RESEARCH ARTICLE
Antioxidant Protection System and Photosynthetic Pigment Composition in Secale cereale Subjected to Short-Term Temperature Stresses
Kateryna O. Romanenko1, Lidia M. Babenko1, *, Oleksandr E. Smirnov2, Iryna V. Kosakivska1
Article Information
Identifiers and Pagination:
Year: 2022Volume: 16
Issue: Suppl-1, M3
E-location ID: e187433152206273
Publisher ID: e187433152206273
DOI: 10.2174/18743315-v16-e2206273
Article History:
Received Date: 14/10/2021Revision Received Date: 2/2/2022
Acceptance Date: 16/3/2022
Electronic publication date: 22/09/2022
Collection year: 2022

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction:
Plants are often exposed to short-term temperature stresses (average 2-4 hours) or “temperature drops”. It is in the first hours of stress (alarm phase of response) that metabolic changes occur, which go on to contribute to the formation of the primary stress-induced response.
Materials and Methods:
Winter rye Secale cereale L. ‘Boguslavka’ plants were grown under controlled conditions in a vegetation chamber. Fourteen-day-old plants exposed to short-term heat (+40°C, 2 h) and cold (+4°C, 2 h) were studied. The dynamics and distribution of free amino acids (AAs), total phenols (TPHs), and flavonoids (TFs), as well as the content of photosynthetic pigments, were all determined according to the standard procedures.
Results:
Accumulation and distribution of secondary metabolites in the organs of winter rye plants depended on the type of temperature, stress, and the plant organ. In the first phase of alarm in cold-resistant winter rye ‘Boguslavka’ after cold stress increased the accumulation of free AAs (GABA, Pro, Asp, Cys, and Val) and decreased the content of flavonoids and chlorophyll. Both types of stress inhibited the accumulation of TPHs, and this effect was more pronounced under heat stress.
Conclusion:
Quantitative and qualitative changes in the accumulation and distribution of low molecular weight protectors indicated that AAs and TPHs are involved in response to both types of short-term temperature stresses in winter rye ‘Boguslavka’ plants. The ability to accumulate free amino acids (GABA, Pro, Asp, Cys, and Val) in the roots after cold stress is considered one of the prerequisites for cold resistance. The photosynthetic apparatus is susceptible to temperature stressors in the alarm phase of response, as indicated by the significantly decreased photosynthetic pigment.
1. INTRODUCTION
Extreme temperature is a widespread abiotic stressor that adversely affects metabolism and inhibits plant growth and productivity [1]. Plants critically depend on ambient temperature, and they survive solely by the cause of internal defense mechanisms [2]. To reduce the effects of temperature damage and improve adaptation to adverse temperature conditions, plants have developed certain physiological and molecular adaptations aimed at regulating metabolism [3]. Plant responses to low and high temperatures are often considered antagonistic. Heat stress causes cellular damage and includes hampering ion homeostasis, ROS production, and subsequent oxidative stress. Cold stress changes the physical state of the membrane bilayer [4]. However, a common response to heat and cold stress activates antioxidant defense systems, which include secondary metabolites: free amino acids (AAs) and total phenolic compounds (TPHs).
Accumulation of free AAs is a nonspecific reaction of plants to abiotic stresses. Free AAs play an important role in the formation of stress resistance as osmoregulators [5]. They are involved in pH regulation, ROS detoxification, synthesis of specific enzymes, and precursors of secondary metabolites and act as energy donors in the tricarboxylic acid cycle [6, 7]. Free AAs act as regulatory and signaling molecules [5] and serve as precursors in the synthesis of phytohormones and low molecular weight nitrogenous substances [6]. TPHs are synthesized mainly in the aboveground organs of plants: leaf and flower tissues, pollen, seeds, and bark. They play an important role in protection from pathogens and abiotic stresses [8]. The activity of a TPHs is determined by the structure of the molecule, specifically the number and position of hydroxyl groups in it. Phenolic compounds are actively involved in the regulation of plant cell growth and differentiation; they stabilize membranes, prevent lipid peroxidation, protein denaturation, and DNA damage, and provide protection against ROS [9]. Membrane stabilization is ensured by the ability of THPs, and especially flavonoids (TFs), to bind to individual integral membrane proteins and phospholipids [10]. The high antioxidant and radical-neutralizing potential of TFs is due to their specific chemical features [11].
The content of photosynthetic pigments (chlorophyll a and b, carotenoids) indicates changes in the photosynthetic apparatus under adverse environmental conditions [12]. The pigment complex actively responds to signals from the environment, and changes in the content and ratio of pigments serve as a marker that allows assessing the impact of a certain factor on the state of the plant. The amount of chlorophyll (a + b) is closely related to the productivity of photosynthesis and is useful as a marker of the effects of stress on plants [13]. The degree of formation and functioning of the photosynthetic apparatus under the action of unfavorable environmental factors characterizes the ratio between chlorophylls a and b. The change in the ratio of chlorophyll occurs mainly due to the lability of chlorophyll a [14]. The ratio of chlorophyll a/b is considered one of the traits of photosynthetic activity and under stress is used as a marker of tolerance [15]. The chlorophyll a + b/carotenoid ratio is another informative indicator of the intensity of damage to the photosynthetic apparatus. Lower ratios correspond to greater damage caused by aging or stressors [16]. Carotenoids are involved in the absorption of low-wavelength spectra of light and in the protection of chlorophyll molecules from oxidative stress [17]. Antioxidant effects of carotenoids are determined by their ability to neutralize the damage induced by the formation of triplet chlorophyll and singlet oxygen [18].
Rye (Secale cereale L.) is a diploid Triticeae species of the Poaceae family that belongs to important annual cereals and ranks first among grains in regions where wheat cultivation is difficult or impossible [19]. Among cereals, winter rye is resistant to adverse environmental factors, including drought and frost, and it also can be grown on poorly cultivated soils [20]. Winter rye is grown as a multi-purpose cereal crop for grain production and forage as a cover crop helping soil erosion, weed control, and building soil organic matter [21]. Rye is characterized by a high level of constitutive frost resistance. Single studies report an increase in antioxidant enzyme activities and proline content in rye seedlings under low temperature stress and freezing [22] as well as cold hardening [23]. The example of two winter rye varieties shows differences in amino acid levels during the cold adaptation and overwintering [24]. However, no studies on the physiological response of rye to high temperatures were found, which is apparently because rye is considered a frost-resistant crop.
Winter rye grows and develops to a fully tillered stage under low positive temperatures. The minimum temperatures for rye germination and growth to the start of tillering fluctuate between 1-5 °C and the temperature optimum for rye development is in the range of 15-22 °C [21]. An increase in temperature during the period of germination and the start of tillering is an extremely negative factor, which leads to wilting, rot, damage by pathogens, and yield loss [25].
Plant stress response is a dynamic process that can be divided into several phases. The first is the alarm phase, usually very short, lasting several hours, in which the stress signaling system and gene expression are activated. Then comes an acclimation phase measured by days, during which plants' protein and energy metabolism change. If the impact of extreme factors continues, plants either die or adapt to them, corresponding to the resistance phase. If the factors stop, the recovery phase begins [26]. Our study analyzes and compares specific dynamics and distribution of free AAs and phenolic compounds in shoots and roots and photosynthetic pigment composition in leaves of young rye plants ‘Boguslavka’ that have been subjected to short-term high and positive low temperature stresses (alarm phase of response). The study suggests that as a result of the activation of signaling systems and changes in gene expression in the alarm phase, a repartition of metabolites occurs under both cold and heat stress. The results of this study should contribute to a better understanding of rye's tolerance to temperature in a changing climate and provide background information for future cultivation management, especially in regions where the difference between day and night temperatures is very large.
2. MATERIALS AND METHODS
2.1. Plant Growth and Treatment
Plants of winter rye Secale cereale L. ‘Boguslavka’ were studied. The genotype is medium-ripe, winter- and cold-resistant. Seeds were obtained from the collection of the National Center for Plant Genetic Resources of Ukraine (Kharkiv). The seeds were sterilized in an 80% ethyl alcohol solution, washed with distilled water, and soaked for 3 hours. They were germinated in a thermostat in cuvettes on water-soaked filter paper at +24°C for 21 hours. Peeled grains were planted in 2-liter containers. Calcinated river sand was used as a substrate. Plants were grown under controlled conditions at a temperature of 20°/17°C (day/night), light intensity 190 μmol/m2•s, photoperiod 16/8 h (day/night), and relative humidity 65 ± 5%. The humidity of the substrate was maintained at 60% of the total moisture content. Watering was performed daily with Knop solution at a rate of 50 ml per vessel. To simulate stress, 14-day-old plants were exposed to a short-term (2 h) effect of temperatures of +40°C and +4°C at the specified mode of humidity and lighting. Control plants were cultivated without any treatment. Samples were taken after temperature stress.
2.2. Determination of Free Amino Acid Content
Amino acids were isolated from dried to absolute dry mass shoots and roots. One gram of plant material was incubated in a 3% sulfosalicylic acid solution and centrifuged at 4000 r/min for 30 min. Qualitative and quantitative content of amino acids was determined by ion-exchange liquid column chromatography on an automatic amino acid analyzer T 339 (Czech Republic). Registration of amino acids in the eluate was carried out by detection with ninhydrin [27].
2.3. Determination of Total Phenols and Flavonoids
Total phenol content was isolated from dried to absolute dry mass shoots and roots and determined using a Folin-Ciocalteu reagent [28], calculated according to the calibration curve of gallic acid and expressed as mg gallic acid equivalents (GAE) per gram of dry weight (mg GAE g-1 DW). Total flavonoid content was determined using a method based on the reaction of flavonoids with zirconyl (IV) nitrate hydrate [29]. Rutin was used to make a calibration curve and measurements were expressed as rutin equivalents (RE) per gram of dry weight (mg RE g-1 DW).
2.4. Determination of Photosynthetic Pigments’ Content
To ascertain the concentration of photosynthetic pigments in leaves in both control and temperature stressed rye plants, extraction was done in 80% acetone and determined by the Wellburn method [30].
2.5. Statistical Analysis
The experiments were performed in three biological replicates, each reproduced independently three times. The experimental data were graphed with Microsoft (Redmond, USA) Excel. Statistical analysis was performed with SPSS 16.0 statistical software (IBM SPSS Statistics, Chicago, USA). The effect of treatment on measurements was analyzed using one-way ANOVA, with P<0,05 used as the cutoff for significance. Figures and tables summarize measurements as mean ± standard error (±SE).
3. RESULTS
3.1. Amino Acid Composition Analysis
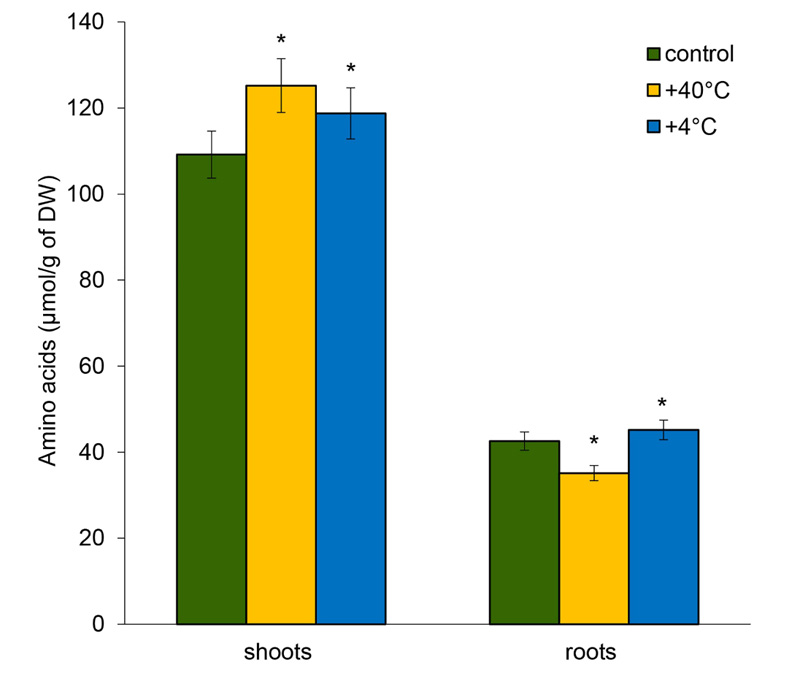
18 free AAs were identified in the shoots and roots of 14-day-old rye plants, including 17 main AAs and the nonprotein GABA that accumulates in plants in response to various environmental stresses. The pool of free AAs was higher in the shoots. After short-term hyper- and hypothermia, the pool of AAs in the shoots increased by 14.7% and 8.8%, respectively. In contrast, in the roots, the total content of free AAs decreased by 17.5% under hyperthermia and increased by 6% during hypothermia (Fig. 1).
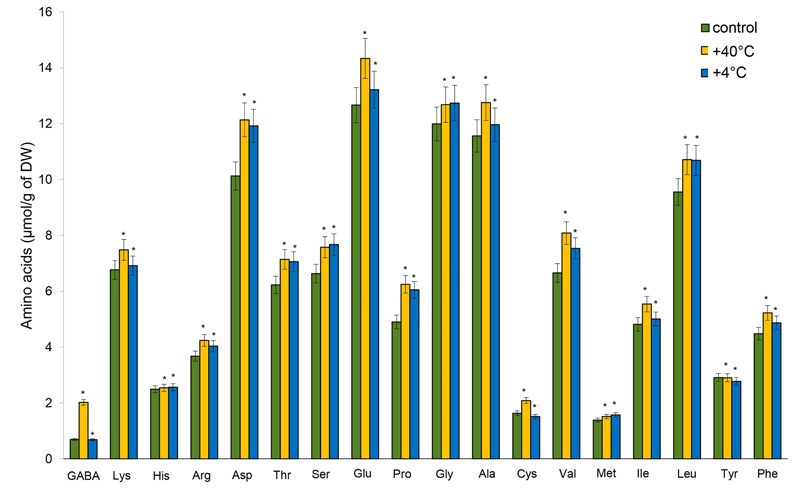
Glutamic (Glu) and aspartic (Asp) acids, glycine (Gly), alanine (Ala), and leucine (Leu) were the dominant AAs in the shoots and roots in all experiments. Changes in the accumulation of AAs were detected in the shoots after hyperthermia: a significant increase in the content of GABA (by 191%), Pro and cysteine (Cys) – by 27.5%, valine (Val) – (by 21%), and Asp – by 20%. The least pronounced changes were recorded for histidine (His) and methionine (Met), and the amount of tyrosine (Tyr) did not measurably change. The accumulation of several AAs increased following hypothermia: Pro – by 23.5%, Asp – by 17.7%, serine (Ser) – by 15.7%, and threonine (Thr) – by 13.3%. The least pronounced changes were determined for His, lysine (Lys) and Met, the content of GABA slightly differed from the control, and the content of Cys decreased (Fig. 2).
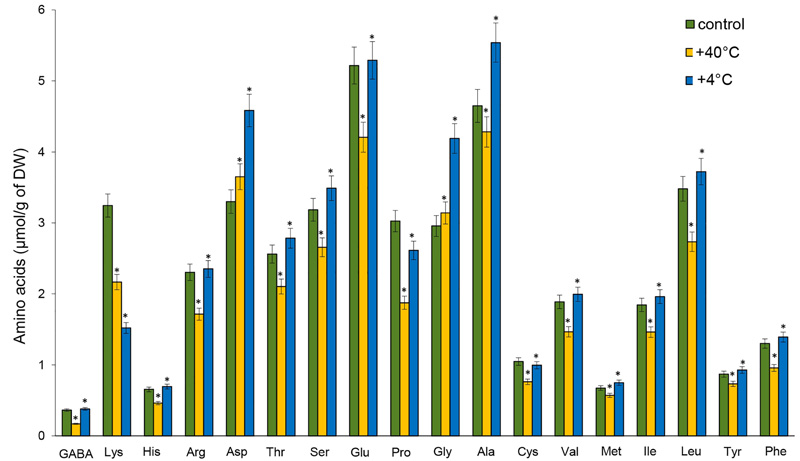
Lower quantities of free AAs were observed in the roots after heat stress, except for Asp and Gly. Among AAs, the levels that decreased the most were GABA (by 53%), Pro (by 38%), Lys (by 33%), and His (by 29.6%). The content of Gly (by 41.6%), Asp (by 40%), and Ala (by 19.2%) increased significantly under the influence of cold stress. On the other hand, the accumulation of Lys (by 53%) and Pro (by 13.6%) declined significantly. The content of GABA, His, arginine (Arg), Met, Cys, Val, and phenylalanine (Phe) did not significantly differ from the control (Fig. 3).
In summary, free AAs of winter rye actively responded to short-term temperature stresses. The nature of the changes depended on the type of stress and the plant organ. After heat stress, the accumulation of free AAs increased in the shoots of rye and decreased in the roots, while cold stress induced an increase in the pool of free AAs both in the roots and in the shoots. GABA, Pro, Asp, Cys and Val responded most dramatically to the change in temperature.
3.2. Changes in the Total Phenolic Compounds Content
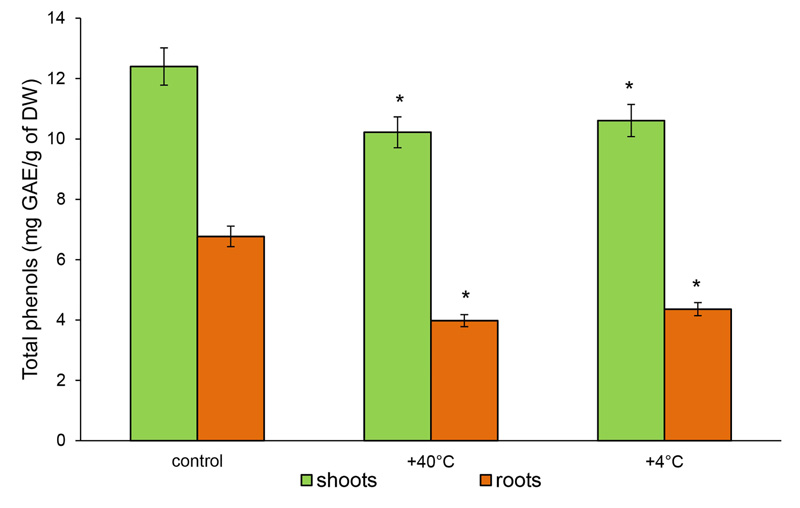
The content of TPHs and TFs in the shoots of control 14-day-old rye plants at 12.4 mg GAE/g DW and 3.62 mg RE/g DW was measured, respectively. In the roots, 6.77 mg GAE/g DW and 1.32 mg RE/g DW were measured, respectively. After hot stress, the content of TPHs decreased in the shoots by 18% (to 10.22 mg GAE/g DW) and in the roots − by 41% (3.98 mg GAE/g DW). The response to cold stress was less pronounced. The level of TPHs in the shoots decreased by 14.5% and in the roots by 35.6% (10.61 and 4.36 GAE/g DW, respectively) (Fig. 4).
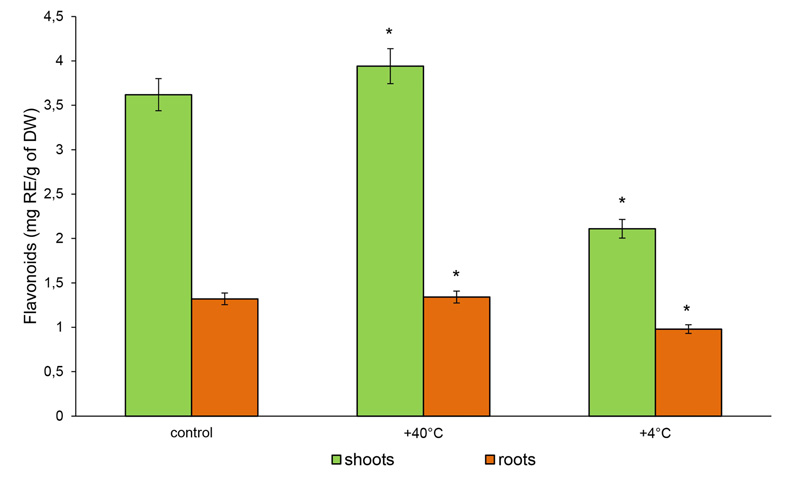
The content of TFs in the shoots of 14-day-old rye plants increased by 9% (3.64 mg RE/g DW), while in the roots, it was not significantly different from the control after hot stress (1.34 mg RE/g DW). Under cold stress, the content of TFs in the shoots was reduced by 41.7% (2.11 mg RE/g DW), while in the roots, it remained similar to the control (0.98 mg RE/g DW) (Fig. 5).
Therefore, phenolic compounds as well as free AAs actively responded to temperature stresses. The magnitude and direction of the change depended on the type of stress and the plant organ. Both stresses inhibited the accumulation of TPHs in the organs of 14-day-old rye plants. On the other hand, the accumulation of TFs in the shoots under heat stress increased, and cold stress decreased the content of TFs in both rye roots and shoots.
3.3. Photosynthetic Pigment Composition
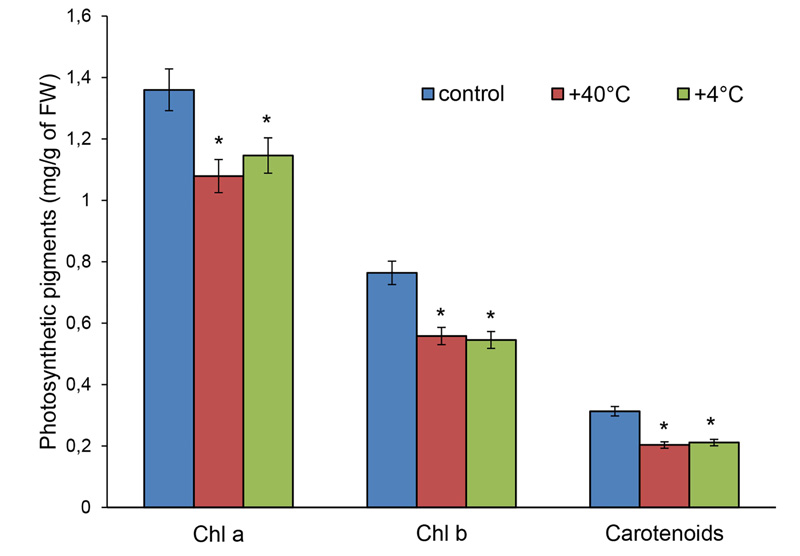
The content of photosynthetic pigments in the leaves of 14-day-old S. cereale plants of ‘Boguslavka’ variety decreased under the action of short-term high and low positive temperatures: chlorophyll a − by 21% and 25%, chlorophyll b − by 27% and 31%, total carotenoids − by 35% and 33%, respectively (Fig. 6).
The amount of chlorophyll a+b under the influence of both stresses decreased 1.3 fold, concordant with a decline in photochemical activity. At the same time, the a/b ratio increased due to a reduction in the chlorophyll b content. Under heat stress, the ratio of the amount of chlorophyll a+b to carotenoids increased by 0.7%, which suggests minimal damage to the photosynthetic apparatus (PhA). Under the action of low positive temperature, the ratio (a+b)/carotenoids decreased by 7%, which indirectly points to damage to the PhA (Table 1).
| Sample | Chl (a+b), mg/g FW | Chl а/b | (a+b)/carotenoids |
|---|---|---|---|
| Control | 2,124±0,07 | 1,78 | 3,8 |
| +40°С | 1,637±0,04* | 1,93 | 3,83 |
| +4°С | 1,571±0,03* | 1,99 | 3,53 |
Thus, the pigment complex of 14-day-old rye plants was quite sensitive to temperature stresses. Cold stress induced a more pronounced decrease in chlorophyll content, and a decrease in the ratio (a + b)/carotenoids indirectly indicated damage in PhA after hypothermia.
4. DISCUSSION
Our study measured nonspecific and specific changes in the nature of the accumulation of AAs in the organs of 14-day-old winter rye plants after temperature stresses (alarm phase of response). Short-term heat stress induced a rise in the pool of AAs in the shoots and a decrease in the roots. On the other hand, after cold stress, the AAs pool increased significantly in the shoots and slightly – in the roots. The changes recorded in the content of AAs after exposure to high temperatures were more pronounced. At the level of individual AAs, GABA, Pro, Cys, Val, and Asp dominated in the shoots of 14-day-old winter rye plants after high temperature stress, while after positive low temperature, Pro, Asp, Ser, and Thr prevailed. In the roots, the content of these AAs decreased significantly compared with the control under both temperature stresses, except for Asp, Ser, Gly and Ala.
Free AAs perform osmoprotective and regulatory functions under stress conditions [31]. Pro and GABA are key osmolites that form the plant responses to abiotic and biotic stresses [32, 33]. An accumulation of Pro in the shoots of 14-day-old winter rye plants after heat and cold stresses were established, and a significant decrease in its content in the roots, especially after hyperthermia. Aghaee et al. [34] showed that low temperature stress induced the accumulation of Pro in the cold-resistant genotype of Oryza sativa. Significant accumulation of Pro and an increase in antioxidant activity were reported in wild Sorghum bicolor genotypes under heat stress [35]. It was previously shown that after cold stress, the pool of free AAs and the content of Pro in shoots and roots in 14-day-old plants of frost-resistance winter wheat ‘Podolyanka’ increased [36]. It was identified that the level of GABA almost doubled in the shoots of 14-day-old rye plants after heat stress, while it did not change after cold stress. There is a relationship between the perception of the stress signal, accumulation of GABA, and physiological responses of plants. GABA is considered involved in signal transduction as a signaling molecule or as a trigger for ROS generation, activation of antioxidant enzymes, interaction with plant hormones, and protection against biotic stressors [37]. An increase in intracellular GABA content induces the expression of signaling and metabolic genes involved in the regulation of growth and stress resistance. Such changes in genome activity positively affect the quality and quantity of the crop [38].
The content of Asp and Gly increased together with GABA in the roots of 14-day-old rye plants under temperature stress. On the contrary, the level of Ser and Ala became elevated under the cold stress, while after heat stress − it decreased. Asp is a precursor in the biosynthesis of asparagine, Thr, Lys, Met, isoleucine (Ill) in higher plants, and such metabolic transformations are activated by abiotic stress [39]. Gly and Ser are two interconverting amino acids that accumulate during photorespiration, which are the precursors for chlorophyll, glutathione, ethanolamine, tryptophan, phosphatidylcholine, and related phospholipids [40]. The synthesis of these AAs in non-photosynthetic cells is considered activated under stress factors [41, 42]. Ala is an activator of the aspartate-derived amino acid pathway in the plant and takes part in carbon and nitrogen metabolisms of the cell [43]. Alanine forms in large quantities under hypoxia in the roots of Arabidopsis thaliana, but after returning the plant to normal conditions, its concentration decreases [44].
The accumulation of AAs under temperature stresses has been reported in a number of studies. For example, an increase in the levels of GABA, Gly, Val, and Ala in seedlings of Arabidopsis was found after the heat and cold stresses [45]. The content of Asp, glutamine (Gln), Val, Arg, Pro, and Gly increased in maturing grains of barley (Hordeum vulgare) after three-hour temperature stress. According to the authors, the elevation in AAs levels was associated with the expression of genes involved in the metabolism of proteins damaged by high temperatures [46].
The results of our study and literature show that the growth of the pool of free AAs and of the content of individual AAS, including Pro, GABA, Asp, and Glu, is a nonspecific response to temperature stresses. Simultaneously, certain specific features depend on the species, tolerance and phase of plant development, plant organs, and the intensity and duration of the stressor.
Phenols and flavonoids, together with free AAs, participate in the protection of plants from adverse impacts [8, 47]. Activity of phenylalanine ammonia lyase and other enzymes involved in the biosynthesis of phenolic compounds has been reported to increase in response to high [48] and low temperatures [49]. It was detected that the content of TPHs decreased in the shoots and roots of 14-day-old rye plants after heat stress, whereas the amount of TFs did not change appreciably. The TPHs level was higher after cold stress as compared with heat stress, while the concentration of TFs in the shoots declined significantly, and in the roots, it remained at the control level. Information on the effect of temperature on the content of TPHs in different plant species is contradictory. For example, an accumulation of TPHs was established after heat stress (+ 35 °C) in plants of Lycopersicon esculentum, for which the optimal temperature is in the range of + 22-26 °C. Similar results were obtained following cold stress (+ 15 °C) in plants of Citrullus lanatus with an optimal growth temperature of + 33-35 °C [50]. It was reported that the leaves of a cold-sensitive grape variety (Vitis vinifera L.) were characterized by a lower content of TPHs than the leaves of a resistant variety [9]. A decrease in the content of TPHs in the leaf tissues of the grape after cold stress (+7 °C and + 10 °C) and its gradual increase during recovery (+18 °C and + 25 °C) was detected [51]. At the same time, enhanced synthesis of TPHs in soybean seedlings was shown under cold stress (+1o and + 10 °C) [52]. However, following recovery (+ 24 °C), [53]. The content of TPHs diminished in the roots of Pisum sativum L. under short-term temperature + 8 °C, but it gradually rose following prolonged exposure to cold [54]. During adaptation to low temperature of the two Triticum aestivum L. varieties, which differ in the levels of frost resistance, a significant increase in the content of TFs, especially in the cold-resistant variety, was noted [55]. Simultaneously, a decrease in the content of TFs was detected at low temperatures in plants of spring and winter wheat [56] and Solanum tuberosum L. [57]. During the hardening of Secale cereale L. seedlings, a gradual increase in the total amount of TPHs was recorded [58]. Our previous studies revealed a slight increase in the content of TPHs and TFs after short-term heat stress (+ 40 °C, 2 h) and no significant changes after short-term cold stress (+ 4 °C, 2 h) in 14-day-old Triticum spelta plants [59].
The absolute content of photosynthetic pigments and their ratio in plants can vary widely depending on environmental conditions, particularly concerning the intensity and spectral composition of light, temperature, nutrition, etc [14]. Photosynthetic pigments are among the first targets of stressors, hence the absolute content of pigments and their ratio after stressor impact is a specific trait of heat tolerance and could further serve as markers during the screening and the creation of tolerant varieties [60]. The content of photosynthetic pigments and the ratio of chlorophyll a/b decreased in wheat flag leaves in the grain filling phase under high temperature. More pronounced changes occurred in the sensitive genotype, which was interpreted as evidence for slower degradation of pigments and better capture of solar energy by the heat-tolerant genotype [61]. In earlier investigations, the effects of short-term temperature stressors on the accumulation of photosynthetic pigments in the leaves of two kinds of 14-day-old winter wheat and wheat spelt were analysed. Heat stress decreased the amount of photosynthetic pigments and increased the ratio of chlorophyll a/b in the Triticum spelta 'Frankenkorn' variety [59] and wheat 'Volodarka' variety [12] studied plants, both of which are known for their cold and frost resistance. This indicates that the pigment complex in these genotypes is sensitive to high temperatures. Simultaneously, an increase in the content of pigments and a decrease in the ratio between chlorophylls a and b were shown in plants of heat-tolerant wheat 'Yatran 60' variety [62]. In terms of total chlorophyll content, the studied rye plants significantly exceeded the previously analyzed winter wheat and spelt plants. Changes in the content and ratio of photosynthetic pigments recorded in our study indicate that in the early stages of the vegetation, the photosynthetic apparatus of winter rye plants of the ‘Boguslavka’ variety responded more actively to low temperatures.
CONCLUSION
It was shown that under simulated short-term temperature stresses (alarm phase of response) at the initial stages of the vegetation, the nature of accumulation and distribution of secondary metabolites in the organs of rye plants is changed, indicative of a primary response to stress. Following heat stress, free AAs accumulated in the shoots. Following cold stress-free AAs pools increase both in the roots and in the shoots. GABA, Pro, Asp, Cys, and Val were dominant following temperature changes. Both temperature stresses inhibited the accumulation of TPHs, while the accumulation of TFs in the shoots under heat stress rose and fell under cold stress, both in the roots and shoots of plants. Quantitative and qualitative changes in the accumulation and distribution of low molecular weight protectors AAs and TPHs in rye plants provide evidence of the involvement of these compounds in the formation of the response to temperature changes. The decrease in the content of photosynthetic pigments indicates the sensitivity of the photosynthetic apparatus of rye plants to temperature regime changes. The results obtained in this study should contribute to a better understanding of the resistance of winter rye plants of the ‘Boguslavka’ variety to short-term temperature changes.
LIST OF ABBREVIATIONS
| Ala | = Alanine |
| AAs | = Amino acids |
| Arg | = Arginine |
| Asp | = Aspartic acid |
| Cys | = Cysteine |
| GABA | = γ-Aminobutyric acid |
| Gln | = Glutamine |
| Glu | = Glutamic acid |
| Gly | = Glycine |
| His | = Histidine |
| Ill | = Isoleucine |
| Leu | = Leucine |
| Lys | = Lysine |
| Met | = Methionine |
| Phe | = Phenylalanine |
| Pro | = Proline |
| Ser | = Serine |
| Thr | = Threonine |
| TPHs | = Total Phenols |
| TFs | = Total Flavonoids |
| Tyr | = Tyrosine |
| Val | = Valine |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No humans or animals were used in the study.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of this study are available within the article.
FUNDING
The study is based on the results of the project Nº ІІІ-82-17.463 "Plant hormonal regulation of the growth and development of cereal plants under the influence of negative climatic factors" (2019-2023), funded by the National Academy of Sciences of Ukraine.
ACKNOWLEDGEMENTS
We thank the Director of the Institute of Plant Physiology and Genetics of the National Academy of Sciences of Ukraine, Prof. DSc. Academician V.V. Morhun for his support, advice, and сritical reading of the manuscript. The authors are grateful to the National Center for Plant Genetic Resources of Ukraine (Kharkov) for providing winter rye seeds for this study.