All published articles of this journal are available on ScienceDirect.
Evaluation of Potential Biodiesel Feedstocks: Camelina, Turnip Rape, Oil Radish and Tyfon
Abstract
Background:
One of the most promising alternative biofuels, competitive with regular petrol, diesel or jet fuel is biodiesel, especially derived from plant oils. Until now, various technological approaches, as well as oil sources, have been proposed for biodiesel production, but an industrially scalable technology with high end-product quality and production efficiency has not been developed and brought to the market yet. Biodiesel is produced in Europe and North America mainly from rapeseed, or canola, sunflower and soybean oil. However, other underutilized plant species could also be considered as potential oil feedstocks for biodiesel. The great perspective holds Brassicaceae family, especially such species as false flax (Camelina sativa) and Ethiopian mustard (Brassica carinata), but many other Brassicaceae crops are still out of sight.
Objectives:
This research has been conducted aiming to identify and compare the productivity of several Brassicaceae crops (camelina or false flax (C. sativa), turnip rape (B. campestris), oil radish (Raphanus sativus var. oleifera) and tyfon (B. rapa ssp. oleifera f. biennis × (ssp. rapifera × ssp. pekinensis)), that are suitable for biodiesel production under conditions of temperate climate regions (Northern America, Europe); and to obtain biodiesel by transesterification of fatty acids present on these species using bioethanol.
Methods and Materials:
Seed oil content, yield and fatty acid profiles have been studied and analysed in different genotypes of C. sativa (10), winter (6) and spring (4) B. campestris, R. sativus var. oleifera (8) and tyfon (5). The most productive crops have been identified: false flax variety ‘Evro-12’ (1620 kg of oil per hectare) and ‘Peremoha’ (1657 kg/ha); winter turnip rape variety ‘Oriana’ (1373 kg/ha), oil radish variety ‘Kyianochka’ (1445 kg/ha) and tyfon varieties ‘Fitopal’ (1730 kg/ha) and ‘Obriy’ (1860 kg/ha). According to chromatographic analysis results, oils of winter turnip rape and tyfon contain high levels (38-42,8%) of erucic (22:1) acid, while oils from spring turnip rape, false flax and oil radish possess high amounts of short-chained fatty acids (not longer than C18) – up to 85,37% in camelina breeding line FEORZhYaFD. Fatty acid ethyl esters (FAEE) were produced from oil of best genotypes and proved to comply with all main quality requirements for diesel.
Results:
Moreover, a new solvent-based technology of high-yield (up to 96%) FAEE production, has been firstly proposed for C. sativa oil conversion.
Conclusion:
Best genotypes that can be used as a plant oil source for biodiesel production have been identified for camelina, turnip rape, oil radish and tyfon species. The data obtained on seed oil content, yield and fatty acid profiles suggested that they are: false flax – breeding form FEORZhYaFD; winter turnip rape - variety ‘Oriana’; oil radish - variety ‘Rayduha’ and tyfon hybrid - variety ‘Fitopal’. Biodiesel samples obtained from these plants fit the Ukrainian standards for diesel fuel and can be used in car engines. The proposed new technological approach to produce fatty acid ethyl esters allows to reduce reaction time and to increase esters yield and quality.
1. INTRODUCTION
Due to the high greenhouse gas emissions and climate change, the development of alternative energy sources to replace fossil fuels becomes as important as never before. Of particular interest is the possibility to replace liquid mineral fuels with biofuels, biodiesel and bioethanol. This idea has been under focused attention for the latest decades. Biodiesel derives from lipids (that include various sources: plant oil, animal fats, algae, waste oil, etc.) and is mainly obtained by the transesterification process, which produces fatty acids alkyl esters, or by hydrocracking. The latter process produces n-alkanes and is mostly used for jet fuel production [1-4].
At present, plants are considered one of the most promising sources of lipids for conversion to biofuel [5]. The idea of using vegetable oils as fuels is not new. The inventor Rudolph Diesel used peanut oil to fuel one of his engines at the Paris Exhibition in 1900. He wrote in 1912 that ‘The use of vegetable oils for engine fuels may seem insignificant today, but such oils may become, in the course of time, as important as petroleum and the coal tar products of the present time’ [6]. Over the last decade, the global annual consumption of vegetable oils has been steadily increasing by millions of metric tons. Main plants, covering more than 80% of world oil production, are oil palm (Elaeis oliefera), soybean (Glycine max), sunflower (Helianthus annuus) and rapeseed (Brassica napus). The main dilemma, however, is that these plants are also cultivated for food purposes and oil supply for the biodiesel industry can potentially compete with food production. For this reason, many researchers are now looking for crops beyond the food production industry that could become an effective source of high quality oil for biofuel purposes [1, 5].
Presently, more than 280 plant species are known to have more or less considerable oil contents in their seeds, fruits, tubers or roots [7, 8]. However, not all of them are suitable for biodiesel production because of different oil content and quality, which depend on the fatty acid profile. Additionally, regional climate conditions set restrictions on the cultivation of different plants and on end-product usage [9-11].
Oil plants amenability for biodiesel production has been reviewed in many articles [4, 12, 13]. The main crops used for biodiesel production are those listed above: oil palm, jatropha, soybean, sunflower and different mustards from Cruciferae family, mostly from Brassica genus [14], including rapeseed, one of the most widely used lipid sources for biofuel conversion in Europe and North America. Some of these plants, like jatropha and oil palm, are long-life crops, which make their management rather expensive; if there was a short life crop with promising oil content and quality, it would be more beneficial for industrial purposes [15, 16].
Brassicaceae crops are highly attractive due to their high resistance to low temperatures and ability to grow under cold climate conditions. These characteristics are extremely important for crop production in most European and North American countries. The economically cost-effective species of this genus are: B. napus (rape, rutabaga), B. oleracea (cabbage, kale, cauliflower, broccoli), B. campestris (syn. B. rapa subsp. oleifera, turnip rape), B. nigra (black mustard), B. carinata (Abyssinian mustard) and B. juncea (brown, oriental, leaf mustard) [17-19]. Besides, there are other underutilized Brassicaceae species that seem to have great potential as the feedstock of oil for biodiesel production. However, the low productivity of such crops does not allow them to become an efficient biofuel source. This is the reason why cultivation and breeding programs for these plants have never been attempted. For example, such a promising plant as pennycress (Thlaspi arvense) was not widely cultivated in the past and was often even considered a weed.
The potentiality of different Cruciferous plants to be cultivated for biodiesel production has been discussed earlier [20-22]. For instance, there are two species that gain particular attention for biodiesel and jet biofuel production: Camelina sativa, or false flax [23-26], and B. carinata [27-31]. Consequently, the most important directions of biofuel research are the elaboration of new high-productive plant oil sources and the improvement of current approaches to biodiesel production.
In this paper, we summarize the outcomes of our four-year-long research devoted to optimize biodiesel production procedures from various cruciferous feedstocks and search for the most efficient plant oil source among them. These studies have been conducted on several genotypes of various Brassicaceae representatives, bred in Ukraine, such as false flax (C. sativa) [32, 33], turnip rape (B. campestis) [34], oil radish (Raphanus sativus var. oleifera) [32, 35, 36] and tyfon (B. rapa ssp. oleifera f. biennis × (B. rapa ssp. rapifera × B. rapa ssp. pekinensis)) [32, 37, 38]. Apart from plant feedstock evaluation, biodiesel production (via bioethanol-based transesterification) technology has also been optimized to improve its efficiency and to enable the use of the obtained fuel in car engines. In addition, the quality of the biodiesel produced from different studied plant oil sources has been tested and analysed.
2. MATERIALS AND METHODS
2.1. Seed Samples and Field Trials
In our study, seeds of four cruciferous species were kindly provided by the M.M. Hryshko National Botanical Garden at the National Academy of Sciences of Ukraine (Kyiv, Ukraine).
In particular, they were from ten C. sativa genotypes (breeding lines FEORZhYaF-1, FEORZhYaF-2, FEORZhYaF-3, FEORZhYaF-4, FEORZhYaF-5, FEORZhYaFD, FEORZhYaFCh, FEORZhYaFChP and varieties ‘Peremoha’, ‘Evro-12’), six winter (breeding lines EOSOFU, EOSOFGl, EOSOFDn, EOSOFVol and varieties ‘Oriana’, ‘Oriana-1’) and four spring genotypes (breeding lines EOSYaF-1, EOSYaF-2, EOSYaFR, Pioner-YuT) of B. campestris, eight R. sativus var. oleifera (breeding lines EORDOFL-2, EORDOFL-3, EORDOF-5, EORDOF-6, EORDOF-8 and varieties Rayduha’, ‘Tambovchanka’, ‘Kyyanochka’) and five genotypes of hybrid culture tyfon (breeding lines EOTFVS, EOTFV and varieties ‘Obriy’, ‘Fitopal’, ‘Orakam’).
Field trials of all the above-mentioned genotypes were conducted on testing plots at the M.M. Hryshko National Botanical Garden (50032’′N, 30033′E). Plot size was 4 m long and 1.5 m wide. Each genotype was cultivated on a separate testing plot (n=3) for 3 years from 2015 to 2018. The soil of the plots was classified as alfisol (рН 6.1-6.8, measured at 1:2 soil to water ratio). The average air temperature and precipitation in this area were +7.6oC and 550-560 mm per year.
Seed total lipid content was measured using the Soxhlet extraction method [39]. Estimated seed oil yield (kg/ha) for each crop was calculated based on lipid content and seed yield data. Oil content was measured for each genotype (n=3) per species across the three harvesting seasons (2015-18) as well as seed yield and estimated oil yield.
2.2. FAMEs Preparation for Gas Chromatography
Seed oil from the 2018 harvest was used for chromatography assay and further biodiesel production tests. Samples for chromatography assay were prepared in at least three replicates (n=3) per genotype, whereas seeds for each repetition were taken from a separate testing plot. Oil extraction from seed samples for GC-analysis was done using the manual press Prom-1 (Olis, Ukraine). Conditions were as follows: mass of press – 20 kg, maximum power gain – 12 t, glass volume for oil 200 cm3, the exposure time for seeds under pressure to get the final product – 5 min. Seeds from the 2018 harvest were taken to extract oil for gas chromatography assay. The obtained oil was transferred to 2 mL vials, samples were left to stand for 48 h at 8°C for precipitation of pigments and then cleaned oil was poured into new vials and used for fatty acids methyl esters (FAMEs) preparation.
In 10 mL glass tube, 60 mg of oil sample, 4 mL of isooctane and 200 μL of 2 M potassium hydroxide dissolved in methanol were added. Then, the tube was vortexed to obtain a clear solution, which meant the completion of reesterefication reaction. To neutralize potassium hydroxide, 1g of sodium sulfate monohydrate was added. After salt sedimentation, the upper layer was transferred into a new 4 mL tube. The obtained isooctane solution contained approximately 15 mg/mL of FAMEs.
2.3. Gas Chromatography Analysis
FAMEs were separated and quantified by Gas Chromatography (GC) with Flame Ionization Detection (FID). The chromatographic analysis was performed with a GC–MS instrument (Agilent 7890B-7697, USA) equipped with a capillary column (60 m × 0.25 mm × 0.2 μm) Zebron ZB-FAMЕ (Phenomenex Inc., USA) using nitrogen as a carrier gas, H2 and air for FID detector. Other chromatographic parameters were set as follows: the vaporizer temperature +250°С, column temperature +185°С. In total, the analysis lasted 40 min. The injection volume was 1 μL. The identification of the individual compounds was performed by frequent comparison with authentic standard mixtures (С10–С24, Supelco) analyzed under the same conditions.
Fatty acids composition was expressed as follows: MUFA –monounsaturated fatty acids, PUFA - polyunsaturated fatty acids, SFA - saturated fatty acids, SCFA - fatty acids with carbon chain length 18 or less, LCFA - fatty acids with carbon chain length more than 18.
2.4. Biodiesel Samples Production
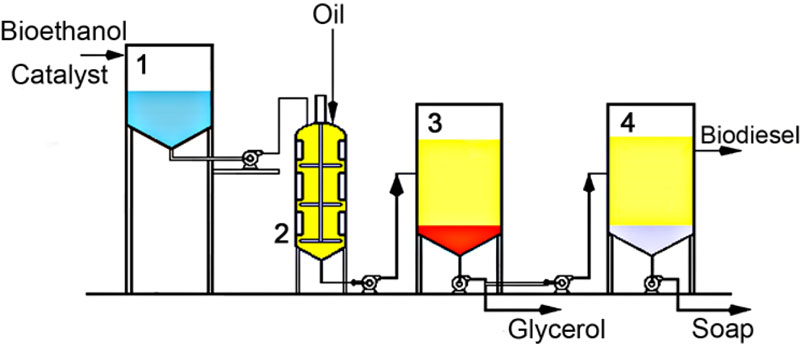
The transesterifìcation reaction of oil was carried out using samples from C. sativa (FEORZhYaFD), B. campestris (cv. ‘Oriana’), R. sativus var. oleifera (cv. ‘Rayduha’) and tyfon hybrid (cv. ‘Fitopal’). All experiments were repeated three times (n=3) for each genotype at each of the different transesterification conditions. For each repetition, oil samples from different testing plots were used, where particular genotypes had been cultivated. Fatty acid ethyl esters (biodiesel) were produced via mixing the required amount of vegetable oil with an alcohol (bioethanol) in the presence of an alkaline catalyst and heating up to +60°C. The volume of the reactor used for transesterification (Fig. 1) was 500 cm3. Two types of experiments were conducted for each oil sample using different catalysts:
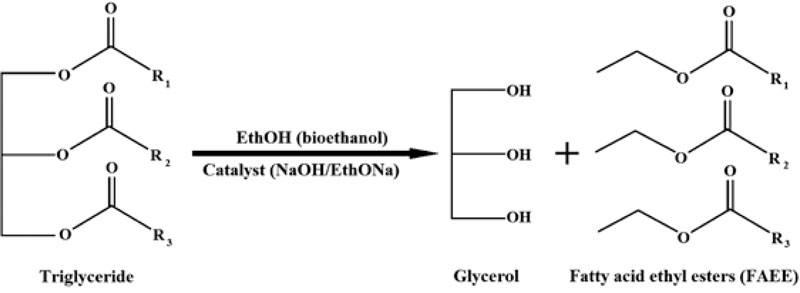
- The plant seed oil was taken in a 1:6 molar ratio to dehydrated bioethanol (C2H5OH). As a catalyst, sodium hydroxide (NaOH) was added in ethanol in different quantities: 0.5, 1, 1.5, 2 and 2.5% (w/w) of plant oil that was used in the reaction;
- The plant seed oil was also taken in a 1:6 molar ratio to dehydrated bioethanol (C2H5OH). Sodium ethoxide (C2H5ONa) as a catalyst was added in ethanol in different quantities from 0.5, 0.8, 1, 1.3, 1.5 to 2% (w/w) of plant oil that was used in the reaction.
The reaction of transesterification lasted 90 min for ‘type a)’ reaction and 30 min for ‘type b)’ reaction. After the reaction was completed, a two-phase system was formed in which higher density glycerol was in the lower phase and fatty acid esters and remainder of alcohol were in the upper layer. The phases were then separated by pouring glycerol (Fig. 2) and the catalyst leftovers were neutralized by adding 10% v/v solution of glacial acetic acid and then washed with water. After that, a new two-phase system was formed in another decanter, where saponificated fats were collated at the bottom. The phases were again separated and, as a result, pure fatty acid ethyl esters were obtained following the dehydration with sodium sulfate.
To improve the technology of FAEE production, other types of reactions were also tested. The main procedure remained as described above, except for the reaction conditions. Camelina oil (280 g) was preliminarily mixed with 70 g of mineral diesel (at 4:1 ratio). A similar approach was described in our patent, using sunflower oil [40]. Sodium hydroxide was used as a catalyst (0.5%, 0.8%, 1.0%, 1.3%; 1.5%, 2.0% as w/v percentage of plant oil amount) dissolved in dehydrated bioethanol, (molar ratio ethanol to plant oil 1:6).
Additionally, the fatty acids conversion efficiency was evaluated for reaction, based on another solvent. As a catalyst, NaOH was added in 0.8 % w/w quantity for production tests with the addition of polyoxyethylene (20) sorbitan monooleate (Tween 80, Polysorbate 80, E433), which was added in 0.5 % w/w to the oil. Similar to the previous reaction, camelina oil was preliminarily mixed with mineral diesel at a 4:1 ratio. The reaction time was 30 min.
Complex catalyst efficiency was tested using winter turnip rape (B. campestris f. biennis) oil. Furthermore, dehydrated bioethanol was used in oil:bioethanol molar ratio 1:6. As a complex catalyst, a 3:1 mixture of sodium ethoxide and monoethanolamine [41] was added in 0.05, 0.07 and 0.1% (w/w) of oil. The reaction time was 60 min. All mentioned productivity tests were conducted
2.5. Biodiesel Samples Testing for Compliance with the Requirements of Ukrainian Diesel Fuel Standards
All biodiesel samples obtained were tested according to Ukrainian regulations on diesel fuel quality (DSTU 7688:2015). The following criteria were used to evaluate the main technical characteristics of produced samples: cetane number, sulfur presence, presence of hydrogen sulfide, corrosive properties, acidity and ash content. Cetane number was identified according to the procedure described in ASTM D613-18a. Measurement of the sulfur amount was recorded by the methods from ASTM D4294-10, EN ISO 20846:2011, EN ISO 13032:2012, as well as the presence of hydrogen sulfide. Corrosive properties were tested on a copper plate as described in ASTM D130-12, the test was scored as ‘passed’ if no significant colour change of copper was observed (group 1a and 1b by ASTM D130-12 classification). Acidity was identified as the amount of KOH in mg per 100 cm3 (0.1 L) of fuel that is needed for sample neutralization. This procedure was performed according to the GOST 5985-59 and the GOST 14141-69 (technical standards issued in the former Soviet Union). Ash content was measured as described in ASTM D482-12.
2.6. Statistical Analysis
All statistical processing of obtained data was conducted using OriginPro 9.1 software. Deviations of all means were calculated as the Standard Deviation (SD). To identify significance of differences in yield, oil content and estimated oil productivity values between studied genotypes, Fisher’s Least Significant Differences (LSDs) were calculated. The LSDs were used to identify homogeneous groups in yield, oil content and estimated oil productivity values separately for each studied species (with p<0.05 significance level). The same was done for fatty acids content data, for which homogeneous groups were identified separately for particular acid content. The LSDs-based comparison, in this case, was also done separately for each species. In terms of analysis of MUFA, PUFA, SFA, SCFA, LCFA content, LSDs were calculated, taking into account data obtained for all studied genotypes of four species.
| Breeding line or variety | Lipid content in seeds, % | Seed yield, kg/ha | Seed lipids yield, kg/ha |
|---|---|---|---|
| FEORZhYaF-1 | 38.2±0.44d | 3386±34.2e | 1293±13.06g |
| FEORZhYaF-2 | 43.9±0.59a | 3658±48.65d | 1606±21.36b |
| FEORZhYaF-3 | 42.6±0.30b | 3348±44.19e | 1426±18.82e |
| FEORZhYaF-4 | 39.5±0.58c | 3967±36.1b | 1567±14.26c |
| FEORZhYaF-5 | 38.1±0.22d | 3947±26.84bc | 1504±10.23d |
| FEORZhYaFD | 42.6±0.55b | 3348±33.15e | 1426±14.12e |
| FEORZhYaFCh | 36.6±0.42e | 3712±33.41d | 1359±12.23f |
| FEORZhYaFChP | 36.0±0.44e | 3386±25.73e | 1218±9.26h |
| ‘Peremoha’ | 42.6±0.37b | 3890±56.41c | 1657±24.03a |
| ‘Evro-12’ | 39.4±0.47c | 4111±27.54a | 1620±10.85b |


3. RESULTS AND DISCUSSION
3.1. Crop Productivity Trials
3.1.1. Camelina (False Flax)
C. sativa has been considered over the recent years as one of the most trendy species for biodiesel production, especially for biojet fuel production. False flax is known to initially originate from Eastern Europe. Some archeological studies showed that camelina was cultivated in the European area since the Bronze Age as an important oilseed crop. Later on, in the 20th century, C. sativa was driven out by sunflower, canola and soybean [23, 42-45]. In particular, camelina has gained focused attention due to its high tolerance to stresses associated with crop cultivation. Currently, C. sativa is one of the most promising candidates for growing in Europe and North America. First of all, camelina is characterized by high resistance to low temperatures, which allows this crop to germinate at +1°С, while seedlings are able to survive at -8-10°С [46-48]. Besides, false flax does not require rich soils to grow and is quite resistant to drought. However, one of the most attractive benefits of this plant is resistance to typical Brassica fungal diseases such as Leptosphaeria maculans and Alternaria brassicae [44]. In addition, there are at least 6 wild species in the Camelina genus, which could be used as a source of useful agronomic traits for the improvement of domesticated false flax [49]. Different growth stages of camelina during field trials are shown in Figs. (3 and 4).
The results of crop productivity trials of C. sativa genotypes from the investigated germplasm collection are provided in Table 1 [32, 33]. Estimated lipid content in seeds varies from 36% to 43.9%, while a study [45] reported that it might vary from 29.6% to 49% depending on the genotype. The highest oil content was identified in seeds of FEORZhYaF-2. Seed yield of different genotypes ranged from 3348 to 4111 kg/ha. The maximal oil yield of false flax studied genotypes reached 1620 kg/ha for ‘Evro-12’, 1567 kg/ha for FEORZhYaF-4 and 1657 kg/ha for the most productive variety ‘Peremoha’. The relatively high productivity, the low requirements for growing conditions and the short vegetation


period make camelina very attractive for crop rotation. It was shown [50] that such an approach could be applicable in the North-Western US, where it could allow running economically sustainable biodiesel production with low environmental impact.
Seed and oil yield of C. sativa vary depending on the growing conditions. Thus, research [51] established that false flax produces up to 1500 kg/ha of seeds under the conditions of Nova Scotia (Canada). At the same time, it was shown [52] that mutant lines of winter camelina can produce up to 2795 kg/ha of seeds, while others reported [47] that yields of this crop could reach up to 3320 kg/ha in Western Canada. In another review, it was mentioned [45] that under optimal conditions, C. sativa can easily give a grain yield of over 3000 kg/ha. These authors also discussed that low camelina seed productivity, which was usually reported, was associated with unfavorable growing conditions (e.g., non-optimal sowing time, drought stress, etc.) of regions, where this crop was cultivated, and might indicate the weak crop establishment. In addition, a study [45] reported that variations in the productivity rates of false flax were predominantly due to the influence of growing conditions.
3.1.2. Turnip Rape
Turnip rape (B. campestris; syn=B. rapa ssp. oleifera) is also called rape and is sometimes confused with rapeseed. It is a highly productive plant culture that could be used both as an oil source and for forage purposes. First of all, winter and spring forms of turnip rape, bred in Ukraine, are characterized by high tolerance to cold. Plants are able to germinate at +2-3 °С, spring plant seedlings can survive at -3-4 °С, while winter forms resist at -6-7 °С and, once small leaf rosette is formed and covered by snow, they can survive frosts down to about -25-30 °С [34]. Such characteristics make turnip rape a very attractive culture for Europe and North America where cold winter seasons or sharp temperature changes occur. Different growth stages of turnip rape during field trials are shown in Figs. (5 and 6) [34].
The results of crop productivity trials for B. campestris f. biennis genotypes are provided in Table 2, those for B. campestris f. annua - in Table 3. Generally, winter plants are more productive than spring ones (1138-1389 kg of seed oil per hectare vs. 737-998 kg).
| Breeding line or variety | Lipid content in seeds, % | Seed yield, kg/ha | Seed lipids yield, kg/ha |
|---|---|---|---|
| ‘Oriana’ | 38.1±0.50a | 4360±64.53b | 1373±20.32a |
| EOSOFU | 35.8±0.26c | 3770±34.31d | 1138±10.36d |
| EOSOFGl | 35.3±0.30c | 4630±36.11a | 1389±10.83a |
| EOSOFDn | 35.9±0.18c | 4390±61.46b | 1321±18.49b |
| EOSOFVol | 37.8±0.21a | 4220±44.73c | 1312±13.91b |
| ‘Oriana-1’ | 36.9±0.27b | 4140±18.63c | 1279±5.76c |
| Breeding line or variety | Lipid content in seeds, % | Seed yield, kg/ha | Seed lipids yield, kg/ha |
|---|---|---|---|
| EOSYaF-1 | 36.2±0.24a | 3240±20.09a | 998±6.19a |
| EOSYaF-2 | 36.4±0.33a | 2400±21.84c | 737±6.71d |
| EOSYaFR | 35.8±0.24a | 2820±32.43c | 857±9.86c |
| Pioner-YuT | 36.3±0.48a | 3180±42.61a | 970±13.00b |
| Breeding line or variety | Lipid content in seeds, % | Seed yield, kg/ha | Seed lipids yield, kg/ha |
|---|---|---|---|
| EORDOFL-2 | 30.1±0.35e | 2640±33.79g | 792±10.14h |
| EORDOFL-3 | 35.3±0.23d | 2720±34.00f | 952±11.90g |
| EORDOF-5 | 35.9±0.53d | 2880±21.60e | 1037±7.78f |
| EORDOF-6 | 37.1±0.48c | 3040±42.86d | 1125±15.86e |
| EORDOF-8 | 41.0±0.25b | 3320±43.49b | 1361±17.83b |
| ‘Rayduha’ | 37.3±0.26c | 3120±21.53c | 1154±7.96d |
| ‘Tambovchanka’ | 40.5±0.52b | 3160±26.86c | 1264±10.74c |
| ‘Kyyanochka’ | 41.8±0.56a | 3440±35.78a | 1445±15.03a |

The difference in the seed lipid content was significantly varying between 35.3 and 38.1% and between 35.8 and 36.4 %, winter and spring turnip rape varieties, respectively. Genotype EOSOFGl produced the highest seed yield – 4630 kg/ha, although with the lowest oil content - 35.3%. The winter turnip rape is known to be one of the most important oilseeds in the Northern European countries, such as Estonia [53]. This crop can produce 2890-3512 kg/ha of seeds, with 44.4-44.9% of oil under the climate conditions of this country. Another study [54] reported an average B. campestris seed yields in Finland from 1347 to 1926 kg/ha.
In the same paper [54], the prospects for turnip rape to be cultivated as a biodiesel feedstock were evaluated, while energy consumption rate had been earlier reported [55] for B. campestris grown in Finland for biofuel (FAMEs) production. A great potential of this oilseed crop in Poland was described [56]. These authors [56] noted that over the period from 2011 to 2016, the total area allocated for turnip rape growth had increased. Thus, B. campestris is a desirable oilseed crop for biofuel purposes in Northern Europe as well as for Ukraine.
3.1.3. Oil Radish
Oil radish (R. sativus var. oleifera) is one of the underused Brassicaceae species. This plant is annual, and does not have a winter form. The spring form is considered to be very productive not only due to the high seed and oil yields, but also due to the efficient biomass growth. Seeds of oil radish can start to germinate at +1–2°С and can resist frosts of about -3°С. However, it cannot resist colder temperatures, starting from -4–5°С and below for a prolonged period of time [32, 34, 35]. R. sativus var. oleifera plants in a blooming phase are shown in Fig. (7), ripening fruits with seeds – in Fig. (8A,B).

The outcomes of the conducted crop productivity trials of R. sativus var. oleifera genotypes are provided in Table 4. Lipid content in seeds of different genotypes varied in a wide range, from 30 to 42%, and the seed yield was 2640-3440 kg/ha. The highest seed oil yield was identified for the variety ‘Kyyanochka’ – 1445 kg/ha, while the lowest value within the analyzed collection was for the variety EORDOFL-2 – only 792 kg/ha, which was quite typical for the less productive spring forms.
Oil radish is characterized by high seed productivity combined with a high oil content at the same time. Some studies reported [57] that it was possible to extract up to 34% of oil from R. sativus seeds, while in this study, it has been shown that oil content can reach up to 42% (variety ‘Kyyanochka’). Earlier, the feasibility of growing oil radish as a biodiesel crop was tested and reported [58]. Based on the results of these authors [58], it can be concluded that the cultivation of R. sativus var. oleifera for biofuel purposes is considered more cost-effective and financially viable, if compared with soybean.
3.1.4. Tyfon (B. rapa Hybrid)
Tyfon hybrid has been created at the M.M. Hryshko National Botanical Garden of the National Academy of Sciences of Ukraine by crossing an original spring forage hybrid ‘Holland greens’ with a winter turnip rape to obtain a frost resistant hybrid [37, 38, 59]. The original hybrid (‘Holland greens’) was created by crossing Chinese cabbage (B. rapa ssp. pekinensis) and rapini (B. rapa ssp. rapifera), or ruvo cabbage, which sometimes is considered to be a turnip rape subspecies or to belong to the same species [59-62]. Since turnip rape (B. campestris) is also classified as B. rapa subspecies (ssp. oleifera), tyfon seems to be a triple intersubspecies hybrid. Tyfon, at a mass blooming phase, is shown in Fig. (9), plants in leaf rosette phase – in Fig. (10). Tyfon is not only a good oilseed crop, but also a good source of forage [32, 38, 59, 63]. Its seeds are able to germinate at +1-3 °С and they can tolerate -6 °С. Moreover, similarly to the winter turnip rape, tyfon can survive at -25-30 °С frosts under the snow. According to earlier reports [59], the life cycle of this hybrid (from seed to seed) is very long, approximately 280-300 days through winter, which may cause some difficulties for its cultivation.


The results of our tyfon productivity trials are provided in Table 5. Seed lipid content ranged from 40.9 to 45.1% and seed yields varied widely from the least productive genotype EOTFV with only 2394 kg of seeds per hectare, to the most productive variety Obriy 4124 kg/ha. Oil yields were also very different, with the highest amounts obtained for cv. Obriy with 1860 kg/ha, followed by Fitopal - 1730 kg/ha.
3.2. Fatty Acid Profiles of Seed Oil
One of the most important traits that play a crucial role in the quality of diesel biofuel is the fatty acid composition of plant oil used for its production [4, 14, 64-66]. Moreover, the fatty acid profile allows not only to predict the quality of the fuel, but it also predetermines oil usage for other technical purposes [16, 17, 22, 67]. Thus, we have conducted a chromatographic analysis of seed lipid samples of each of the above-mentioned plant genotypes.
3.2.1. Camelina
C. sativa oil composition is atypical if compared to the most Brassicaceae representatives that usually contain a very high amount of erucic (22:1) acid [17, 18, 67]. False flax oil contains only 1.28-2.37% of this acid. Erucic acid is considered an unwanted oil component, since in quantities over 5%, it could be potentially harmful to human health [19]. Taking into account this fact, all examined camelina genotypes oils are suitable not only for technical usage, but also for human food purposes.
The other important feature of false flax oil composition is a significantly high content of such polyunsaturated acids, such as linoleic (18:2) and linolenic (18:3) – up to 21.98% (FEORZhYaFChP) and up to 38.27% (FEORZhYaFD) respectively. These acids have relatively short chains that determine their high biofuel volatility. However, linoleic and linolenic acids have more than one double bond in the molecule structure, which reasons their low oxidizing stability. Consequently, such fuel cannot be stored for long.
High amounts of palmitic (16:0) acid (9.11-11.08%), as well as gondoic (20:1) acid - 9.53-12.91%, have also been detected in the test samples. Other fatty acids were present in minor quantities and are summarized in Table 6 as “Other Saturated Fatty Acids” (SFA) and “Other unsaturated fatty acids”(UFA).
| Breeding line or variety | Lipid content in seeds, % |
Seed yield, kg/ha |
Seed lipids yield, kg/ha |
|---|---|---|---|
| EOTFVS | 41.7±0.32c | 3376±36.12b | 1408±15.07c |
| EOTFV | 41.3±0.55c | 2394±25.38d | 989±10.48e |
| ‘Obriy’ | 45.1±0.35a | 4124±49.49a | 1860±22.32a |
| ‘Fitopal’ | 42.8±0.56b | 4042±58.61a | 1730±25.09b |
| ‘Orakam’ | 40.9±0.56c | 2914±28.27c | 1192±11.56d |
| Breeding line or variety | С16:0 | C18:1 | С18:2 | С18:3 | С20:1 | С22:1 | Other SFA | Other UFA |
|---|---|---|---|---|---|---|---|---|
| FEORZhYaF-1 | 9.41e | 16.72c | 20.09d | 34.07d | 10.78e | 1.47e | 4.21c | 2.76d |
| FEORZhYaF-2 | 9.53e | 18.47a | 20.03d | 32.5e | 12.5b | 1.55e | 2.58g | 2.84cd |
| FEORZhYaF-3 | 10.48c | 13.8f | 20.58c | 32.45e | 12.84a | 1.74d | 4.8a | 3.32b |
| FEORZhYaF-4 | 11.08a | 13.19g | 21.86a | 31.35f | 12.91a | 1.85c | 4.43c | 3.34b |
| FEORZhYaF-5 | 9.83d | 12h | 20.96b | 34.97c | 12.46b | 2.02b | 4.06d | 3.71a |
| FEORZhYaFD | 9.92d | 14.14e | 20.45c | 38.27a | 9.53f | 1.28f | 3.66e | 2.75d |
| FEORZhYaFCh | 10.77b | 13.22g | 21.62a | 33.11d | 11.74d | 1.77d | 4.34c | 3.43ab |
| FEORZhYaFChP | 9.11f | 15.28d | 21.98a | 32.86e | 11.42d | 2.37a | 3.89d | 3.1b |
| ‘Peremoha’ | 9.78d | 17.32b | 21.19b | 32.27e | 12.05c | 1.38e | 3.08f | 2.93c |
| ‘Evro-12’ | 9.66de | 13.05g | 19.76d | 35.56b | 11.65d | 1.86c | 4.7b | 3.76a |
3.2.2. Turnip Rape
The analyzed oil fatty acid compositions of spring and winter turnip rape (B. campestris) genotypes are presented in Tables 7 and 8. Significant differences have been identified within annual and biennial forms. The main difference resides in the content of dominating fatty acids, which differ significantly for winter and spring genotypes. In the case of winter forms, it was erucic (22:1) acid (from 38% in oil of EOSOFGl to 42.8% in ‘Oriana-1’), for spring forms – oleic (18:1) acid (from 43.54% in EOSYaFR to 46.92% in EOSYaF-2). The gondoic acid’s content varied from 11.1-12.77% in winter genotypes oil to 6.17-7.98% in spring turnip rape.
Polyunsaturated fatty acids are mainly represented by linoleic (18:2) and linolenic (18:3) acids. The other UFAs are present in minor or trace quantities only. Linolenic (18:3) acid content in winter genotypes was only 6.53-9.09% and 8.51-9.08% in spring genotypes. As for linoleic (18:2) acid, its value ranged from 11.88-14.34% in lipid samples of biennial plants, while its level for annual turnip rape was significantly higher - 19.62-21.17%. Such variations in seed oil fatty acid composition could be determined not only by genetic factors but also by the field cultivation conditions since this plant has different vegetation periods.
The differences observed in seed fatty acid profiles of winter and spring turnip rape have also been reported in earlier studies [17, 18, 68-70]. The content of erucic acid (22:1) for B. campestris f. biennis varied from 34.51-55.5%, while oleic (18:1) acid content reached only 9.56-27.4%. The opposite situation was observed for spring genotypes. Their seed oil contained oleic fatty acid (C18:1) at higher levels (29-32.5%) and erucic fatty acid (C22:1) in much lower quantities than in winter turnip rape – 23.5-29% [68-70]. Another spring variety (cultivar Tobin), which was bred as a special low erucic genotype, showed an extremely high level of oleic acid (58.8%) while the erucic acid (C22:1) level was very close to zero (0.3%) [17].
| Breeding line or variety | С16:0 | C18:1 | С18:2 | С18:3 | С20:1 | С22:1 | Other SFA | Other UFA |
|---|---|---|---|---|---|---|---|---|
| ‘Oriana’ | 2.53b | 20.28c | 11.88e | 6.53f | 12.77a | 42.63d | 3.56a | 0.95b |
| ‘Oriana-1’ | 2.08e | 17.71f | 12.94d | 8.85b | 11.57c | 42.8a | 3.38b | Tr |
| EOSOFU | 2.45c | 20.69b | 12.81d | 6.9e | 11.61c | 40.87b | 2.89d | 1.81a |
| EOSOFGl | 2.41c | 21.34a | 14.34a | 8.36c | 11.86b | 38d | 3.28c | Tr |
| EOSOFDn | 2.35d | 19.54d | 14.04b | 9.09a | 11.37c | 39.55c | 3.33b | Tr |
| EOSOFVol | 2.99a | 18.15e | 13.28c | 7.99d | 11.1d | 40.71b | 3.24c | 0.55c |
| Breeding line or variety | С16:0 | C18:1 | С18:2 | С18:3 | С20:1 | С22:1 | Other SFA | Other UFA |
|---|---|---|---|---|---|---|---|---|
| EOSYaF-1 | 3.83a | 46.15a | 21.07a | 9a | 6.17c | 11.21c | 2.78a | Tr |
| EOSYaF-2 | 3.25b | 46.92a | 20.16b | 9.08a | 6.82b | 11.11c | 1.92c | 0.62b |
| EOSYaFR | 3.19b | 43.54b | 19.62c | 8.51b | 7.98a | 13.77a | 2.57b | 0.82a |
| Pioner-YuT | 3.39ab | 44.28b | 21.17a | 9.07a | 6.68b | 11.74b | 2.69ab | Tr |
| Breeding line or variety | С16:0 | C18:1 | С18:2 | С18:3 | С20:1 | С22:1 | Other SFA | Other UFA |
|---|---|---|---|---|---|---|---|---|
| EORDOFL-2 | 5.49a | 33.86c | 16.93a | 11.94b | 9.85b | 16.33b | 3.87e | 2.44a |
| EORDOFL-3 | 5.16b | 33.84c | 15.93c | 12.74a | 9.87b | 16.7b | 3.86e | 2.53a |
| EORDOF-5 | 5.42a | 36.64b | 15.8c | 10.92d | 9.84b | 14.98c | 4.11d | 2.28b |
| EORDOF-6 | 5.59a | 37.14b | 16.51b | 10.73d | 9.62c | 13.81d | 3.99e | 2.28b |
| EORDOF-8 | 5.42a | 32.45a | 17.04a | 11.54c | 9.84b | 17.3a | 3.78f | 2.58a |
| ‘Rayduha’ | 5.59a | 37.89a | 14.14d | 10.07e | 10.36a | 15.48c | 4.37c | 2.12c |
| ‘Tambovchanka’ | 5.58a | 37.33ab | 16.92a | 10.54c | 9.32d | 13.06d | 5.13a | 2.1c |
| ‘Kyyanochka’ | 5.46a | 33.54c | 16.57b | 11.92b | 9.18d | 15.94c | 4.92b | 2.43a |
| Breeding line or variety | С16:0 | С18:1 | С18:2 | С18:3 | С20:1 | С22:1 | Other SFA | Other UFA |
|---|---|---|---|---|---|---|---|---|
| ‘Obriy’ | 2.15a | 18.08c | 13.56a | 7.46a | 11.05b | 42.28b | 3.26ab | 2.65a |
| ‘Orakam’ | 2.15a | 18.78b | 13.05b | 7.4a | 10.72c | 42.27b | 3.34a | 2.33b |
| ‘Fitopal’ | 2.23a | 18.71b | 13.26ab | 6.99b | 10.85c | 42.8a | 3.19ab | 2.5a |
| EOTFV | 2.23a | 19.92a | 13.35ab | 7.5a | 11.46a | 39.95d | 3.02b | 2.44ab |
| EOTFVS | 2.16a | 20.02a | 12.82b | 6.6b | 11.06b | 41.63c | 3.1b | 2.42ab |
3.2.3. Oil Radish
Fatty acid profiles of seed oil have been accomplished for eight oil radish (R. sativus var. oleifera) genotypes (Table 9). Within all analyzed samples, oleic (18:1) acid was found as the main component of oil (32.45-37.89%). The content of erucic (22:1) acid was estimated in the range from 13.06% (‘Tambovchanka’) to 17.3% (EORDOF-8). The level of linoleic (18:2) acid was very similar to the amount of erucic acid and varied from 14.14% (‘Rayduha’) up to 17.04% (EORDOF-8). Linolenic (18:3) acid’s portion amounted to 10.07-12.74%, while the level of gondoic (20:1) acid comprised 9.18-10.36%. The fraction of palmitic (16:0) acid in the seed oil of variety ‘Rayduha’ reached 5.59%. Other minor SFAs were detected from 3.86% to 5.13% in total and UFAs – up to 2.53%.
By considering data of the present study and those provided by previous publications on the subject, we note that fatty acid profiles of seed lipids of this crop demonstrate significant variations. For example, in the present study, erucic (22:1) acid content was determined within the range of 13.06-17.3%, which corresponds to the earlier published data of other authors – 16.37% [71] and 15.4% [72]. However, according to other studies, R. sativus var. oleifera seed oil may contain C22:1 from 1.2-16.3% [73] to 31.76% [58] and 33.3% [74]. Other fatty acid profiles also vary, but not so significantly, e.g., oleic (18:1) acid has been mostly detected within 27.9-34.53% range, although in some cases, it was only 4.5% [73]. In the present stud,y content of this acid has been identified in the range of 32.45-37.33%. Based on all findings mentioned above, general consideration is the same for most of the cases: oil of oilseed radish is not suitable for food production or human consumption due to the relatively high content of erucic acid [19], and can be used for industrial purposes only.
3.2.4. Tyfon (B. rapa Hybrid)
The seed lipid composition of 5 genotypes of hybrid tyfon is presented in Table 10. Comparison of fatty acid profiles between tyfon and winter turnip rape, one of its parental species, reveals high similarity. The high content of erucic (22:1) acid was also identified in cv. ‘Fitopal’ (up to 42.8%), while oleic (18:1) acid level ranged from 18.08% (‘Obriy’) to 20.02% (EOTFVS). As it was reported earlier, the fatty acid composition of tyfon is very similar to the fatty acid profiles of its parental genotypes, as they both are subspecies of B. rapa [38, 75].
Apart from that, the content of linolenic (18:3) acid in tyfon genotypes is lower than in turnip rape, in which it reaches up to 9.09%. The overall level of other UFAs comprises not more than 2.65%. Conversely, UFAs are lower in B. campestris f. biennis. Finally, gondoic (20:1) acid was present in all tyfon genotypes in the amount from 10.72 to 11.46 %.
3.3. General Comparison of the Suitability of Studied Plant Genotypes for Biodiesel Production
Properties and, consequently, the quality of biodiesel, are directly determined by the fatty acid composition of the plant feedstocks. In practical terms, it means presence/absence and proportions of particular fatty acids. It is well known that the carbohydrate chain length of a fatty acid molecule determines its melting point and ignition temperature as well as the products of the transesterification [1, 4, 12, 13, 76]. Additionally, the presence of a double bond in the fatty acid molecule increases compound volatility, but decreases oxidative stability [3]. In general, the ratio between different fatty acids’ amounts determines properties of oil and properties of the end-product, such as biodiesel, lubricant material, etc [21, 22, 67]. Since different structural characteristics of fatty acids (length of carbon chains and the number of double bonds) have a determining impact on biodiesel quality, we suggest to evaluate oil composition profiles by comparing total values of such groups of fatty acids [4, 67, 76].
Thus, based on the obtained data on the seed oil fatty acid composition, the levels of each main fatty acid category were calculated for all studied genotypes: MUFA, PUFA, SFA plus separate classification by fatty acid chain length: short-chained (C18 and less or SCFA) and long-chained (C18 and more or LCFA). All these features are presented in Table 11, while the average amounts of appropriate fatty acid class for each species are illustrated in Fig. (11).
| Species | Breeding line or variety | MUFA | PUFA | SFA | SCFA | LCFA |
|---|---|---|---|---|---|---|
| Camelina | FEORZhYaF-1 | 29.26h | 56.62c | 13.62b | 83.17b | 16.33g |
| FEORZhYaF-2 | 32.89g | 54.99d | 12.12c | 82.61bc | 17.39fg | |
| FEORZhYaF-3 | 28.81h | 55.91cd | 15.28a | 80.83c | 19.17f | |
| FEORZhYaF-4 | 28.35h | 56.14cd | 15.51a | 80.85c | 19.15f | |
| FEORZhYaF-5 | 26.85hi | 59.26b | 13.89b | 80.56c | 19.44f | |
| FEORZhYaFD | 25.21i | 61.21a | 13.58b | 85.37a | 14.64h | |
| FEORZhYaFCh | 27.15hi | 57.74c | 15.11a | 81.96bc | 18.04fg | |
| FEORZhYaFChP | 29.43h | 57.58c | 12.99d | 81.56bc | 18.44fg | |
| ‘Peremoha’ | 31.06gh | 56.08cd | 12.86d | 82.81bc | 17.19fg | |
| ‘Evro-12’ | 26.94hi | 58.7bc | 14.36ab | 81.21bc | 18.79fg | |
| Mean | 28.6±2.24 | 57.42±1.88 | 13.93±1.13 | 82.09±1.46 | 17.86±1.51 | |
| Winter turnip rape | ‘Oriana’ | 75.68a | 19.36j | 6.09g | 42.23h | 58.9a |
| ‘Oriana-1’ | 72.08c | 21.79i | 5.46h | 42.48h | 56.85a | |
| EOSOFU | 73.17bc | 21.52i | 5.34h | 43.82h | 56.21a | |
| EOSOFGl | 71.2c | 22.7i | 5.69h | 47.41g | 52.18b | |
| EOSOFDn | 70.46c | 23.13hi | 5.68h | 45.99gh | 53.28ab | |
| EOSOFVol | 69.96d | 21.82i | 6.23g | 43.32h | 54.69ab | |
| Mean | 72.09±2.1 | 21.72±1.31 | 5.75±0.35 | 44.21±2.06 | 55.35±2.46 | |
| Spring turnip rape | EOSYaF-1 | 63.53e | 30.07ef | 6.61g | 81.54bc | 18.67fg |
| EOSYaF-2 | 64.85e | 29.86ef | 5.17h | 80.72c | 19.16f | |
| EOSYaFR | 65.29e | 28.95f | 5.76h | 76.17d | 23.83e | |
| Pioner-YuT | 62.7ef | 30.24e | 6.08g | 79.3c | 19.72f | |
| Mean | 64.09±1.19 | 29.78±0.57 | 5.9±0.6 | 79.4±2.36 | 20.35±2.36 | |
| Oil radish | EORDOFL-2 | 61.85ef | 29.5ef | 9.36f | 70.59ef | 30.12c |
| EORDOFL-3 | 62.28ef | 29.33ef | 9.02f | 70.02f | 30.61c | |
| EORDOF-5 | 63.17ef | 27.29g | 9.53ef | 71.28ef | 28.71cd | |
| EORDOF-6 | 62.29ef | 27.8fg | 9.58ef | 72.46e | 27.21d | |
| EORDOF-8 | 61.49f | 29.26f | 9.2f | 68.72f | 31.23c | |
| ‘Rayduha’ | 65.36e | 24.7h | 9.96e | 70.31f | 29.71c | |
| ‘Tambovchanka’ | 61.25ef | 28.02fg | 10.71e | 73.49e | 26.49d | |
| ‘Kyyanochka’ | 60.45f | 29.13ef | 10.38e | 70.34f | 29.62c | |
| Mean | 62.27±1.49 | 28.13±1.61 | 9.72±0.59 | 70.9±1.49 | 29.21±1.64 | |
| Tyfon | ‘Obriy’ | 72.85bc | 22.23i | 5.41h | 42.54h | 57.95a |
| ‘Orakam’ | 73.06abc | 21.49i | 5.49h | 42.65h | 57.39a | |
| ‘Fitopal’ | 73.8abc | 21.31i | 5.42h | 42.49h | 58.04a | |
| EOTFV | 72.76c | 21.86i | 5.25h | 44.26gh | 55.61ab | |
| EOTFVS | 74.15abc | 20.4i | 5.26h | 42.9h | 56.91a | |
| Mean | 73.32±0.62 | 21.46±0.69 | 5.37±0.11 | 42.97±0.74 | 57.18±0.99 |
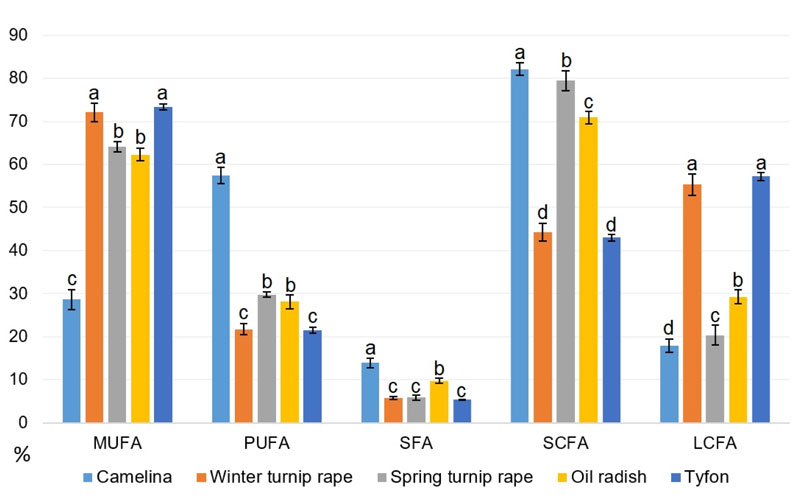
Our data in Fig. (11) show that tyfon and winter turnip rape fatty acid patterns are rather close, they both possess the highest values of long-chained fatty acids comparing to other species. The highest proportion of short-chained fatty acids has been identified in camelina and spring turnip rape oils, while false flax possesses at least two-fold the amount of PUFA comparing to other studied species. It is worth to note that camelina has the highest content of saturated fatty acids (SFA) probably because of the highest content (up to 11.08%) of palmitic (16:0) acid. The highest MUFA levels were found in tyfon and winter turnip rape genotypes.
Practically it is possible to obtain biodiesel via transesterification from the majority of plant oils and other lipid sources, e.g., animal fats [77]. The problem is that not all such ‘fuels’ are suitable for usage in engines. For normal engine performance, obtained biodiesel must correspond to the same technical requirements as traditional mineral diesel [1, 78, 79]. Besides, fatty acid esters usually demonstrate higher density and viscosity at low temperatures, which cause additional restrictions to the use of such fuel under cold weather conditions [76, 78, 80].
Since the goal of this investigation is to obtain FAEE using bioethanol instead of more toxic methanol, it poses stricter limitations for plant oil source, because FAEEs are less volatile than FAMEs [76, 81]. Therefore, feedstock for biodiesel production should contain a significantly higher proportion of SCFAs than LCFAs. At the same time, fatty acids should be unsaturated, preferably monounsaturated, although polyunsaturated fatty acids could also be suitable, as they do not negatively affect the storage time [1, 4, 80].
The problem of low oxidative stability could potentially be bypassed by creating special storage conditions, by mixing the biofuel of interest with fuel from other feedstock or by performing on some production stage oil hydroprocessing to saturate double bonds with H2 [24, 26, 82]. However, the latter approach has not been well elaborated yet. Consequently, all these approaches, especially the last one, can lead to price increase and cause other unfavourable market conditions for the biofuel.
Oil feedstocks with high levels of LCFAs could also be used for biodiesel production, but, as it has been mentioned above, such fuel would have lower volatility at low temperatures and, as a consequence, lead to worse performance of the engine.
The other useful application for such seed lipids is the production of lubricant materials. In such a case, the presence of long-chained fatty acids is even desirable, but on the condition that the majority of fatty acids are monounsaturated; in an ideal situation, it could be just one dominating MUFA, e.g., erucic (22:1) acid [67, 76]. Moreover, the content of polyunsaturated fatty acids should be as low as possible, since oxidative stability is crucial for lubricants which are often exposed to high temperatures for a long time [22]. Having taken into account all the above-mentioned requirements, we decided to analyze particular genotypes of each studied species by comparing MUFA, PUFA, SFA, SCFA, LCFA values, given in Table 11.
Having considered the data in Fig. (11), it could be concluded that winter turnip rape and hybrid tyfon with dominating ‘heavy’ fatty acids and the highest MUFA content have better prospects for usage as lubricant feedstock. False flax, spring turnip rape and oil radish that contain high proportions of SCFAs could be a good oil source for biodiesel production, especially due to the high amounts of MUFA contained in spring turnip rape and oil radish. At the same time, the main disadvantage of B. campestris f. annua is its rather low productivity – only 737-998 kg of seed oil per hectare that makes its industrial cultivation financially unprofitable. Probably, further breeding of spring turnip rape with higher productivity crops could eliminate this obstacle.
In general, camelina seed lipids contain a large amount of PUFA, which can pose some difficulties for fuel storage. Low oxidation stability of C. sativa derived biodiesel can be improved by adding synthetic antioxidants, like butylated hydroxyanisole (BHA), 2,6-di-tert-butyl-4-methylphenol (BHT), propyl gallate (PrG) and tert-butylhydroquinone (TBHQ). It was shown that these antioxidants make a positive impact on fuel storage time when they are added to camelina-derived biodiesel in the amount of 500-3000 ppm [83]. On the other hand, oil of this crop is characterized by the highest content of short-chained FAs, including one at the highest level, that is C16:0, amongst the studied species
Industrial crop productivity of camelina is high enough compared to other studied crops, so it could be a very promising feedstock for biodiesel production. In addition, great attention of researchers to this crop creates more possibilities for further improvement of false flax using not only classical breeding approaches, but also by applying novel molecular genetic techniques [15, 50]. In particular, FEORZhYaFD can be considered as the most promising C. sativa genotype from the studied collection due to the highest content of short-chained fatty acids (85.37%), low amount of SFA (13.58%) and high crop productivity (1426 kg of oil per ha), as well as the highest oil content in seeds among all studied false flax genotypes – 42.6%. It was shown recently [84] that it was possible to increase the content of short-chained and very-short-chained (C10-C14) fatty acids in camelina oil by introducing seed-specific transgenes from Cuphea species to C. sativa and by RNAi silencing of genes for acyl-acyl carrier protein synthases. These modifications made it possible to produce false flax lines that were able to accumulate up to 28 mol% of C8:0, C10:0, C12:0, C14:0, with dominating capric (C10:0) acid – up to 25 mol% of total fatty acid content. Oil of such modified camelina could be easily converted either to biojet fuel or to biodiesel (probably with high volatility) [84].
Oil radish variety ‘Rayduha’ seems to be one of the most promising options for biofuel production due to rather favourable features, such as high crop productivity (3120 kg of seeds per ha), high SCFA content (70.31%), the lowest content of PUFA (24.7%) and the highest value of MUFA (65.36%) within all studied R. sativus var. oleifera genotypes.
Since winter turnip rape and tyfon have a significant amount of LCFA, they meet the criteria for lubricant feedstock. Variety ‘Oriana’ (B. campestris f. biennis) has a good potential as an oil source due to high crop productivity (1373 kg of oil per ha), the highest MUFA content (75.68%) and the lowest level of PUFA (19.36%) among all studied winter turnip rape genotypes. The same conclusion could be made for tyfon variety ‘Fitopal’ that produces high oil yield (1730 kg/ha) and contains 73.8% of MUFA.
This allows to conclude that the most appropriate genotypes for further biodiesel production tests are C. sativa – FEORZhYaFD; R. sativus var. oleifera - ‘Rayduha’; B. campestris f. biennis - ‘Oriana’; tyfon hybrid - ‘Fitopal’.
3.4. Biodiesel Production and Characterization
All our experiments were conducted using a pilot facility, which works according to the scheme illustrated in Fig. (12), except for procedures of glycerol purification and alcohol reuse, which were not included in our experiment, but suggested here as potential steps to be introduced on an industrial scale.
Presently, oil transesterifìcation (or reesterification), which occurs with alcohol (methanol, ethanol, etc.) in the presence of a catalyst, solvent or under supercritical conditions, represents the main technology for biodiesel production [4, 77, 78, 85]. The general scheme of this process and reaction formula are provided in Figs. (1 and 2).
There are two main technological approaches to obtaining biodiesel via transesterification. The first one, traditional, is based on a reaction induced by adding the catalyst (usually alkali or base catalyst) into a mixture of oil with alcohol in a molar ratio from 1:4 – 1:20. Such type of transesterification occurs at the temperatures below the methanol boiling point of 64.5oC, if this alcohol is used. The reaction does not require extra pressure. The other methodology does not require a catalyst but involves other agents to facilitate the reaction: either the addition of a solvent or the creation of supercritical conditions. One of the most widely used solvents for reesterification is tetrahydrofuran, which is added being dissolved in alcohol. In this case, the reaction lasts for 5-10 min at +30 oC. The disadvantages of such a method are: increased cost of the end-product, high-flammability and volatility of tetrahydrofuran and, consequently, the requirement of special hermetic sealed equipment to work with. Another non-catalyst approach is based on creating supercritical conditions, that include significantly high temperatures (up to 350-400 oC), high pressure (up to 80 atm.) and usually high molar ratio of alcohol added to the oil (42:1) [1, 4, 5, 76, 86].
There are also enzymatic transesterification approaches which involve lipases of different origin instead of a catalyst [87, 88]. Alternatively, a novel method of biodiesel (biojet fuel) production, which is currently progressing very actively, is based on hydroprocessing (hydrocracking) of plant oils to obtain mainly n-alkanes suitable for fuel in jet engines [24, 26, 82]. According to a recent analysis, camelina-derived biojet fuel could provide up to 800 million gallons of fuel per year in the USA, which would potentially reduce greenhouse gas emission to 67-80% of its current level, compared with mineral oil-based jet fuel [89].
| Testing criteria | Ukrainian standard | C. sativa | R. sativus var. oleifera | B. campestris | Tyfon |
|---|---|---|---|---|---|
| Cetane number | ≥48 | 48 | 48 | 46 | 46 |
| Sulfur content, % | ≤0.05 | 0.02 | 0.015 | 0.02 | 0.02 |
| Presence of hydrogen sulfide | Absent | Absent | Absent | Absent | Absent |
| Copper plate testing | Passed | Passed | Passed | Passed | Passed |
| Acidity (KOH mg per 100 cm3 (0.1 L) of fuel) | ≤5 | 4.4 | 4.0 | 4.0 | 4.0 |
| Ash content, % | ≤0.01 | 0.006 | 0.005 | 0.006 | 0.006 |
| Esters yield | Not regulated | 87% | 86% | 85% | 86% |
The ‘classic’ technologies for transesterification are still in demand due to their simplicity in implementation and a relatively predictable economic effect [85]. Thus, our study has been mostly focused on the catalytic reesterification of plant lipids, especially on FAEE production based on bioethanol. Currently, FAME is a common type of biodiesel, since the methanol-based reaction is faster, compared with ethanol-based, and it produces shorter chain ester from the same fatty acid, than ethanol or another alcohol, such as butanol, etc [4, 76]. Methanol is known to have high toxicity for the human body and could easily form highly-explosive mixtures with air. In addition, the possibility of diesel-biodiesel-alcohol blends is also discussed nowadays [4].
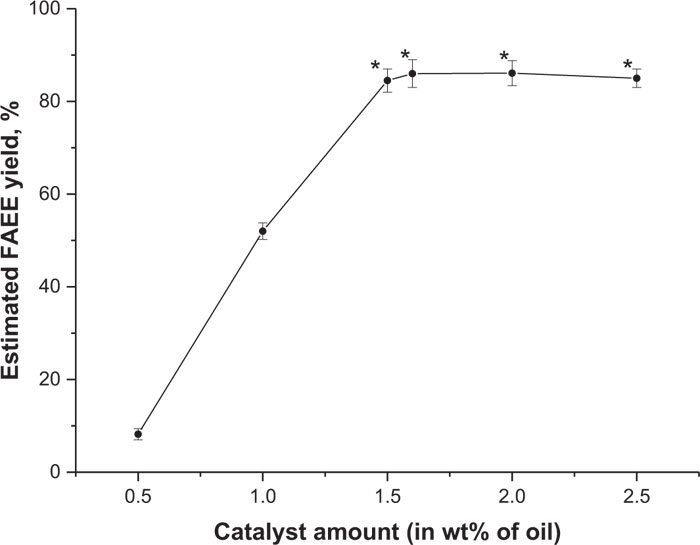
In our experiments, the first reaction type was based on NaOH usage as a catalyst (described in Section 2.6). Camelina oil (breeding line FEORZhYaFD) was used in the series of experiments to find out the optimal amount of catalyst that would allow obtaining maximal FAEE output. False flax was chosen as a model oil feedstock object, because the procedure for biodiesel production from its oil is well described and its fatty acid composition is very close to other common biofuel crop – soybean [90]. In each experiment, different quantities of NaOH (0.5, 1, 1.5, 1.6, 2 and 2.5% w/w of plant oil) were dissolved in ethyl alcohol, which was taken in a 6:1 ratio to plant oil. The curve of conversion rate was built based on the obtained results of these tests (Fig. 13). Fatty acid esters yield increased rapidly from 8.2% to 84.5%, following the increase of the catalyst amount up to 1.5%. The highest conversion rate reached was 87%, with 1.6% of the catalyst and started to decrease to an average of 85% of esters with 2.5% of NaOH.
In a subsequent step of our investigation, biodiesel samples were produced from oil of variety ‘Rayduha’ (R. sativus var. oleifera), v. ‘Oriana’ (B. campestris f. biennis) and v. ‘Fitopal’(tyfon). To do that, transesterification was carried out under the conditions that were considered optimal (1.5-1.6% w/w of NaOH) according to the results of the previous experiment. The obtained fuel samples were further examined for compliance with the requirements of the Ukrainian diesel fuel standards (Table 12). The highest conversion rate was registered for C. sativa oil – 87% w/w, while the lowest one was determined for winter B. campestris oil – 85%. The obtained fatty acid esters fit almost all main requirements of the Ukrainian standards, except for biodiesel samples produced from B. campestris and hybrid tyfon seed lipids, which had a lower cetane number than required: 46 vs 48. Other studies devoted to the production of biodiesel from the oil of R. sativus var. oleifera (FAMEs) reported that their obtained fuel samples had high acidity regardless of the previously mentioned FA profiles peculiarities [71, 74, 91]. In these papers, carbon residue amounted to 0.01-0.4% w/w levels, while in the present study, the ash content was only 0.015% w/w. Data on esters yield was reported in the other study [57], where it equaled 86% w/w (FAMEs), similar to the value in the present study, but for FAEE.
It was shown in the case of B. campestris oil that it was possible to reach 97% yield of FAME, if biodiesel is produced under the following conditions: 9:1 methanol to oil ratio, 1 wt% of NaOH as a catalyst and 75oC reaction temperature, that is above the boiling temperature of this alcohol [92]. Authors of this research also mentioned that such fuel might fit all the ASTM D6751-15a requirements. Lately, it was established that it was possible to produce only 81% of biodiesel (also methyl esters-based) from turnip rape oil. On the other hand, this experiment has been carried out under different conditions (alcohol oil ratio – 6:1, 70 oC, unknown catalyst amount) that probably determined the decreased esters yield [93]. Alternatively, as reported earlier [94], the use of 2.5% w/w of sodium methoxide as a catalyst enabled to reach 84% of FAMEs yield from B. campestris oil. Authors of that study also identified that the B50 blend of that fuel had only 44 cetane number, while B100 showed it at a higher level – 53. Therefore, based on the described findings and data, presented in Table 12, we conclude that the technology of biodiesel production (either FAME or FAEE) from turnip rape oil requires further research and improvement.
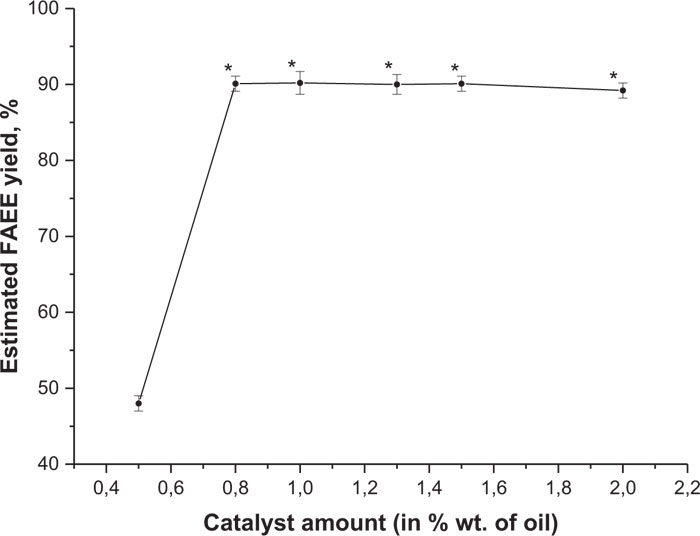
The second reaction type was based on using sodium ethoxide (C2H5ONa) as a catalyst which was added in quantities of 0.5%, 0.8%, 1%, 1.3%, 1.5% and 2% w/w of plant oil weight. The general design of these experiments remained the same as it was described above for the other catalyst (NaOH). The mean values of the conversion rate of camelina seed lipids to FAEE with different catalyst amount are provided in Fig. (14). Ester yield at 0.5% w/w of the catalyst was very low, approximately 48%, but by adding a higher sodium ethoxide amount (0.8% w/w), it increased rapidly up to 90.1%. The higher conversion rate (avrg. 90.2%) was obtained with 1% w/w of the catalyst, which is not proved to be significantly different from the previous (0.8% w/w) sample. With more sodium ethoxide esters, the yield slowly decreased.
| Testing criteria | Ukrainian standard | C. sativa | R. sativus var. oleifera | B. campestris | Tyfon | |
|---|---|---|---|---|---|---|
| Cetane number | ≥48 | 52 | 51 | 49 | 49 | |
| Sulfur content, % | ≤0.05 | 0.02 | 0.02 | 0.02 | 0.02 | |
| Presence of hydrogen sulfide | Absent | Absent | Absent | Absent | Absent | |
| Copper plate corrosion test | Passed | Passed | Passed | Passed | Passed | |
| Acidity (KOH mg per 100 cm3 (0.1 L) of fuel) | ≤5 | 3.9 | 3.7 | 3.6 | 3.6 | |
| Ash content, % | ≤ 0.01 | 0.006 | 0.005 | 0.007 | 0.005 | |
| Esters yield, % | Not regulated | 91% | 90.5% | 89.7% | 89.5% | |
The following fuel samples were produced using oil of another studied oilseed species under the reaction conditions identified as optimal (0.8% w/w of C2H5ONa). The biodiesel derived from all these oil sources was tested to comply with the main requirements of the Ukrainian standard for diesel (Table 13). It is important to note that the cetane number increased up to 52 for the false flax sample (which at the same time possessed the highest acidity). The lowest cetane number was registered for winter B. campestris and tyfon samples. In general, the quality of the biodiesel samples, compared to the previous method, has improved. It is important to note that in both experiments, camelina oil-based biodiesel samples possessed the highest acidic value due to the formation of the highest amount of PUFA ethyl esters. This leads to low oxidation stability that negatively impacts the fuel storage lifespan. As it was reported earlier, camelina biodiesel shows the poorest oxidative stability if compared with fuel from other common feedstocks (e.g., soybean, palm, sunflower, etc.) [90]. Apart from that, other advantages of false flax-derived biodiesel make it still very attractive as a fuel for engines.
In other studies, it was shown that camelina biodiesel (FAMEs) possessed 42.7-52.8 cetane number [46, 51, 95, 96], which is consistent with our results. Different standards require various minimal values of this parameter. The strictest limit is described in EN 14214 2012+A1:2014, that does not accept cetane number below 51, while ASTM D6751-15a sets this limit at 47. Ukrainian diesel fuel standard DSTU 7688:2015, which is based on both these documents, defines the cetane number limit as ‘not lower than 48’, to which most of the produced biodiesel samples comply with (Tables 12 and 13).
3.5. Improvement of FAEE Obtaining Technology
3.5.1. Solvent Input to Reaction Mass
The two main factors that negatively impact the efficiency of ethanol-based biodiesel production are the very high esters yields and the longer reaction time, 1.5 h for FAEE production vs 0.5 h for FAME. For this reason, we have conducted several experiments to evaluate possible approaches to improve the existing FAEE production technology. Hence, the methodology, described earlier for sunflower oil transesterification [40], was adopted for camelina oil conversion. The addition of mineral diesel as a solvent to plant oil can increase the conversion rate and reduce the reaction time. In the case of sunflower oil transesterification, the best results were obtained when oil and diesel were mixed in proportion 4:1 [40]. Based on these data, such a ratio was used in our experiments.
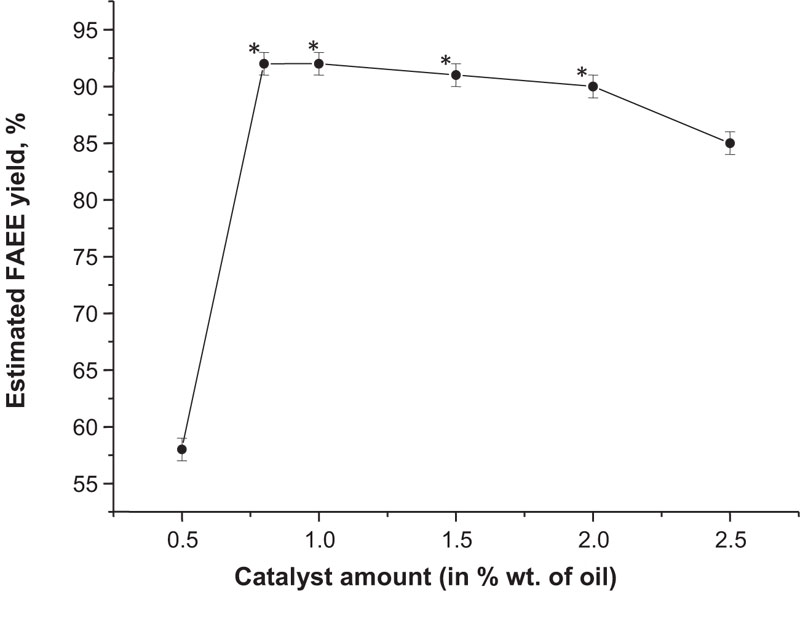
The productivity test was conducted using the same facilities as previously reported, therefore the temperature and pressure conditions remained unchanged. In addition, oil and bioethanol proportion remained the same (1:6 mol.) and NaOH was used as a catalyst. Preliminary, plant oil was mixed with mineral diesel at 4:1 w/w ratio. Camelina oil was chosen for further tests because, in previous experiments, its biodiesel had shown the best quality. Additional measurements were conducted on different amounts of sodium hydroxide to find out the best conditions for the FAEE yield (Fig. 15). In the first type of reesterification reaction, the most effective biodiesel production was reached at 1.6% w/w of the catalyst (Fig. 13), but in this case, a similar amount of NaOH (1.5% w/w) resulted in the practically same performance (91% conversion rate) compared to its lower concentrations (0.8% and 1.0%) which allowed to obtain up to 92% of FAEE. These findings suggest that a lower amount of catalyst is required to reach the highest efficiency, if solvent-based technology is applied.
The other approach, described originally previously [40], is based on the use of anhydrous polyoxyethylene (20) sorbitan monooleate (Tween 80, Polysorbate 80). This compound is highly soluble in water, lipids and ethanol, but insoluble in mineral oils [97]. For this reason, we conducted other test reactions with the same reagents as previously, but with Polysorbate 80 presence at 0.5% w/w concentration to plant oil. This procedure allowed to reduce the reaction time to 30 min, increase esters yield to 96% and generally, a better phase separation was observed. The obtained samples were evaluated for compliance with the Ukrainian standards (Table 14).
Another study was aimed to test engine performance with camelina-derived FAMEs. The test showed that the use of 100% biodiesel decreases engine power by 14.39% compared with mineral diesel. B7 fuel (7%-additive of biodiesel to
| Testing criteria | Ukrainian standard | 4:1 oil and diesel mixture | Same mixture with Polysorbate 80 |
|---|---|---|---|
| Cetane number | ≥48 | 54 | 54 |
| Sulfur content, % | ≤0.05 | 0.02 | 0.02 |
| Presence of hydrogen sulfide | Absent | Absent | Absent |
| Copper plate testing | Passed | Passed | Passed |
| Acidity (KOH mg per 100 cm3 (0.1 L) of fuel) | ≤5 | 3.8 | 3.8 |
| Ash content, % | ≤0.01 | 0.005 | 0.005 |
| Esters yield, % | Not regulated | 92% | 96% |
regular fuel), which is approved to be used by EU standards, decreases engine power by 4.97%. It was also detected that FAME burning generated about 20% more CO2 than regular diesel because of more complete biodiesel oxidation, since FA methyl esters contain oxygen in their molecules, which provides better combustion. Besides, fuel consumption increased by 12.21% with B7 compared with mineral diesel [98]. It was also reported that the test of oil radish methyl esters-based biodiesel revealed only a 20% increase in fuel consumption for B100, while this rate for B20 was similar to the one for mineral diesel [58].
None of such tests were performed for camelina FAEE. Studies on comparison of FAME and FAEE, produced from soybean oil, which also contained C18-fatty acids, have revealed that the FAEE-driven engine showed a lower fuel consumption rate than the FAME-driven engine. In addition, soybean FAEE burning showed less NOx emission than FAMEs, but still higher than regular diesel [99, 100]. Since FAEE has a higher calorific value than FAME, which is closer to mineral diesel [86], ethyl esters-based biodiesel could show better engine performance than methyl ester-based, especially in fuel consumption rates and engine power. Besides, a comparison of different FAME and FAEE (derived from camelina) blends with mineral diesel, revealed that ethyl esters-based fuel possessed higher lubricity, which can also have a positive impact on the engine [95].
3.5.2. Transesterification using Complex Catalysts
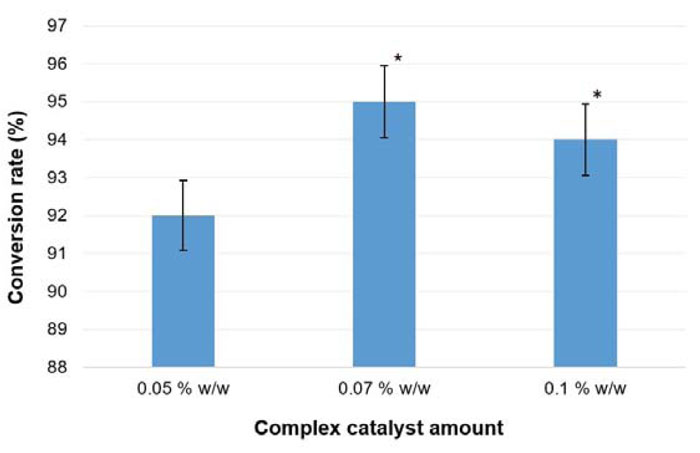
The alternative approach tested for oil with long-chained fatty acids (e.g., winter turnip rape oil) transesterification included the use of a complex catalyst. This technology is described in a study [41], where sodium ethoxide and monoethanolamine (3:1 w/w) mixture was proposed as a complex catalyst. Previously, it was shown that the addition of amines had a positive impact on the efficiency of biofuel production [101]. In this experimental reaction, the conditions were kept as described in section 3.4 (except for the catalyst). To find out optimal conditions of biodiesel production, the complex catalyst was added in different amounts: 0.05%, 0.07% and 0.1% w/w of the plant oil weight. The FAEE conversion rate for each tested production variant is shown in Fig. (16).
The obtained biodiesel samples were tested to comply with the major requirements of the Ukrainian standard for diesel (Table 15). It was established that the cetane number of produced fuel increased up to 51.5, comparing with the previous testing, where it reached only 49 (B. campestris column in Table 14). In the current experiments, the conversion rate reached 95% (at 0.07% w/w complex catalyst presence), which is lower compared with the Tween-80 method results (96%). Furthermore, the reaction time was reduced. Transesterification with the complex catalyst lasted for 1 h, while for a single-catalyst approach, 1.5 h was needed. Besides, the solvent-based approach allowed to reduce the reaction time more significantly – down to 0.5 h.
FAEE produced from oil of winter turnip rape had a lower acidity comparing with biodiesel, obtained from camelina seed lipids. This could be explained by a lower level of PUFA in B. campestris oil. Fuel acidity is tightly connected to the oxidative stability of biodiesel, which makes a significant impact on the storage stability of the esters. The present study focused on rapeseed biodiesel storage demonstrated that FAEEs were slightly more stable than FAMEs. Fuel acidity for both types of esters had been increasing over the 24 month period of the study. After the 2-year storage, FAME and FAEE biodiesel showed insignificant differences in engine performance, compared with fresh fuel samples [102]. The obtained results make it clear that FAMEs have no preferences over FAEEs in terms of fuel storage stability.
| Testing criteria | Ukrainian standard | 0.05% w/w | 0.07% w/w | 0.1% w/w |
|---|---|---|---|---|
| Cetane number | ≥ 48 | 51 | 50 | 51.5 |
| Sulfur content, % | ≤ 0.05 | 0.02 | 0.02 | 0.02 |
| Presence of hydrogen sulfide | Absent | Absent | Absent | Absent |
| Copper plate testing | Passed | Passed | Passed | Passed |
| Acidity (KOH mg per 100 cm3 (0.1 L) of fuel) | ≤ 5 | 3.5 | 3.6 | 3.8 |
| Ash content, % | ≤ 0.01 | 0.007 | 0.006 | 0.007 |
| Esters yield, % | Not regulated | 92% | 95% | 94% |
Improvement of every step of the biodiesel production process will help to improve the whole technology. For instance, modeling of physical processes that are carried out could give insight into solutions, which will help to optimize this technology. One of such studies [103] offered a thermodynamic model that could predict liquid–liquid equilibrium of the system consisting of water, ethyl-biodiesel (FAEE) and ethanol. As one of the examples of FAEE-fuel, authors have chosen oil radish-based biodiesel. Such data could be applied for the improvement of the washing step that takes place during biodiesel production. Another example of such modeling is research devoted to statistical optimization and kinetic study of biodiesel production also from R. sativus [104]. As a result of that research, the authors established optimal conditions for catalytic production of FAMEs from the oil of radish, these were: 50oC, 9:1 methanol:oil molar ratio, wt% to % w/w of catalyst (NaOH) and 30 min reaction time. Those conditions theoretically should allow obtaining up to 94.58 wt% to % w/w of methyl esters, which would meet ASTM D6751-15a requirements. In addition, there are various ways for biodiesel quality improvement via adding compounds that may enhance oxidative stability, cetane number, lubricity, combustion properties, etc [105]. The use of nanocatalysts for efficient and sustainable biofuel production has recently been piloted and published [106]. It is an interesting and promising field of study that may have good prospects for practical application in the future. In the present study, both solvent and complex catalyst methods have demonstrated good performance and have improved reaction efficiency. Nevertheless, the implementation of both methods at an industrial scale of production requires additional steps of esters purification from solvent or catalyst residues, which will inevitably and negatively affect the economic feasibility of the whole technology. A possibility of combining both proposed approaches is also under consideration.
CONCLUSION
Seed oil content, productivity and fatty acid profiles have been analyzed on different genotypes of camelina (C. sativa), winter and spring turnip rape (B. campestis), oil radish (R. sativus var. oleifera) and tyfon (hybrid winter oil turnip), bred in Ukraine. The most productive genotypes have been identified for each species: camelina varieties ‘Evro-12’ and ‘Peremoha’; winter turnip rape variety ‘Oriana’, oil radish variety ‘Kyyanochka’ and tyfon varieties ‘Fitopal’ and ‘Obriy’. Tyfon and winter turnip rape had very similar seed lipid profiles with the highest amounts of long-chained and monounsaturated fatty acids. The largest short-chained fatty acids levels were identified in camelina and spring turnip rape oil samples. Camelina seed oil possessed the highest level of polyunsaturated fatty acids.
We have selected the most promising genotypes that could potentially be biodiesel raw oil source due to their desirable fatty acid profiles and high productivity: breeding form FEORZhYaFD (C. sativa), variety ‘Rayduha’ (R. sativus var. oleifera), variety ‘Oriana’ (B. campestris f. biennis) and variety ‘Fitopal’ (tyfon). Fatty acid ethyl esters (FAEE) were produced from oil of chosen genotypes by transesterification using two different types of catalyst (sodium hydroxide and sodium ethoxide) with bioethanol as the essential alcohol.
Two additional approaches, based on solvent addition and complex catalyst usage, have been tested to improve FAEE production efficiency. Camelina (genotype FEORZhYaFD) oil was used to test the solvent-based process, involving preliminary mixing of mineral diesel with plant oil. Another method of FAEE production involved the usage of the complex catalyst (sodium ethoxide and monoethanolamine in 3:1 w/w ratio) instead of a single compound. Implementation of both proposed approaches showed increased esters production efficiency and reduction of the reaction time. Finally, the quality of obtained fuel samples showed full compliance with the Ukrainian diesel fuel standard, which suggests that these types of biodiesel can be considered suitable for diesel engines.
LIST OF ABBREVIATIONS
| SFA | = Saturated Fatty Acids |
| UFA | = Unsaturated Fatty Acids |
| MUFA | = Monounsaturated Fatty Acids |
| PUFA | = Polyunsaturated Fatty Acids |
| SCFA | = Short-Chained Fatty Acids |
| LCFA | = Long-Chained Fatty Acids |
| FAME | = Fatty Acid Methyl Esters |
| FAEE | = Fatty Acids Ethyl Esters |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of this study are available within the article.
FUNDING
This work was supported by the project (No. 0115U001644) of the Program on Problems of Sustainable Development, Rational Environmental Management and Environmental Conservation (2015-2019) of the National Academy of Sciences of Ukraine.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We acknowledge Dr. V. Kyrylenko (Institute of Food Biotechnology and Genomics, National Academy of Sciences of Ukraine, Kyiv) for giving suggestions aimed to improve the manuscript language.


