14 hours under continuous illumination resulted in a 50% [65] or even much high (5-fold) increase [68] in TBARS concentration. Such a big difference is due to the fact that in the second study, the TBARS content was determined not only in the leaves, but also in the incubation medium, considering the possible leakage of the metabolite in the medium due to the damage of the membranes [68].
Under long-term accumulation of Cd ions, an increase in TBARS content was observed in several plant species, e.g., in barley (Hordeum vulgare) and Arabidopsis thaliana [63, 69]. Cultivation of pea plants for 3 to 7 days in Hogland's solution with the addition of 40 μM Cd nitrate led to the accumulation of Cd in the leaves at a concentration of 25-89 μg / g DW and a moderate increase in the TBARS content by 14-18%, while the lower concentration of 4 μM did not cause a significant increase in TBARS. Cd accumulation in leaves was accompanied by a 1.2 to 1.3-fold increase in H2O2 content [8]. Also, a more than 2-fold increase in protein oxidation and activation of proteolytic degradation was found in pea leaves after 14 days of treatment with 50 µM Cd chloride [70].
A 2.5 to 3.5-fold increase in the TBARS content was found in leaves of tomato plants that were cultivated for 11 days on a liquid medium with 25 or 50 µM Cd chloride [12]. In tobacco leaves, similar effects, i.e., a 1.5 to 3.0 fold increase in TBARS, were found by application of 300 and 500 µM Cd chloride for 5 days, while 100 µM Cd had no significant effect. The H2O2 level was increased 3.6-fold in leaves of plants treated with 500 µM Cd chloride, but the effect was less pronounced at lower concentrations. No changes in the PCG content were found in these experiments [11]. Another study demonstrated a 1.2 to 1.4 fold increase in TBARS levels in old (but not in young) leaves of tobacco grown for 11 days in the presence of 25 or 50 µM Cd, followed by a slight decrease for 100 µM of Cd [13].
Taken together, the data reveal that changes in biochemical indicators, e.g., the H2O2 or TBARS content, correlate with the visible damage to plants (see above), suggesting that oxidative stress is an important component of Cd toxicity. The levels of Cd at which plants show symptoms of oxidative damage differ markedly between species, and tobacco is among those that can tolerate relatively higher levels of Cd in leaves.
The comparison of the effects of short- (our data) and long-term [11, 13] exposure of tobacco to Cd chloride shows that the manifestation of oxidative stress depends not only on the plant species and the tissue concentration of Cd, but also on the duration of the treatment. Especially, in our experiments, after 2 hours of treatment, the increase in TBARS levels was lower than after long-term exposure, although the Cd content in the leaves was higher. Also, after 2 hours, there was a Cd concentration-dependent increase in protein oxidation (Fig. 2), which was absent upon long-term treatment [11]. After 12 hours, both stress indicators returned to control levels (with the exception of increased oxidation of proteins after application of the highest concentration of Cd chloride), suggesting activation of protective mechanisms.
4.3. Changes in the Content of Low Molecular Weight Antioxidant
An increase in the concentration of ROS in the cell induces a protective response that includes changes in the concentration of low molecular weight antioxidants (Asc/DHA, GSH/GSSG, tocopherols, etc.), which are involved in maintaining cellular redox status and the activation of enzymes capable of neutralizing ROS [8, 10, 20, 42, 45, 71]. Also, a well-known protective mechanism against the toxic effects of heavy metals, especially of Cd, is the activation of phytochelatin synthase, a constitutively expressed enzyme responsible for the synthesis of PC from GSH. Activation of PC synthesis occurs within minutes after exposure to Cd and is independent of de novo protein synthesis [23, 20, 47, 72]. Respectively, Cd uptake can lead to depletion of the GSH pool, which is used as a substrate for PC synthesis, which in turn leads to a disturbance of the cellular redox balance [1, 8, 23, 28, 34, 36, 73].
In leaves of pea plants that had been exposed to Cd in hydroponic culture for 7 days, the GSH content demonstrated a concentration and time-dependent decrease, while the GSSG level showed no significant changes compared to the control [8]. Also, in tomatoes that were treated with 10, 25 and 50 µM Cd chloride for 7 days, the decrease in the GSH content and the increase in the GSSG content were observed, resulting in a significant decrease in the GSH / GSSG ratios of 12.02 (control) to 4.73 (50 µM Cd) [12].
The low molecular weight antioxidants were also mobilized as a result of tobacco plants' treatment with Cd for 5 days. The total ascorbate pool in the leaves was reduced by 19%, but increased by 48 and 90% after exposure to 100, 300 and 500 µM Cd chloride, respectively. In control plants and after application of 100 µM Cd, the percentage of Asc in the ascorbate pool (calculated as (Asc x 100%)/(Asc + DHA)) was 34% and increased after application of 300 and 500 µM Cd to 52 and 58%. The induction of non-protein thiol levels (including GSH, PC and other SH compounds) were significantly higher, increasing 8.5- and 15-fold when exposed to 300 and 500 µM Cd, respectively [11]. Taking into account that GSH serves as a substrate for DHA reduction to Asc by DHAR, the increase in Asc content can be explained by the activation of GSH synthesis.
In our study, we estimated the content of Asc and DHA and then calculated the total ascorbate content (Asc + DHA) in tobacco leaves after short-term exposure to Cd chloride (Fig. 3). After 2 hours of treatment, the total ascorbate content did not change, and the percentage of Asc in the total pool remained the same after the application of 100 and 500 µM Cd chloride, but 5000 µM Cd chloride caused a decrease in the relative level of Asc from 60 to 45%. Similar data were obtained for cell cultures of Arabidopsis thaliana, in which no change in Asc concentration was observed during the first 3 hours after the addition of Cd although a rapid H2O2 accumulation occurred. In contrast to Asc, glutathione levels completely diminished within 1 hour [47].
After 12 hours of treatment compared to the control, the total ascorbate content decreased by 18%, remained unchanged or increased by 85% after application of 100, 500 and 5000 µM Cd chloride, respectively (Fig. 3). The increase in the ascorbate pool should be considered as a part of the protective response, and it appears that the intensity of the response depends on the Cd concentration. In contrast to the long-term exposure to Cd [11], the increase in the ascorbate pool observed in our study was caused by the increase in the content of DHA. Accordingly, the percentage of Asc in the total ascorbate pool dropped below 17%. It can be assumed that this effect of Cd is caused by the depletion of the GSH pool, which is necessary for the recovery of Asc from DHA, since in the early stage of the Cd stress response GSH is intensively consumed for the synthesis of PC.
4.4. The Activity of Antioxidative Enzymes upon Cd Stress
The accumulation of Cd not only affects the concentration of low molecular weight antioxidants, but also alters the expression and activity of enzymes that are involved in maintaining redox homeostasis in the plant cell [5, 20].
A 12-hour incubation of sunflower leaf segments in 0.5 mM Cd chloride led to a decrease in APX, CAT, DHAR, GR and SOD activity by 18-30% [65]. In barley plants, stimulation of SOD, POD and CAT activities was reported after long-term exposure to 1 and 5 mM Cd [69]. APX, CAT, GR and SOD activities increased in pea leaves exposed to 4 and 40 µM Cd. With increasing treatment time from 1 to 7 days, the CAT activity gradually decreased, while the APX activity increased [8]. The treatment of tomato seedlings for 7 days with Cd chloride led to differential changes: the activities of APX, POD and SOD were decreased or not changed after the treatment with 1 µM Cd and increased after the treatment with 50 µM Cd. CAT activity was not altered [12].
The following changes in the activity of enzymes were found in the tobacco plants after treatment with 100, 300 and 500 µM Cd chloride for 5 days [11] or with 10, 25, 50 and 100 µM Cd chloride for 11 days [13]. The APX activity increased only after treatment with 500 µM Cd chloride for 5 days. The POD activity was enhanced, and a particularly strong increase (up to 11-fold) was shown after exposure to 500 µM Cd chloride. After 5 days of treatment with 300 µM Cd chloride, the total CAT activity decreased. The treatment with 500 µM Cd chloride led to some increase in CAT activity without reaching the level of the untreated plants. Conversely, no significant alterations in the CAT activity were found after 11 days of treatment. After 5 days, the SOD activity gradually grew with the increase in the Cd concentration. A similar effect was also observed in old (but not in young) leaves after 11 days of Cd treatment [11, 13].
Taking together, the data presented above show that the long-term exposure to Cd mainly leads to an increase in the activity of various antioxidant enzymes in the leaves of tobacco and other plants, especially when Cd salts are used in high concentrations (> 100 µM), which is accompanied by a significant accumulation of Cd in the tissues. In contrast, in our study, no changes in APX and POD activity were observed after short-term (2 and 12 hours) treatments despite the accumulation of high Cd concentrations in leaves. In addition, the CAT activity decreased (with the exception of reactivation of CAT by application of 500 µM Cd for 12 hours compared to 2 hours - see Results). Accordingly, it appears that activation of antioxidant enzymes is not a component of the primary protective response to Cd.
4.5. Source of ROS Production and Stress Signaling
In the plant cell, ROS are produced in several compartments, namely in chloroplasts, peroxisomes, mitochondria and the plasma membrane. Different types of stress activate the production of ROS, which not only damage the cell, but also represent signal molecules that trigger protective reactions [21, 34, 35, 38, 41]. Similar to other stressors, Cd increased intracellular levels of H2O2 and other ROS [1, 12, 21, 44, 46, 63].
In particular, Cd induces various changes in the structure and organization of the photosynthetic apparatus, including the obstruction of electron transfer in photosystem II, which leads to an overproduction of ROS in chloroplasts [20, 48].
Another source of H2O2 associated with photosynthesis is photorespiration in peroxisomes [5, 20, 74]. Peroxisomes can participate in the toxicity of Cd by overproducing H2O2, which could diffuse outside these organelles. In pea plants exposed to 50 µM Cd chloride for 28 days a 2-fold increase in the content of H2O2 in peroxisomes was demonstrated. The activity of glycolate oxidase, an enzyme, which generates H2O2 in the photorespiratory pathway, was significantly enhanced. However, the excessive generation of H2O2 was fully compensated by the induction of APX and GR [74].
Plasma membrane NAD(P)H oxidases are also involved in H2O2 generation upon short- and long-term Cd stress. A rapid (15 min) and concentration-dependent increase in their activity and transcription were found in Arabidopsis thaliana cell cultures exposed to Cd [47].
In tobacco cell culture treated with 3000 µM CdCl2 for 8 hours, three waves of oxidative stress were observed: (i) transient, NAD(P)H oxidase-dependent accumulation of H2O2; (ii) increased production of superoxide radicals in mitochondria; (iii) fatty acid hydroperoxide accumulation concomitant with the necrotic type of cell death [50]. In contrast, no serious damage was observed in our experiments after the application of 5000 µM Cd for 12 hours, demonstrating that the effects observed in cell suspension cultures can differ significantly from those in leaf tissues.
In leaves of pea plants that were cultivated with 50 µM Cd chloride for 14 days, there was a six-fold increase in the content of H2O2, which was mainly produced in the plasma membrane of transfer, mesophyll and epidermal cells and in the tonoplasts of bundle sheath cells. In mesophyll cells, a small accumulation of H2O2 in mitochondria and peroxisomes was also observed. Experiments with inhibitors suggested that the main source of H2O2 could be an NAD(P)H oxidase [46].
The generation of ROS in chloroplasts and peroxisomes is linked to photosynthesis, i.e., it occurs only under light. Accordingly, the oxidative stress observed in our experiments cannot be associated with the production of ROS in these cell compartments since we treated tobacco plants with Cd in the dark. Therefore, we believe that the source of H2O2 in our experiments is the activation of NAD(P)H oxidases in the plasma membrane.
CONCLUSION
Despite the accumulation of high Cd concentrations, only a relatively weak increase in the oxidation of proteins and lipids was found in tobacco leaves in our experiments after 2 hours of treatment. These effects were transient and completely disappeared after 12 hours. Accordingly, the primary generation of ROS induced by Cd does not appear to be a deleterious manifestation of Cd toxicity but rather a component of stress signaling that causes activation of the protective response. The severe oxidative stress occurs under chronic exposure to high Cd concentrations as a result of the light-dependent overproduction of ROS in chloroplasts and peroxisomes as well as the generation of H2O2 by the plasma membrane NAD(P)H oxidases in combination with the depletion of the antioxidative defense. The role of ROS-dependent signaling in Cd-stress requires further investigation.
LIST OF ABBREVIATIONS
| PCG | = Protein Carbonylic Groups |
| TBARS | = Thiobarbituric Acid Reactive Substances |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
The research was supported by the Ministry of Education and Science of Ukraine (grant Nos 0112U002334, 0122U001217).
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ACKNOWLEDGEMENTS
Declared none.


