RESEARCH ARTICLE
Identification and Biological Properties of the Pathogen of Soft Rot of Tomatoes in the Greenhouse
Yuliia Kolomiiets1, Ivan Grygoryuk1, Lyudmila Butsenko2, Vita Bohoslavets1, Yaroslav Blume1, 3, Alla Yemets1, 3, *
Article Information
Identifiers and Pagination:
Year: 2020Volume: 14
First Page: 290
Last Page: 298
Publisher ID: TOASJ-14-290
DOI: 10.2174/1874331502014010290
Article History:
Received Date: 07/08/2020Revision Received Date: 09/09/2020
Acceptance Date: 17/09/2020
Electronic publication date: 15/12/2020
Collection year: 2020

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Recently, in the greenhouses of Ukraine, a sharp increase in the infestation of tomato plants with soft (wet) rot has been registered.
Purpose:
To identify the pathogen of the soft rot, study its biological properties and develop practical recommendations for plant protection measures.
Methods:
The work was performed using classical microbiological and biochemical research methods. The API 20E (Bio Merieux) test system was used to study the physiological and biochemical properties of the bacteria. The study of bacterial fatty acids was carried out by mass-spectrometry.
Results:
Using a bacteriological analysis of tomato plants selected in greenhouses of the Kyiv region, for the first time, virulent strains from this crop were identified and assigned, according to their physiological, biochemical, morphological and cultural characteristics, to the Pectobacterium carotovorum species. The fatty acid profile of the total cell lipids of the isolated strains was distinguished by the presence of 1,13‒1,17%, of 3-hydroxytetradecanoic acid, which is typical for P. carotovorum subsp. carotovorum. It was found that the isolated strains were sensitive to oxychloride and copper hydroxide-based fungicides with 10,5 to 17,5 mm growth inhibition zones.
Conclusion:
The causative agent of soft (wet) rot of tomatoes in greenhouses is P. carotovorum subsp. сarotovorum. Fungicides with oxychloride and copper hydroxide are recommended as the active agents to protect tomato plants from the pathogen.
1. INTRODUCTION
In the vegetable growing nowadays, a steady trend of growth of bacterial plant diseases affecting various types of vegetable crops, is taking place. Unlike micromycetes, these pathogens are hardly predictable and cause significant crop losses. Tomato plants are affected by multiple bacterial pathogens, the spread of which is facilitated by changeable natural and climatic conditions as well as by the structural and functional specifics of the plants [1]. Over recent years, climate change has facilitated the proliferation of bacterial diseases into temperate climate zones and an increase in their severity. If the average daily air temperature in the summer increases by 3-4°C, the prevalence of bacterioses doubles and the infestation of plants grows by 30-50% [2].
Another important natural climate factor for the spread of bacterial diseases is increasing the length of the growing seasons and the frost-free periods. Under such conditions, the increase of occurrence of bacterial diseases is due to both typical and common in Ukraine pathogens and to the pathogens that previously had not been spotted on the territory of our country. In particular, there is an increased degree of damage to tomato plants by such species as Clavibacter michiganensis subsp. michiganensis, Pectobacterium carotovorum, Pseudomonas corrugata, P. syringae pv. tomato, Xanthomonas vesicatoria and Pantoea agglomerans [3].
Favourable agro-meteorological conditions of the growing season are reported to facilitate the spread of the pathogenic bacteria of the genera Pectobacterium, Dickeya [4-6], which cause the soft (wet) rot of tomatoes. The disease is particularly damaging for tomatoes cultivated in the greenhouses, where up to 67% of the plants can become affected.
The pathogen P. carotovorum is reported to affect a wide range of host plants, in particular, potatoes, cabbage, lettuce, onions and carrots. It is widespread in various climatic zones, including the whole of Europe [7-12]. Most often, the lesion develops through small damage of the fruit skin or in the places where pests feed. When seedlings get damaged, the bacteria, under favourable conditions, can destroy the seedlings rather quickly. They penetrate the plant mainly through surface wounds; as a result, the stems get rotted most often near the substrate, and the fruits – near the pedicels. At first, a transparent spot appears on the affected fruit, then the spot gets pressed in and the skin cracks. The affected tissue of stems and fruits softens, liquefies and becomes dark brown; after 2‒3 days, the fruit rots into a liquid mass with an unpleasant smell [13]. Similar symptoms are caused by the blackleg pathogens Dickeya dianthicola and D. solani, and such similarity significantly complicates their identification and differentiation. If the fruit is infected while it is on the plant, it gradually dries off, and ultimately, only the skin remains. When the fruit is open, the formation of necrosis of the seed shell and fruit vessels can be observed; this wet rot is similar to the necrosis of the core of the tomato stem, the pathogen of which is Pseudomonas corrugate [14].
Usually, the development of the soft rot starts with the secretion by phytopathogens of a set of exoenzymes, primarily pectinases and cellulases, which are known to depolymerize the cell wall of the plant. The infections caused by Pectobacterium lead to maceration of parenchyma cells through the secretion of pectate lyases enzymes, polygalacturonases, pectin lyases, pectin methylesterases, cellulases and proteases. Such maceration is followed by the development of symptoms of softening of the affected plant tissues. The maceration depends on the external temperature and humidity. Optimal temperatures for the development of the disease are 25 - 30 °C [15].
The lack of timely diagnostics and express test systems for identifying phytopathogenic bacteria in seeds, seed material and plantings, the use of non-traditional farming systems that do not control plant bacterioses, lack of modern, highly effective protective measures against fungi, but not bacteria and lack of plant protectants lead to a gradual accumulation of infected material in fields, seeds, and weeds [2]. Pectobacterium spp. is a taxon that consists of strains with a number of different phenotypic, biochemical and genetic indicators, with a wide range of host plants and pathogenicity systems, which makes it difficult to diagnose this pathogen and choose suitable methods and ways for protecting tomato plants. Currently, the control of the spread of soft rot pathogens is based on steaming or sterilizing the soil in the greenhouse, as well as reducing the proportion of nitrogen and increasing potash fertilizers, removing diseased plants in nurseries, reducing the temperature and relative humidity of the air, careful sorting and culling of tomato fruits damaged by diseases 3‒5 days after the harvesting before storage [16]. Under industrial production conditions, fungicides are used against pathogens of bacterial diseases since there are no special preparations with antibacterial activity registered in Ukraine. For the control of the bacterial diseases of tomato plants, the preference is given to sulfate, oxychloride and copper hydroxide [17], which, however, have been only partially effective and have not ensured complete quality control of the soft rot pathogens. In this regard, the development of effective methods for detecting and identifying the pathogen is an important element in the overall plant protection and tomato seed certification programs.
The aim of this work is to identify, investigate the biological properties and develop practical recommendations for plant protection against the pathogen of soft (wet) rot of tomatoes.
2. MATERIALS AND METHODS
2.1. Plant Material
Plants with affected, liquefied and softened dark brown stems with a liquid mass and an unpleasant smell, and chlorotic or necrotic light yellow leaves with abundant watery spots were selected at commercial greenhouses in the Kyiv region.
2.2. Isolation of the Pathogen
For microbiological analysis, small pieces of affected tissue (capturing areas of affected and healthy tissue) were consistently washed with sterile running water and placed on potato agar in Petri dishes and incubated in a thermostat at 26‒28 °C for 48‒72 h. For comparative system studies, we used the type strain from the collection of the Department of Phytopathogenic Bacteria of the D. K. Zabolotny Institute of Microbiology and Virology at the National Academy of Sciences of Ukraine – P. carоtovorum subsp. carotovorum (Jones 1901) Waldee 1945 emend. Gardan et al. 2003, UCM B-1075Т (АТСС15713, ІСМР 5702, NCPPB 312).
2.3. Morphological and Cultural Properties of the Pathogen
The morphology (size, shape, colour and growth patterns) of colonies was recorded after 24‒48 hours of growth on a dish with a potato agar at +28°C. Gram staining was performed using a commercial IVD kit (Merck, Germany). The mobility of bacteria was determined by the “crushed drop” method on the one-day culture. The prepared slide was examined under binocular microscope Sigeta MB-201 (Sigeta, Ukraine) using immersion oil. Cell morphology was studied under a transmission electron microscope JEM-1400 (Jeol, Japan) at the Center for Open Access at the D. K. Zabolotny Institute of Microbiology and Virology at the National Academy of Sciences of Ukraine.
2.4. Kovac-oxidase Test
The commercial set of Oxidase Reagent (Bio Merieux, France) with N,N,N',N'-tetramethyl-p-phenylene-diamine (TMPD) was used. Fresh bacterial culture (18‒24 h) was spread over the TMPD-treated filter paper. Within 10-30 seconds, a dark blue or purple colour appeared, which confirmed the oxidation of the reagent.
2.5. Redox (o/f) Glucose Utilization Test (Hugh-Leifson Test)
A commercial set of Micro-La-Test (Erba Lachema) was used.
2.6. API Test System
To study the properties of the soft rot pathogen of tomatoes, API 20E test systems were used to identify gram-negative bacteria (BioMerieux, France). The results were estimated according to the results table, and identification was performed using the list of profiles API 20Е [18].
2.7. Pectolytic Activity Test
One-day cultures of separate isolates were transferred to sterile potato disks. The presence or absence of maceration was determined visually or by touching the disks with a bacteriological loop after one day of incubation.
2.8. Analysis of Fatty Acid (FA) Profile of Total Cellular Lipids
To obtain methyl esters, raw bacterial cells grown on the potato agar, were suspended in methanol with 1.5% of sulfuric acid. Methanolysis was performed in sealed ampoules at 80°C for 1 hour. The FA methyl esters were extracted with 3 ml of hexane. The extracts were evaporated on a vacuum rotary evaporator. Methyl esters of fatty acids were divided by Agilent 6890N/5973 inert mass-spectrometer system, HP-5MS column 30 m × 0,25 mm × 0,25 mm, temperature - 150-270°C with a gradient of 4 °C, carrier gas – helium, in the Open Access Center at the D. K. Zabolotny Institute of Microbiology and Virology of the National Academy of Sciences of Ukraine. The peaks were identified by comparing the time of their content with the time of the content of standard samples of FA methyl esters (Serva, Germany), as well as by the integrated mass spectrum database NIST 02. The FA content was determined as a percentage of the total peak area [19].
2.9. Study of Pathogenic Properties of the Pathogen
The ability to induce a hypersensitivity reaction was determined by injecting the suspension of bacterial cells with a titer of 107 CFU/ml under the epidermis of tobacco leaves [19]. The pathogenicity of separated isolates was assessed by artificial infection of the stem and leaves of tomato plants [19].
2.10. Screening of Preparations with Antimicrobial Activity
The effect of the antimicrobial preparations on the tomatoes soft rot pathogen was studied by diffusion in a solidified potato agar. The experiment was performed in Petri dishes with holes made by cork drill in the center of the agar. The test preparations were added at the concentrations recommended by the manufacturer. The one-day bacterial suspension with a titer of 109 CFU/ml was radially spread, and the dishes were incubated in a thermostat for 48 h at 28°C. The degree of sensitivity was determined by the diameters of the zones of test culture growth inhibition. If they were higher than 30 mm, the strains were susceptible, 15‒30 mm – intermediate, if less than 15 mm – resistant [20].
Statistical calculations of the experimental data were performed using the Statistica 6.0 software.
3. RESULTS
The tomato plants picked from the greenhouse had blackening tissues in the basal aboveground part of the stem. On the cross-cutting section of the stem, the darkening of the vascular system and the damage of the parenchyma with rotting of the stem were observed. The leaves had specific wet, oily spots of various sizes. The fruits had watery gray-green or brown spots, and the plants had an unpleasant smell.
The bacterial samples were taken from the rotting stems of tomato plants on edge between the affected and healthy tissues (Fig. 1). Bacteria isolated from the affected plants formed colonies of white to cream colour on the potato agar. Most isolates did not cause maceration of potato disks, neither did they form dry rots. The gram-negative motile rods that could metabolise glucose under anaerobic conditions and cause maceration of potato pieces, specifically the appearance of brown watery spots at the site of application, were selected for subsequent research.
 |
Fig. (1). Tomato plant stems with soft rot lesions (natural infection). |
 |
Fig. (2). Tomato plants stems artificially inoculated with bacteria isolated from the plants affected by soft rot. |
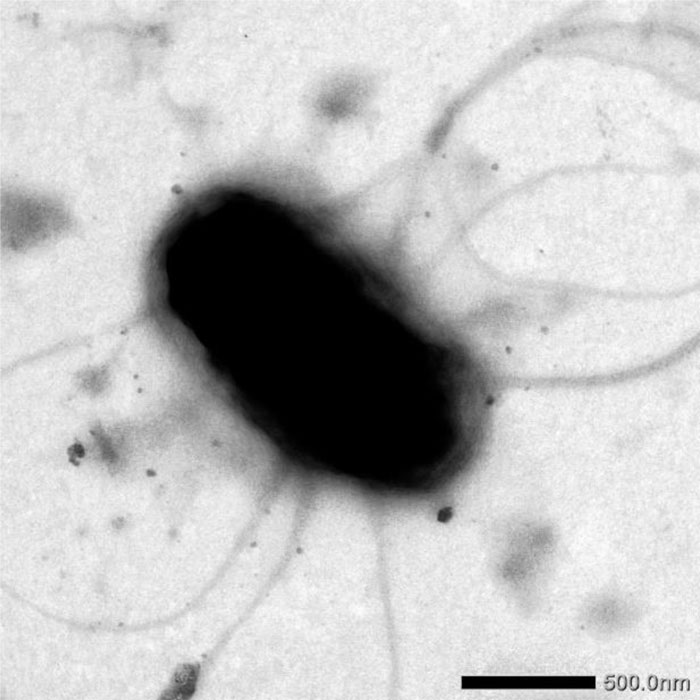 |
Fig. (3). The electrophonogram of the cell of the soft rot pathogen. |
The pathogenicity tests showed that soft rot symptoms were developed within 72 h after the inoculation of tomato plants with the separated isolates (Fig. 2). It is worth noting that bacteria, re-isolated from artificially inoculated tomato plants, had the same morphological and cultural characteristics.
Since all the isolates virulent for tomatoes were similar in terms of morphological and cultural properties, we selected two isolates TKO-2 and TKO-7, for further identification. The TKO-2 and TKO-7 isolates, grown on the potato agar, formed translucent, shiny convex colonies of light gray colour with smooth edges 2‒4 mm in diameter. An in-depth study of the biological properties of virulent isolates of bacteria showed that they were facultatively anaerobic single rods of 0,5‒1,0 x 1‒3 microns in size, motile by means of peritrichous flagella (Fig. 3), do not form spores, are oxidase-negative, catalase-positive, and have pectolytic activity.
The biochemical analysis of the TKO-2 and TKO-7 isolates has shown that growing on media with L-arabinose, D-glucose, D-sucrose and L-rhamnose formed an acid without gas. In addition to carbohydrates, the studied bacteria fermented β-galactosid and diluted gelatin (Table 1), which was similar to the typical strain of Pectobacterium caratovorum subsp. carotovorum UCM B-1075T and corresponded to the literature data for the bacteria of the species P. carotovorum [18]. Based on the results of the conducted biochemical analyses, the separated isolates were identified as the causative agent of the soft rot of tomatoes P. carotovorum subsp. carotovorum.
| Test/enzyme | Strains Isolated in Greenhouses in the Kyiv Region | P. carotovorum subsp. carotovorum UCM B-1075Т |
P. carotovorum according to the Literature Data |
|
|---|---|---|---|---|
| ТКО-2 | ТКО-7 | |||
| The form of cells | Rods | Rods | Rods | Rods |
| Gram reaction | ‒ | ‒ | ‒ | ‒ |
| Motility | Motile | Motile | Motile | Motile |
| Pigment formation | ‒ | ‒ | ‒ | ‒ |
| Fermentation of glucose: | ||||
| Aerobic | + | + | + | + |
| Anaerobic | + | + | + | + |
| Pectinase | + | + | + | + |
| Oxidase | ‒ | ‒ | ‒ | ‒ |
| API 20E tests | ||||
| β-galactosidase | + | + | + | a/d |
| Arginine dihydrolase | ‒ | ‒ | ‒ | ‒ (5% +) |
| Lysine decarboxylase | ‒ | ‒ | ‒ | ‒ (5% +) |
| Ornithine decarboxylase | ‒ | ‒ | ‒ | ‒ (5% +) |
| Utilization of citrates | + | + | + | a/d |
| Formation of Н2S | ‒ | ‒ | ‒ | + |
| Urease | ‒ | ‒ | ‒ | ‒ |
| Tryptophane deaminase | ‒ | ‒ | ‒ | ‒ |
| Formation of indole | ‒ | ‒ | ‒ | ‒ |
| Formation of acetoin (reaction of Voges-Proskauer) | ‒ | ‒ | ‒ | a/d |
| Gelatinase | + | + | + | + |
| Utilization: | ||||
| D-glucose | + | + | + | + |
| D-mannitol | + | + | + | + |
| inositol | + | + | ‒ | var. |
| D-sorbite | ‒ | ‒ | ‒ | + |
| L-rhamnose | + | + | + | + |
| D-sucrose | + | + | + | a/d |
| D-melibiose | + | + | + | + |
| Amygdaline | + | + | + | + |
| L-arabinose | + | + | + | + |
| Reduction of nitrates | ‒ | ‒ | ‒ | ‒ |
The study of the fatty acid composition of total cell lipids of the type P. carotovorum UCM B-1075Т and selected strains of P. carotovorum subsp. carotovorum TKO-2 and TKO-7 confirmed the presence of fatty acids with carbon chain lengths C12 - C18, particularly: unsaturated – cis-9-hexadecenoic (C16:1) and cis-11-octadecenoic (C18:1); saturated – dodecanoic (C12:0), pentadecanoic (С15:0), hexadecanoic (C16:0) and octadecanoic (C18:0) (Table 2). Hydroxy acids were represented by 3-hydroxytetradecanoic acid (3-ОН С14:0) at a concentration of 1,13‒1,17%, which is typical for soft rot pathogen P. carotovorum subsp. carotovorum.
For primary screening of antimicrobial activity against isolated bacteria, 15 chemicals were tested. In the experiments, preparations with the active substance copper hydroxide showed the greatest antibacterial activity against P. carotovorum subsp. carotovorum TKO-2 and TKO-7 strains, and growth inhibition zones ranged from 14 to 17 mm. Preparations with the active substance copper chloroxide showed moderate activity against P. carotovorum subsp. carotovorum TKO-2 and TKO-7 strains with growth inhibition zones from 10,5 to 13,5 mm. Most of the studied fungicides, recommended for processing plantations, did not affect the pathogen of soft rot of tomatoes (Table 3).
| Fatty Acids | Average amount of FA, % of the Total Content |
|||
|---|---|---|---|---|
| Formula | Name | ТКО-2 | ТКО-7 | P. carotovorum UCM B-1075Т |
| С 12:0 | dodecanoic | 3,73 | 3,78 | 3,62 |
| 3OH С 14:0 | 3-hydroxytetradecanoic | 1,17 | 1,13 | 1,14 |
| С 15:0 | pentadecanoic | 1,77 | 1,89 | 1,71 |
| С 16:1 | cis-9-hexadecenoic | 33,40 | 34,90 | 34,67 |
| С 16:0 | hexadecanoic | 36,56 | 35,06 | 35,44 |
| С 17:0 cyclo | cis-9,10- methylenehexadecanoic | 1,37 | 1,32 | 0,95 |
| С 18:1 | cis-11-octadecenoic | 21,22 | 20,98 | 21,52 |
| С 18:0 | octadecanoic | 0,78 | 0,94 | 0,95 |
| Preparations of Fungicidal Action, Concentration | Diameter of Zone of Inhibition, (mm, M±SE) |
Sensitivity Pattern | |
|---|---|---|---|
| ТКО-2 | ТКО-7 | ||
| Copper chloroxide, 350 g/l | 10,5±0,4 | 13,0±0,5 | Intermediate |
| Kresoxim-methyl, 500 g/l | 0 | 0 | Resistant |
| Aluminium tris (ethyl phosphonate) 75%, folpet 25% | 3,0±0,08 | 3,5±0,08 | Resistant |
| Copper hydroxide, 770 g/l | 14,0±0,2 | 17,0±0,5 | Intermediate |
| Sulfur, 800 g/l | 3,0±0,08 | 0±0 | Resistant |
| Metalaxyl, 80 g/l, mancozeb, 640 g/l |
8,0±0,4 | 6,5±0,2 | Resistant |
| Azoxystrobin, 250 g/l | 0±0 | 0±0 | Resistant |
| Difenoconazole, 250 g/l | 7,5±0,3 | 7,5±0,3 | Resistant |
| Thiophanate-methyl, 500 g/l | 7,5±0,3 | 7,5±0,2 | Resistant |
| Mancozeb, 800 g/l | 7,5±0,2 | 7,5±0,3 | Resistant |
| Benomyl, 500 g/l | 3,0±0,08 | 3,0±0,06 | Resistant |
| Mancozeb, 302 g/l, propamocarb hydrochloride, 248 g/l | 6,0±0,2 | 8,5±0,2 | Resistant |
| Phenhexamide, 500 g/l | 0 | 0 | Resistant |
| Thiamethoxam, 250 g/l, acetamiprid, 100 g/l, cymoxanil, 250 g/l, metalaxyl, 100 g/l |
7,5±0,2 | 6,5±0,3 | Resistant |
4. DISCUSSION
Pathogens of bacterial soft (wet) rot (SRP) belong to the genera Pectobacterium and Dickeya. These necrotrophic bacterial pathogens are spread widely amongst various farm crops. The pathogens can be isolated from infected plants, soil, and water; they can also be found on the surface and inside insects [21]. Due to the fact that SRP can significantly impair the growth, development and productivity of the plants, these bacteria have been attributed to the ten most harmful bacterial pathogens in agriculture [22]. For tomatoes under storage and transport conditions, the main pathogens of soft rot (rotting of fruits, tomato stalks, black leg) are P. carotovorum subsp. carotovorum, P. atrosepticum [21] and several species of Dickeya [23]. In Europe, blackleg and soft rot diseases can cause relatively high tomato crop losses both in the field and in the storage. Moreover, some studies have confirmed that P. atrosepticum and P. carotovorum subsp. carotovorum are among the most frequent pathogens causing soft rot and/or black leg in the temperate climate zones [6, 10, 24-26]. The symptoms caused by different SRPs in tomatoes are often hard to distinguish. SRP bacteria can cause systemic infection and death of the host plant. They usually produce a significant amount of extracellular enzymes that destroy the cell wall of the plant (for example, cellulases, pectinases, pectin methyl esterases, pectate lyases, polygalacturonases, phospholipases, proteases) and allow bacteria to enter the vascular compartments and lead to maceration (rotting) of the host plant tissues [22, 23]. Among all Pectobacterium spp. species that infect plants, P. carotovorum subsp. carotovorum has the widest range of hosts in the world, while P. atrosepticum is associated mainly with tomatoes grown in temperate climate [21]. Bacterial pathogens of soft rot cause watery lesions, rotting, softening of tissues and wilting of parts of the host plant, in particular stems, leaves, fruits and brushes of tomatoes [27, 28]. The bacteria isolated from the affected tomato plants were found similar to the species P. carotovorum subsp. carotovorum. According to the identified morphological characteristics, the isolates were facultative anaerobes, gram-negative, straight mobile rods, these features were consistent with previous experiments [29]. These isolates showed a positive hypersensitivity reaction, but some authors reported that P. carotovorum strains caused negative and positive effects of the hypersensitivity test on tobacco [30]. The isolates revealed high pectolytic activity, which is essential in the pathogenesis of this genus [31].
The choice of effective methods of protection against bacterial diseases is complicated by the similarity of the symptoms and by the cross infestation of growing plants with several different pathogens. Rapid withering away from plant tissues, leaf spotting and rotting are the main manifestations of damage by various species of the genus Pectobacterium. In order to facilitate the identification and investigation of the properties of isolates P. carotovorum subsp. carotovorum TKO-2 and TKO-7, the API 20E test system (BioMerieux, France), was used. It is based on 21 standardized biochemical tests for identifying phytopathogenic facultatively anaerobic gram-negative bacteria. The results of analysis showed that isolates of P. carotovorum subsp. carotovorum had similar biochemical characteristics. Nine of the twelve generally accepted API 20E tests were negative, in particular, nitrate reduction, indole production, urease, acetoin, hydrogen sulfide, arginine dehydrolase, lysine decarboxylase, ornithine decarboxylase, tryptophandeaminase. Whereas the isolates showed the ability to hydrolyze β-glucosidase, gelatins and citrates, inositol and D-sorbitol assimilation tests were negative, although bacterial strains had the ability to use D-glucose, L-arabinose, D-mannitol, D-sucrose, D-melibiose, amygdalin, and L-rhamnose. Similar results are presented in the works of Terta et al. [32], Kettani-Halabi et al. [33] and are considered as diagnostic biochemical signs.
Identification methods based on the study of FA, protein profile and metabolic processes were quite sensitive. About 300 FA and associated components are found in bacteria. The type and relative concentrations of individual FA strictly depend on the genotype and are characteristic of a particular type of bacterium or its strain. Identification of phytopathogens by the spectrum of fatty acids coincides with the data of DNA–DNA and DNA–rRNA hybridization studies, which confirms its high specificity [34].
Taking this into account, the identification of the FA profiles of the bacterial membrane lipids, allows to determine their generic and species identity. In the studied pathogenic strains, the main FA in the spectra of total lipids were hexadecanoic, hexadecenoic, octadecanoic and octadecenoic acids. The presence of 3-hydroxytetradecanoic acid was found in both strains, which is a characteristic feature of the family Pectobacteriaceae. The results obtained regarding the fatty acid composition of P. carotovorum cell lipids isolated from tomatoes affected by soft rot are consistent with the literature data. According to De Boer and Sasser [35], Erwinia carotovora strains contain cis-9-hexadecenoic (C 16:1), cis-11-octadecenoic (C 18:1), dodecanoic (C 12:0), 3-hydro xytetradecanoic (3-OH C 14:0) and tetradecanoic acid (C 14:0). Strains E. carotovora ssp. atroseptica and E. carotovora ssp. carotovoraican were differentiated based on three different ratios of fatty acids. Strains E. carotovora ssp. carotovora had a ratio >3,71 for C12:0/C14:0, <4,87 ‒ C16:0/C12:0 and <2,7 ‒ C16:1/C18:1, whereas strains E. carotovora ssp. atroseptica had contrasting values for each of the ratios.
Under industrial production conditions, fungicides are used against pathogens of bacterial diseases, since no special preparations with antibacterial activity have been recommended among those registered in Ukraine. Minor antibacterial activity against isolated strains of the soft rot pathogen of tomatoes P. carotovorum subsp. carotovorum TKO-2, TKO-7 showed only oxychloride and copper hydroxide. The mechanism of copper ions action involves the denaturation of proteins, destroying enzymes that are crucial for the functioning of bacterial cells [17]. The work by Pradnyarani et al. [36] showed high antibacterial activity of copper hydroxide on the growth of P. carotovorum subsp. carotovorum bacteria under in vitro conditions. Preparations based on copper sulfate were characterized by an average value, while carbendazim and mancozeb were characterized by the absence of zones of bacterial growth suppression in the tested concentrations.
Copper compounds, as before, remain indispensable agents in the fight against bacterial pathogens in vegetable growing, although the literature describes some of the risks of their constant use, which are associated with the accumulation and contamination of soils and a high content of their residues in food products. On the other hand, copper plays a special role as an important element in the physiological and biochemical processes of higher organisms [17]. Therefore, the use of copper in all its varieties must be controlled and correspond to the environmentally friendly conditions and needs of plants.
CONCLUSION
On the basis of morphological, cultural, physiological and biochemical characteristics, isolates TKO-2 and TKO-7 of phytopathogenic pectolytic bacteria from the Ukrainian greenhouse were attributed to the species P. carotovorum subsp. carotovorum. On the basis of the verification of the antibacterial effect of plant protection products, it is recommended to use preparations with active substances oxychloride and copper hydroxide to fight the development of the pathogen of soft rot of tomatoes – P. carotovorum subsp. carotovorum.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This work was financially supported by the project “Induced resistance and control of phytopathogenic bacteria in the latest biotechnologies for growing vegetable crops using growth stimulants with an elicitor activity” (2020-23 р.р.) (No. 0120U102106) of the Ministry of Education and Science of Ukraine.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We acknowledge Dr. V. Kyrylenko (Institute of Food Biotechnology and Genomics, National Academy of Sciences of Ukraine, Kyiv) for giving suggestions aimed to improve the manuscript language.
REFERENCES
| [1] | Raichuk T. Bacterial diseases of tomato in Kyiv region. Planter 2013; 4: 26-8.https://agrotimes.ua/article/bakterialni-hvorobi-tomata-na-kiy ivshchini/ |
| [2] | Ihnatov AN, Yehorova MS, Khodykina MV. The spread of bacterial and phytoplasmic plant diseases in Russia. Plant protection and quarantine 2015; 5: 6-10.https://cyberleninka.ru/article/n/raspros tranenie-bakterialnyh-i-fitoplazmennyh-bolezney-rasteniy-v-rossii |
| [3] | Patyka VP, Pasichnyk LA. Phytopathogenic bacteria: fundamental and applied aspects. Bulletin of the Uman National University of Horticulture 2014; 2: 7-11. Available from: https://cyberleninka.ru/article/n/ fitopatogenni-bakteriyi-fundamentalni-i-prikladni-aspekti |
| [4] | Akbar A, Ahmad M. Azra Neelam, Khan SZ, Ahmad Z. Characterization of the causal organism of soft rot of tomatoes and other vegetables and evaluation of its most aggressive isolates. Am J Plant Sci 2015; 6: 511-7. |
| [5] | Benada M, Boudalia S, Boumaaza B, Khaladi O, Guessas B. Variability of aggressiveness and virulence of Erwinia carotovora subsp. carotovorum causing the soft rot on potato tubers in the western of Algeria. Int J Plant Biol 2018; 9(1): 52-8. |
| [6] | Zaczek-Moczydłowska MA, Fleming CC, Young GK, Campbell K, O’Hanlon R. Pectobacterium and Dickeya species detected in vegetables in Northern Ireland. Eur J Plant Pathol 2019; 154: 635-47. |
| [7] | Cariddi C, Sanzani SM. A severe outbreak of bacterial lettuce soft rot caused by Pectobacterium carotovorum subsp. carotovorum in Apulia (Italy). J Plant Pathol 2013; 95: 441-6. |
| [8] | Onkendi EM, Moleleki LN. Characterization of Pectobacterium carotovorum subsp. carotovorum and brasiliense from diseased potatoes in Kenya. Eur J Plant Pathol 2014; 139: 557-66. |
| [9] | Moretti C, Fakhr R, Cortese C, et al. Pectobacterium aroidearum and Pectobacterium carotovorum subsp. carotovorum as causal agents of potato soft rot in Lebanon. Eur J Plant Pathol 2016; 144: 205-11. |
| [10] | Dees MW, Lebecka R, Perminow JI, et al. Characterization of Dickeya and Pectobacterium strains obtained from diseased potato plants in different climatic conditions of Norway and Poland. Eur J Plant Pathol 2017; 148: 839-51. |
| [11] | Naas H, Sebaihia M, Orfei B, Rezzonico F, Buonaurio R, Moretti C. Pectobacterium carotovorum subsp. brasiliense and Pectobacterium carotovorum subsp. carotovorum as causal agents of potato soft rot in Algeria. Eur J Plant Pathol 2018; 151: 1027-34. |
| [12] | Oztruk M, Aksoy HM, Potrykus M, Lojkowska E. Genotypic and phenotypic variability of Pectobacterium strains causing blackleg and soft rot on potato in Turkey. Eur J Plant Pathol 2018; 152: 143-55. |
| [13] | Ghazzawi Al A, Ghoura Abu M, Beig Al N, Jaloul A, Mando J. First report of Pectobacterium carotovorum subsp. carotovorum causing stem rot on greenhouse tomatoes in Syria. J Plant Pathol 2012; 94(4): S4.85-S4.105. |
| [14] | Caruso A, Licciardello G, La Rosa R, Catara V, Bella P. Mixed infection of Pectobacterium carotovorum subsp. carotovorum and P. carotovorum subsp. brasiliensis in tomato stem rot in Italy. J Plant Pathol 2016; 98(3): 661-5. |
| [15] | Nazerian E, Sijam K, Meor Ahmad ZA, Vadamalai G. Characterization of Pectobacterium carotovorum subsp. carotovorum as a new disease on lettuce in Malaysia. Australas Plant Dis Notes 2013; 8(1): 105-7. |
| [16] | Czajkowski R, Pérombelon MCM, van Veen JA, van der Wolf JM. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: a review. Plant Pathol 2011; 60: 999-1013. |
| [17] | Kolomiiets YV, Grygoryuk IP, Butsenko LM, Kalinichenko AV. Biotechnological control methods against phytopathogenic bacteria in tomatoes. Appl Ecol Environ Res 2019; 17(2): 3215-30. |
| [18] | Patyka VP, Butsenko LM, Pasichnyk LA. Application of commercial test-systems to identify gram-negative facultatively anaerobic bacteria. Agricult Sci Practice 2016; 1: 43-8. |
| [19] | Patyka V, Pasichnyk L, Butsenko L, et al. Express diagnostics of phytopathogenic bacteria and phytoplasmas in agrophytocenosis 2019; 86. |
| [20] | Kolomiiets JV, Grygoryuk IP, Butsenko LM. Bacterial diseases of tomatoes plant in terms of open and covered growing of Ukraine. Ann Agric Sci 2017; 15(2): 213-6. |
| [21] | Czajkowski R, Ozymko Z, de Jager V, et al. Genomic, proteomic and morphological characterization of two novel broad host lytic bacteriophages ΦPD10.3 and ΦPD23.1 infecting pectinolytic Pectobacterium spp. and Dickeya spp. PLoS One 2015; 10(3)e0119812 |
| [22] | Mansfield J, Genin S, Magori S, et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol 2012; 13(6): 614-29. |
| [23] | Toth IK, van der Wolf JM, Saddler G, Lojkowska E, Hélias V, Pirhonen M, et al. Dickeya species: an emerging problem for potato production in Europe. Plant Pathol 2011; 60: 385-99. |
| [24] | De Boer SH, Li X, Ward LJ. Pectobacterium spp. associated with bacterial stem rot syndrome of potato in Canada. Phytopathology 2012; 102(10): 937-47. |
| [25] | Dees MW, Lysøe E, Rossmann S, Perminow J, Brurberg MB. Pectobacterium polaris sp. nov., isolated from potato (Solanum tuberosum). Int J Syst Evol Microbiol 2017; 67(12): 5222-9. |
| [26] | de Neergaard E, Harding S, Czajkowski R. as a causal agent of potato blackleg in Greenland. Eur J Plant Pathol 2020; 157: 425-31. |
| [27] | Umunna OE, Austin AA. An overview of characterization and identification of soft rot bacterium Erwinia in some vegetable crops. Greener J Biol Sci 2016; 6(3): 46-55. |
| [28] | Wang J, Wang Y, Zhao T, Dai P, Li X. Characterization of the pathogen causing a new bacterial vein rot disease in tobacco in China. Crop Prot 2017; 92: 93-8. |
| [29] | Maisuria VB, Nerurkar AS. Characterisation and differantiation of soft rot causing Pectobacterium carotovorum of Indian origin. Eur J Plant Pathol 2012; 136(1): 87-102. |
| [30] | Ngadze E, Brady CL, Coutinho TA, van der Waals JE. Pectinolytic bacteria associated with potato soft rot and blackleg in South Africa and Zimbabwe. Eur J Plant Pathol 2012; 134: 533-49. |
| [31] | Crepin A, Barbey C, Cirou A, et al. Biological control of pathogen communication in the rhizosphere: A novel approach applied to potato soft rot due to Pectobacterium atrosepticum. Plant Soil 2012; 358: 27-37. |
| [32] | Terta M, Azelmat S, M’hand RA, Barakate M, Bouteau F, Ennaji MM. Molecular typing of Pectobacterium carotovorum isolated from potato tuber soft rot in Morocco. Ann Microbiol 2012; 62(4): 1411-7. |
| [33] | Kettani-Halabi M, Terta M, Amdan M, El Fahime M, Bouteau F, Ennaji MM. An easy, simple inexpensive test for the specific detection of ectobacterium carotovorum ssp. carotovorum based on sequence analysis of the pmrA gene. BMC Microbiol 2013; 13: 176-82. |
| [34] | Narayanasamy P. Microbial plant pathogens-detection and disease diagnosis. Microbial plant pathogens-detection and disease diagnosis 2011; 255. https://www.springer.com/gp/book/ |
| [35] | De Boer S, Sasser M. Differentiation of Erwinia carotovora ssp. carotovora and E. carotovora ssp. atroseptica on the basis of cellular fatty acid composition. Can J Microbiol 2011; 32(10): 796-800. |
| [36] | Pradnyarani N, Kulkarni MS, Kirankumar KC, Mesta RK, Chethankumar K. Evaluation of different agrochemicals / antibiotics under in vitro against Pectobacterium carotovorum subsp. carotovorum (Erwinia carotovora). carotovorum (Erwinia carotovora) causing tip over disease of banana Int J Chem Studies 2018; 6(4): 75-8. |








