All published articles of this journal are available on ScienceDirect.
Dosed Exposure to Low Temperature as a Breeding Background in The Selection of Gene Pool Breeds of Chickens for Viral Vaccines Production
Abstract
Background:
One of the ways to utilize the potential of local breeds is to use them as producers of raw materials for the bio-industry (production of vaccines and diagnostics for animals and humans).
Methods:
Breeding of Russian White (PRWC) laying hens in 5 generations was carried out with the aim of increasing the output of allantois-amniotic fluid of embryos with a selective background (cooling of hatching eggs during the sensitive period of embryogenesis). In F5, the thermoregulation capabilities of 7-day-old PRWC chickens were studied in comparison with Amrox chickens under the influence of a daily stress factor (cooling at +10oC for 30 minutes) and raising at +22oC. The level of allantois-amniotic fluid output and biological activity of the Newcastle disease virus in F5 PRWC embryos in comparison with F0 and commercial line embryos was evaluated.
Results:
Day-old PRWC chicks responded to exposure to low sublethal temperature with muscle shivering, but, unlike Amrox chickens, they were active and retained the reflex of searching for food. PRWC chickens raised at low temperatures up to 7 days of age maintained the same growth rate as chickens raised at a common regime but used less residual yolk, which practically did not decrease the body temperature, and change the level of thyrotrophic hormone in response to the dosed exposure at low temperatures at 7 days of age. The content of doses of the Newcastle disease virus (EID50) in the extraembryonic fluid of F5-embryos of PRWC was 32.3 times higher than that of commercial line ones.
Conclusion:
PRWC chicks in the early neonatal period are more reactive at low temperature and have better thermoregulation mechanisms. PRWC embryos can be recommended for use in the production of various viral vaccines as well as diagnosticums.
1. INTRODUCTION
The disappearance or reduction of the number of local breeds leads to a narrowing of genetic variability and a decrease in the genetic diversity of poultry due to the loss of specific genes and their combinations [1]. The genes responsible for, among other things, resistance to various diseases, high viability of poultry and resistance to extreme climatic conditions in which local breeds are selected, are going towards extinction. A prerequisite for reliable conservation of gene pool breeds of poultry is the economically justified application of their distinctive valuable traits. One of the ways to utilize the potential of local breeds is using them as producers of raw materials for the bio-industry (production of vaccines and diagnosticums for animals and humans).
The best raw materials for the production of vaccines are known to be SPF eggs (free of specific pathogens), but their use is associated with a number of constraints; the main one is their high cost. They are produced in strict isolation and do not contain antigens and antibodies against 18 agents, a list of which is provided in the European Pharmacopoeia [2]. A special feature of SPF eggs is the complete absence of poultry vaccination. The largest producer of SPF eggs in the world is the breeding and genetic company “Valo Biomedia” (https://www.valobiomedia.com/).
Very high requirements for management and reproduction of such populations lead to the fact that existing flocks have to be reproduced using a limited number of related individuals, which can lead to inbreeding depression and, as a result, to the decrease of egg productivity and reproduction rates, including egg fertilization. It should also be noted that for the production of a number of vaccines, “clean” eggs (obtained with a “sparing” vaccination scheme) are successfully used. Chickens of gene-pooled breeds can be used for getting such “clean” eggs [3].
The use of gene pool breeds as a model population for the development of methods for creating specialized flocks or as producers of raw biomaterials has great prospects, since the epidemic and epizootic situations in the world are becoming more complex every year, and, as a result, the need for vaccines is increasing. It should be noted that, despite the widespread use of the culture method, the use of developing chicken embryos is indispensable for the production of a number of vaccines [2]. Therefore, the development of methods for producing specialized poultry populations for use in the bio-industry and the production of “clean” eggs with an increased yield of allantois-amniotic fluid is an extremely urgent task.
Selection work with the population of Russian White hens (PRWC) from the “Genetic collection of rare and endangered breeds of chickens” of RRIFAGB is a successful example of this approach. This population was created in RRIFAGB by A. N. Sokolova [4, 5] in the second half of the XX century by selecting resistance at low temperature in the first days of life (15-22оC in the first 5 days with a gradual decrease to 14-11оC by 21-30-day age) with hens kept in winter at a temperature below 0oC, but while maintaining laying performance at a high level. As a result of such selection in conditions of critical low temperatures, genotypes appeared that differed not only in terms of thermal resistance of neonatal chicks, but also in increased resistance to a number of neoplastic diseases, such as Marek's disease, leukemia, and carcinomas [4-6].
Modern studies on this population have shown that PRWC embryos also have differences in embryonic development and have the highest yield of allantois-amniotic fluid (raw material for the production of viral embryonic vaccines for animals and humans) and the titer of the vaccine virus in it compared to the embryos of chickens of other gene-pool breeds and commercial egg lines [7].
The research objective was to evaluate the effectiveness of using the dosed impact of low temperature, with the PRWC chickens as example, as a breeding background for creating a specialized population of chickens of gene-pool breeds, producers of raw materials for viral vaccines production.
2. MATERIALS AND METHODS
The research was conducted on chickens namely, (Gallus gallus domesticus) PRWC and Amrox (control) bred in the “Genetic collection of rare and endangered breeds of chickens RRIFAGB” (Collection). Chickens were kept in individual cages, with individual control of egg performance. Artificial insemination was used for new generations of chicks. Hens were kept at feeding systems usual for this farm. The main criterion for selection in the breeding work with PRWC for 5 generations was the average yield of extraembryonic liquid from 3 – 5 pieces 12.5–day-old embryos from each hen with a selective background: cooling of eggs at the 5.5th day of incubation to 20oC for 6 hours. For reproduction of the next generation, only hens with egg production and egg weight not lower than the average for the flock, whose embryos gave at least 0.200 ml/g of egg weight and at least 10 ml of extraembryonic fluid per embryo were used. We used cocks obtained from mothers and having sisters with the same indicators of the allantois-amniotic fluid output of their embryos. The selection level was within the range of 24-26% for hens and 5-6% for cocks; the average number of hens and cocks after selection was 420 and 60 heads, respectively.
2.1. Experimental Design of Thermoregulation Capabilities
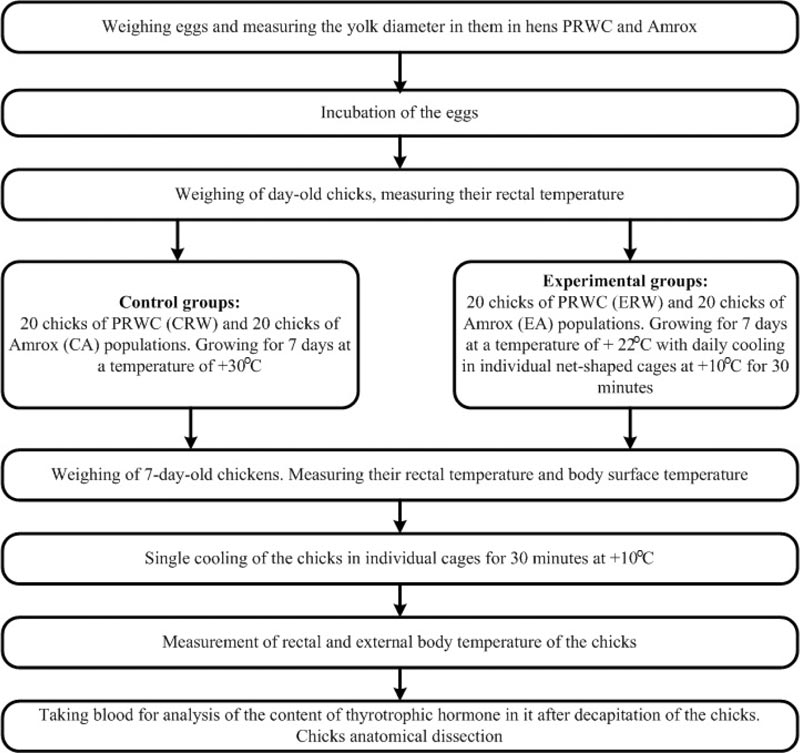
Studies of thermoregulation capabilities at low-temperature conditions were performed on neonatal chicks (n = 40) of PRWC and Amrox breed (n = 40). Chicks of the Amrox breed (control) were used for comparison, as their egg productivity among the gene pool breeds is the closest to the level of productivity of the PRWC. In addition, Amrox were not selected for resistance to low ambient temperatures (Fig. 1).
2.2. Measurements
Eggs and chicks, as well as residual yolk and lungs, were weighed on “HL-400EX” electronic scales (“A&D Company Ltd.”, Japan). The yolk diameter was evaluated without eggs breaking using an ultrasonic portable scanner, “Raskan” (“Rateks”, Russia) [7]. Eggs were incubated in laboratory conditions at the accepted temperature for chickens of the gene pool flock according to a standard operating procedure (1-2 days-38.0oC, 3-10 days-37.8oC, 17-21 days-37.2oC) in “REMIL-C” incubator (“REMIL”, Russia).
The body’s surface temperature in the head and lateral projection areas (10-12 images of each chick) was measured using a thermal imager “Thermal Expert” FL 13mm f/1.0 (“Thermal Expert”, South Korea). Rectal temperature was measured using an electronic thermometer “Microlife MT 3001” (“Microlife”, China). Heat loss after the dose exposure at low-temperature was calculated as the difference between the temperature before and after cooling, expressed in absolute or relative values. The reaction of 7-day-old chicks to cooling also was evaluated by the level of thyrotrophic hormone in their blood, since it plays an important role in thermoregulation of the body at low temperature [8-12]. The analysis to evaluate the content of thyrotrophic hormone in the blood was carried out in the research laboratory “Diagnostics” (www.explana.ru). Blood sampling was performed in 7-day-old chicks immediately after the dose exposure to the stress factor (30 minutes at 10oC) and their decapitation. Measurement of “background” values of thyrotrophic hormone was performed in chicks that were not subjected to cooling.
2.3. Virological Research
The Newcastle disease virus was used as a model for studies of the volume of allantois-amniotic fluid of embryos and the titer of the vaccine virus [13]. Since the selection work for creating a specialized population for the purpose of bio-industry has been carried out for a number of years, we were able to evaluate hens by the volume of allantois-amniotic fluid of their embryos and the titer of the vaccine virus in it in F5 and compare the data obtained with those in F0.
The volume of allantois-amniotic fluid and the titer of the vaccine virus for Newcastle disease (“La Sota” strain) were evaluated in the “All-Russian Research Veterinary Institute of Poultry Science”, Branch of the Federal State Budget Scientific Institution Federal Scientific Center and “All-Russian Research and Technological Poultry Institute” of the Russian Academy of Sciences (ARRVIPS). To determine the amount of the virus-containing fluid, 10-day-old embryos were infected with the Newcastle disease virus strain “La Sota”. Before infection, the inoculation side of the egg was swabbed with alcohol and flamed. A small hole was punched. A syringe needle was inserted to a depth of 20 mm into the allantois cavity and 0,1 cm3 of the inoculum (Newcastle disease virus in a 1:10000 dilution) was injected. After the infection of the embryos, the hole in the shell was filled with molten paraffin. Infected embryos were incubated in a thermostat for 96 hours at a temperature of 37.0-37.5oC. Embryos that died within the first 24 hours were disposed of. After 96 hours of incubation, the embryos were cooled at a temperature of 4-6oC for 16-18 hours. Before opening, the shell was disinfected and flamed; then the virus-containing fluid was harvested with a pipette.
To determine the biological activity of the Newcastle disease virus, the method of titration of a virus-containing fluid was used. Ten-fold dilutions from 10-1 to 10-10 were prepared for research. Each dilution infected 10-day-old chicken embryos in the allantois cavity (5 embryos per each dilution) in a volume of 0,1 cm3. As a control, we used five non-infected embryos. The embryos were incubated for 5 days at a temperature of 37oC and relative humidity of 60-70%. The death of embryos in the first 24 hours after the infection was considered non-specific and they were disposed of. After 120 hours of incubation, the embryos were cooled down for 18 hours at a temperature of 4-6oC, then the allantoic fluid from each embryo was harvested and examined in a hemagglutination reaction with a 1% suspension of rooster red blood cells. The virus titer was determined using the Kärber method.
The unit of infectivity used was the mean (50%) embryo infective dose (EID50) — that was the amount of virus capable of infecting 50% of inoculated eggs, the titer of the virus suspension and was expressed as the number of infectious units per unit volume. Data of the titer of the vaccine virus for Newcastle disease (“La Sota” strain) in F0 chicken embryos were obtained using the same methods.
2.4. Statistical Analysis
Statistical processing of the results was performed in Microsoft Excel and Statistica 10.0. The average values for the groups (M) and the standard error of the averages (±SEM) were calculated. The reliability of differences was assessed by the Student's t-criterion. The differences were considered statistically significant at p < 0.05.
3. RESULTS AND DISCUSSION
3.1. Thermoregulation Capabilities of PRWC Chickens
Modern programs for the conservation of gene pool poultry breeds involve studying specific traits of breeds for their further use for selection purposes. The ability to adapt and maintain productivity over a wide temperature range is an important, economically significant trait. When solving the problem of thermal resistance of birds, two approaches are considered: the first one is based on the study of epigenetic adaptation [13-16], and the second approach is selective-genetic [3, 17]. The possibility of selection for resistance to extreme ambient temperatures has been confirmed [5, 18, 19].
It was found that in PRWC chickens, increased thermal resistance was inherited by the dominant type with monogenic inheritance [4, 20]. The role of chemical thermoregulation decreased in the generations of thermoresistance selection, but the role of physical thermoregulation increased. This was achieved by reducing heat transfer capability, in particular, by reducing the relative weight of the lungs [4]. The thermoregulatory capabilities of this chicken population were studied in comparison with the most similar type of productivity breeds, which was not selected for this trait.
As presented in Table 1, the egg weight of Amrox hens was lower, because there was no selection for this trait. The yolk diameter of the eggs of these hens was on average 3% higher (p <0.01), and the yolk percentage was 4% higher than in the eggs of PRWC hens. At the withdrawal from the incubator, there were no statistically significant interbreeding differences in the body temperature of the chicks. The live weight of daily PRWC chicks was higher due to the weight of the egg, but the highest growth rate by 7-day age was shown by the chicks of the control group (Amrox), 18% higher than PRWC.
Interbreeding differences became noticeable by the difference in behavioral responses in day-old chicks after the first dose exposure to the stress factor (10oC for 30 minutes). PRWC and Amrox chicks, 10 heads of each breed, were exposed to sub-lethal low temperatures in group trays. Neonatal chicks of the Amrox breed showed classic hypothermia signs [21, 22], such as huddling, muscle shivering and torpor. The chicks closed their eyes and fell on their sides in torpor. Day-old PRWC chicks were found to move actively; despite muscle shivering, they had a well-expressed reflex of searching for food.
| Parameters | PRWC | Amrox breed | ||
|---|---|---|---|---|
| CRW | ERW | CA | EA | |
| Number of chicks | 20 | 20 | 20 | 20 |
| Average egg weight, g | 61.1a ± 0.5 | 56.4b ± 0.6 | ||
| Egg yolk: - diameter, cm; - yolk percentage (% egg weight) |
2.88c ± 0.01 26.9a ± 0.1 |
2.96d ± 0.02 30.9b ± 0.2 |
||
| Body weight of a day-old chick, g | 42.7a ± 0.5 | 39.2b ± 0.6 | ||
| Rectal temperature of a day-old chick, оС | 39.6 ± 0.08 | 39.6± 0.20 | ||
| Body weight of a 7-day-old chick, g | 69.9 ± 1.1 | 68.2c ± 1.4 | 67.6e ± 1.6 | 62.4df ± 1.5 |
| Relative body weight gain, % | 62.0b ± 2.1 | 62.0 ± 3.0 | 80.0a ± 2.8 | 66.0b ± 2.3 |
| Rectal temperature of a 7-day-old chick, оС: - at room temperature; - immediately after cooling |
41.0±0.06 40.0±0.02 |
41.4±0.07 41.1±0.04 |
41.3±0.05 39.4±0.15 |
41.3±0.05 40.9±0.17 |
| Heat loss: - absolute (rectal temperature), оС; - relative (body surface temperature), % |
1.0a ± 0.1 25.4± 1.0 |
0.2b ± 0.04 24.4a ± 0.6 |
1.9ag ± 0.1 26.6 ± 0.8 |
0.4b ± 0.1 28.6b ± 0.9 |
| Residual yolk sac: - weight, g; - percentage (% to body weight) |
0.19d± 0.04 0.30h ± 0.05 |
0.35e ± 0.08 0.52a ± 0.07 |
0.06с± 0.02 0.10c± 0.02 |
0.16df ± 0.02 0.23bd ± 0.03 |
| Lungs: - weight, g; - percentage (% to body weight) |
0.56 ± 0.02 0.82 ± 0.03 |
0.58 ± 0.02 0.86c ± 0.03 |
0.56 ± 0.02 0.85c± 0.02 |
0.59 ± 0.02 0.94d ± 0.02 |
| Thyrotrophic hormone in the blood, ulU/ml: - after cooling; - «background» value |
0.0070 0.0084 |
0.0076 0.0078 |
0.0162 - |
0.0282 - |
Probably, the mechanism of epigenetic adaptation was functional in both experimental groups as a result of the dosed effect of the stress factor (low temperature). Therefore, after short-term exposure to low temperature at the age of 7 days, the chicks of both experimental groups, in comparison with the control, slightly decreased their rectal body temperature. Minimal heat loss on the body surface was recorded in PRWC chicks (Fig. 2).
In response to low-temperature exposure, EA chicks reduced their relative body weight gain by 14% compared to CA chicks. And although the absolute weight of the lungs in all groups did not have significant differences, their percentage in the EA group increased (p < 0.01). Amrox chicks of both groups also used the residual egg yolk more actively in comparison with PRWC chicks, and this is fully consistent with the literature information. According to the literature data, the adaptive humoral mechanisms are activated at low temperature, in particular, the level of thyrotrophic hormone increases, lipolysis processes are activated, and the absorption of the yolk sac increases in chicks [23, 24]. The use of lipids as energy to maintain the body temperature is accompanied by a higher level of oxygen consumption, which probably explains the increase in lung proportion.
PRWC chickens under the influence of a low-temperature stress factor did not change the growth rate (62% both in ERW and CRW groups), and practically did not use the residual yolk; however, the percentage of the lungs increased, but slightly. PRWC chicks of both the groups also did not respond to changes in the level of thyrotrophic hormone in response to the dosed low-temperature exposure. We suppose that PRWC neonatal chicks significantly react at a low temperature with better thermoregulation mechanisms (mainly physical, not chemical). They better adapt to low-temperature conditions in the early postnatal periods. This trait can be used for further selection work with PRWC when creating a chicken population with increased adaptive abilities under hypothermic stress.
3.2. Quantity and Biological Activity of Allantois-amniotic Fluid in PRWC Embryos
As a result of long-term selection of PRWC under sub-lethal low temperatures, not only genotypes that differ in thermal resistance of neonatal chicks appeared, but it was also found that, in comparison with other gene pool breeds, PRWC embryos had a higher yield of allantois-amniotic fluid and the titer of the vaccine virus in it [6, 7].
| Parameter | Sufficient biological activity of the virus-containing fluid used for the preparation of the vaccine series | Commercial lines hens * | Hens of the maternal form CD in commercial laying cross «Lohmann LSL» [7] |
Hens of the maternal form SP-9 in commercial laying cross «SP-789» [13] |
PRWC | |
|---|---|---|---|---|---|---|
| F0 [7] | F5 | |||||
| Number of embryos | 30 | 15 | 50 | 60 | ||
| Extraembryonic fluid relative volume, ml / g of egg weight | 0.157a ± 0.004 | 0.180d ± 0.007 | 0.191b ± 0.002 | 0.228c ± 0.001 | ||
| Newcastle disease virus titer (“La Sota” strain), lgEID50/cm3 | 9.0 for live vaccines 10.3 for inactivated vaccines |
9.1- 9.5 | 9.0a ± 0.21 | 9.20a ± 0.28 | 9.33a ± 0.20 | 10.51b ± 0.17 |
* ARRVIPS data
Therefore, the work on creating a specialized population for bioindustry was started with PRWC. The selection work aimed at increasing the volume of allantois-amniotic fluid in embryos was conducted with both cocks and hens. Analysis of the data related to the output of extraembryonic fluid revealed that the maternal effect on the transmission of such trait as the level of output of extraembryonic fluid in embryos, was fundamental. The phenotypic coefficient of heritability of relative fluid output (ml/g egg weight) for hens was 0.25, while for cocks, its value was 0.19 [7].
The selection of poultry for increased resistance to diseases is difficult because of low heritabilities, antagonism between this trait and chicken productivity, and the complexity of its evaluation. Another difficulty is rapid evolution to more virulent forms among disease-causing microorganisms. However, successful selection work has been carried out for 5 years at PRWC to increase the level of allantois-amniotic fluid output (in absolute and relative volume) in 12.5-day-old embryos. Selection for this trait allowed to increase the number of hens with a relative fluid volume of 0.200 ml/g of egg weight and higher in their eggs [25, 26]. When comparing cocks evaluated in F0 with their progeny in F4, it was found that the frequency of occurrence of daughter hens with a high output of extraembryonic fluid increased by an average value of 25.2%. This proves that the selection of hens to increase the yield of vaccine raw materials from their embryos (allantois-amniotic fluid) is quite effective. The positive effect is achieved both by increasing the output of extraembryonic fluid from the embryo itself, and by increasing the total number of embryos per hen due to an increase in the egg productivity of hens (egg production and egg weight). Intensive selection work with PRWC has led to an increase in egg production for 5 generations of selection by 15%, and egg weight - by 10%. The volume of allantois-amniotic fluid increased by 14% in the absolute value (ml) and by 12% – in the relative value (ml/g of egg weight).
According to the results of virological studies, it was found that PRWC embryos have a higher biological activity of the virus-containing raw material collected from them (allantois-amniotic fluid) than embryos from laying hens of commercial lines of egg-type chickens. Thus, the unit volume (cm3) of extraembryonic fluid of PRWC embryos contained 10.2 – 25.7 times more doses of Newcastle disease virus (EID50) in comparison with embryos obtained from chickens of commercial layer lines (Table 2).
Selection work to create a specialized chicken population for bioIndustry was effective. Such a population has not only an increased yield of vaccine raw materials from their embryos, but also higher biological activity of the virus in it (+11.5% in F5 compared to F0). PRWC hens outperformed commercial line hens in terms of Newcastle disease virus (EID50) doses per unit volume of allantois-amniotic fluid of their embryos by 2.1 times in F0 (Table 2). After 5 generations of selection, the gap increased; the biological activity of the virus in the extraembryonic fluid of PRWC embryos was 32.3 times higher than that of commercial line hens and 20.4 times higher than that of the maternal form of Russian layer cross SP-789.
Also, when testing embryos of PRWC in the ARRVIPS laboratory, it was found that these embryos, in contrast to embryos of commercial line chickens, which do not always give stable results, could be successfully used as diagnostics for reovirus infection and infectious bronchitis in chickens. The level of biological activity of the Newcastle disease virus in PRWC embryos allows us to recommend them for the production of both live and inactivated vaccines.
CONCLUSION
Using the example of the PRWC gene pool population, the efficiency of using the dose at low temperatures as a breeding background for creating a specialized population of hens – producers of raw materials for the manufacture of viral vaccines- was evaluated. As a result of breeding, PRWC chicks have acquired a wide range of reaction norms at low temperatures in the early neonatal period and better thermoregulation mechanisms.
The effectiveness of PRWC selection to increase the yield of vaccine raw materials (allantois-amniotic fluid) for the production of viral vaccines for farm birds has been proved. The results of virological studies of PRWC embryos allow us to recommend them for use in the production of a number of viral vaccines (alive and inactivated), as well as diagnosticumes.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study carried out in accordance with the Ethical Guidelines for in vivo Experiments of Russian Research Institute of Farm Animal Genetics and Breeding — Branch of the L.K. Ernst Federal Science Center for Animal Husbandry (RRIFAGB).
HUMAN AND ANIMAL RIGHTS
No humans were used in this research. All the experiments on animals were in accordance with "Guidelines for Accommodation and Care of Animals. Environment, Housing and Management". http://docs.cntd.ru/document/1200127789 (Accessed July 08, 2020).
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
Research conducted on the topic AAAA-A18-118021590129-9.
CONFLICT OF INTERESTS
The authors declare no conflict of interests, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


