All published articles of this journal are available on ScienceDirect.
High-Efficiency Ukrainian Strains of Microalgae for Biodiesel Fuel Production (Overview)
Abstract
Background:
In Ukraine, the research works focused on the study of green microalgae as an alternative source of feedstock for biodiesel production started in 2009. The screening of the Ukrainian Microalgae Culture Collection (IBASU-A) revealed a number of promising strains of species belonging to Chlorella, Chloroidium, Desmodesmus, and Parachlorella (Chlorellaceae and Scenedesmaceae) genera. The main criteria for strain selection were: accumulation of a significant volume of lipids, high kinetic characteristics (specific growth rate and productivity), resistance to stresses and biological contaminations. Some novel algal strains with relevant properties and optimized to the local climatic conditions were also isolated from different regions of Ukraine. Biotechnological studies showed a rather high potential of many of them, especially those of species from Tetradesmus and Desmodesmus (Scenedesmaceae) genera. Recently, some strains of species belonging to Monoraphidium, Raphidocelis, and Selenastrum (Selenastraceae) genera have also been isolated and the search for strains with a high biomass production continued.
Objective:
Moving from an overview of the oleaginous high-efficiency strains present in the largest algal collection in Ukraine (IBASU-A) and a critical analysis of problems related to biodiesel fuel production by microalgae, here we report preliminary data on biomass productivity, lipid amount and fatty acid profiles of some Ukrainian strains of Selenastraceae family.
Methods:
Traditional techniques were used for the isolation of new strains. The study of growth characteristics was carried out under intensive conditions and the daily increase of algal biomass was determined by the gravimetrical method. The data obtained on growth characteristics were compared with those of the well-known producers of biomass. The fatty acid composition in the most promising strains was determined by the gas-liquid chromatography.
Results:
Three new algal strains, M. minutum IBASU-A 574, Monoraphidium sp. IBASU-A 377 and Raphidocelis subcapitata IBASU-A 358, of Selenastraceae, adapted to regional climatic conditions, were isolated from different regions of Ukraine. They showed a higher efficiency in biodiesel production with respect to strains of Chlorellaceae and Scenedesmaceae earlier investigated. The biomass productivity of M. minutum IBASU-A 574, Monoraphidium sp. IBASU-A 377 and Raphidocelis subcapitata IBASU-A 358 was 1.84, 0.84 and 1.32 g DW L-1·day-1, while the lipid contents were 33.65%, 29.43% and 23.14%, respectively. Their fatty acid profiles included mainly C16:0, C18:2 and C18:3, all of interest for biodiesel production.
Conclusion:
The Ukrainian Microalgae Culture Collection has been supplemented with strains of species from family Selenastraceae showing high-efficiency for biodiesel production and adaptation to local environmental conditions.
1. INTRODUCTION
The limited stocks of native oil, coal and gas and the increasing emission into the atmosphere of carbon dioxide and other polluting substances from the combustion of fossil fuels make the search for alternative sources of energy a pressing need. Solar energy, wind power, the energy of waves and tidal currents, thermonuclear fusion processes and renewable biomass-derived fuels (biofuel) are considered as alternative sustainable sources. In the last few decades, some active scientific research works on generation, processing and usage of biofuel, especially biodiesel as the most needed and perspective energy resource, have been carried out worldwide. Agriculture oil crops (rice, sunflower, soybean, olive, corn, sugarcane), non-food crops (willow, switch grasses, forest and waste residues), animal fats, lipids from bacteria, yeasts, filamentous fungi and algae are used as raw materials for biofuel production. With respect to terrestrial oleaginous plants, microalgae show faster cell growth, higher photosynthetic efficiency and higher biomass productivity. Thanks to their ability to grow in fresh, brackish, seawater and even field wastewater and city sewages, microalgae can be cultivated on an industrial scale in areas unsuitable for agriculture, so they do not compete with crops for arable land use. Moreover, they could be grown in various types of cultivators, which are environmentally safe and controlled [1-6]. The interest in microalgae for biodiesel production is based on their ability to accumulate high amounts (20-50% of dry biomass) of neutral lipids, mainly in the form of Triacylglycerol (TAG). TAG concentration is known to significantly depend on the growth characteristics of strains and cultivation conditions [7, 8]. Some species of Bacillariophyta (e.g. Chaetoceros muelleri Lemmerm., Phaeodactylum tricornutum Bohlin) and Chlorophyta (e.g“Chlorococcum humicola (Nägeli) Rabenh.”, Chlorella vulgaris Beijr., Botryococcus braunii Kütz., Monoraphidium sp., M. minutum (Nägeli) Komárk.-Legner., Nannochloropsis sp., Oocystis sp., Scenedesmus bijugatus Kütz., Tetraselmis sp.) are considered as promising candidates for algal-based biofuels (bioethanol, biogas, biodiesel) [6, 9-14]. However, the need for algal oleaginous strains that have high growth potential and show robustness in different environmental conditions and the high cost of algal-based biofuel versus conventional diesel remain obstacles to be overcome.
Many projects aimed at screening high lipid algal producers were implemented in EU countries, the USA, the Middle East, China, India, Japan, and Australia and other parts of the world. In the frame of Aquatic Species Program (ASP) funded by the Department of Energy of the United States (US DOE) from 1978 to 1996, the most comprehensive research works were carried out. They resulted in a collection of 300 biotechnologically valuable strains, including promising lipid producers found among the representatives of Bacillariophyta and Chlorophyta [6]. Large scale screening of wild strains from the West Pacific Ocean and the southeast Chinese coastline present in FACHB (Freshwater Algae Culture Collection of the Institute of Hydrobiology Chinese Academy of Science) was conducted to search for fast-growing oleaginous strains [15]. The exploitation of algal collections such as SAG (Sammlung von Algenkulturen der Universität, Göttingen, Germany), CCALA (Culture Collection of Autotrophic Organisms Institute of Botany, Czech AS), CCAP (Culture Centre of Algae and Protozoa, Ambleside, UK), PCC (Pasteur Culture Collection of Cyanobacteria, France) in Europe, UTCC (The University of Toronto Culture Collection of Algae and Cyano bacteria, Canada), UTEX (Culture Collection of Algae at the University Texas at Austin, USA) NIES (Collection Microbial Culture at the National Institute of Environmental Studies, Japan) has provided important information related to oil productions and ways to enhance lipid productivity in these organisms [16-20]. These are crucial factors to reduce the difference in prices between renewable and conventional fuels.
In Ukraine, the research works focused on the microalgae biomass as an alternative source of feedstock for biodiesel production started in 2009 in the frame of scientific project of NAS of Ukraine: Biomass as Raw Material for Fuel [21, 22].
The aim of this work is to report data relative to biomass productivity, lipid amount and fatty acid profiles of some recently isolated Ukrainian strains of Selenastraceae family as well as to provide an overview of the oleaginous high-efficiency algal strains present in the largest algal collection in Ukraine (IBASU-A) and to critically analyse the problems related to biodiesel fuel production by microalgae.
2. AN OVERVIEW OF THE LARGEST MICROALGAE CULTURE COLLECTION IN UKRAINE – IBASU-A
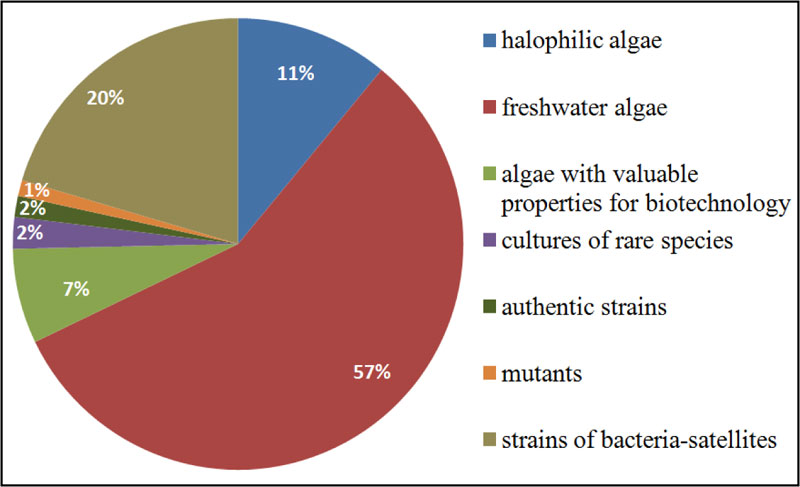
The Microalgae Culture Collection of M.G. Kholodny Institute of Botany, NAS of Ukraine (IBASU-A), is the largest algal collection in Ukraine. The collection was established in the 1960s and aimed at gaining information on morphology, systematics, ecology and biogeography of Dunaliella Teod. species for their potential use as a source of β-carotene, bioactive compounds, pharmaceuticals, and the use of microalgal mass culture as feed for animals and fishes [23]. Later on, the assortment of strains was gradually increased to meet the topics of new scientific projects. In the 1980s and 1990s, strains of species belonging to Chlorellaceae and Scenedesmaceae families were isolated from different regions of Ukraine, as well as from other countries (Germany, Georgia, Israel, and Russia). Some strains were obtained from such well-known collection namely: SAG (Göttingen, Germany), CALU (St-Petersburg, Russia), CCALA (Třebon, Czech Republic), CCAP (Ambleside, UK), UTEX (Austin, USA). Many of them were used in different applied projects as test-objects for an assessment of toxicity and carcinogenicity of synthetic organic substances or as biological agents in the biodegradation of detergents or biological treatment/post-treatment of different types industrial waste [24, 25]. A number of the Dunaliella strains were used for mass cultivation in some space biology projects [26]. The IBASU-A has been ranked as part of the Ukrainian national heritage since 2013. It holds 500 strains of halophilic, freshwater and aerophytic microalgae, most of them (469 strains) are representatives of green algae (Chlorophyta), the basis of species diversity of Ukrainian algal flora [27]. There are also some strains of species from Cyanoprokaryota (5), Euglenophyta (5), Eustigmatophyta (5), Bacillariophyta (1) and Charophyta (17). Overall, 300 strains originated from various regions of Ukraine. In addition, 270 strains of satellite bacteria isolated from algal cultures are also stored in this collection. IBASU-A consists of several separate collections, including sets of authentic (type) strains, mutants, cultures of rare species, and strains for biotechnological purposes. This collection provides material for both basic and applied research [28] (Fig. 1).
3. MATERIALS AND METHODS
3.1. Screening of IBASU-A Strains Collection for Promising Candidates for Algal-Based Biodiesel Production and Comparison with Strains from other Collections
At the initial stage of our studies, we screened the strains present in IBASU-A collection to identify promising candidates for biodiesel production. According to original and published data, the main criteria for this selection were: the active growth of algae, their tolerance to stress factors and resistance to biological contamination and accumulation of a rather significant amount of lipids. To select the fast-growing strains, the express-method, including algal cultivation under uniform culture growing conditions with visual determination, was initially used [29]. Then a study of the growth characteristics of selected strains was carried out under intensive conditions during 10 days at temperature 26-32°C, constant light intensity of 100 µmol m-2 s-1 and constant aeration. The initial cell density in the flask was 5·106 cells mL-1. The daily increase of algal biomass was estimated by microscopic cell counting or determining dry cell weight (DW) gravimetrically [30]. The growth characteristics (specific growth rate and biomass productivity) were calculated according to the formulas [31] on the basis of the obtained data. They were compared with those of known high biomass producers Tetradesmus dimorphus (Turpin) M.J. Wynne IBASU-A 254 (=Scenedesmus acutus Meyen CALU 24; = Acutodesmus dimorphus (Turpin) P. Tsarenko), and Ch. vulgaris IBASU-A 189 (= CALU 157) obtained from CALU (St-Petersburg, Russia).
As a result of this screening, a new collection of strains as potentially high biomass producers was established. Overall, it included 33 strains of 18 species from Botryococcus Kütz. (1), Chlorella Beijr. (5), Chloroidium Nadson (2), Desmodesmus (Chodat) S.S. An, T. Friedl et E. Hegew. (9), Euglena Ehrenb. (2), Monoraphidium Komárk.–Legner. (2), Parachlorella Krienitz, E. Hegew., Hepperle, Huss, Rohr et Wolf (6) and Tetradesmus G.M. Sm. (=Acutodesmus (E. Hegew.) P. Tsarenko) (6 strains) genera. This new collection included strains obtained from other collections (15 strains from CALU, UTEX, SAG, CCAP and CCALA) or isolated from territories of other countries (7) and Ukraine (10).
The optimal media and growing conditions for these strains were determined before carrying out further experiments.
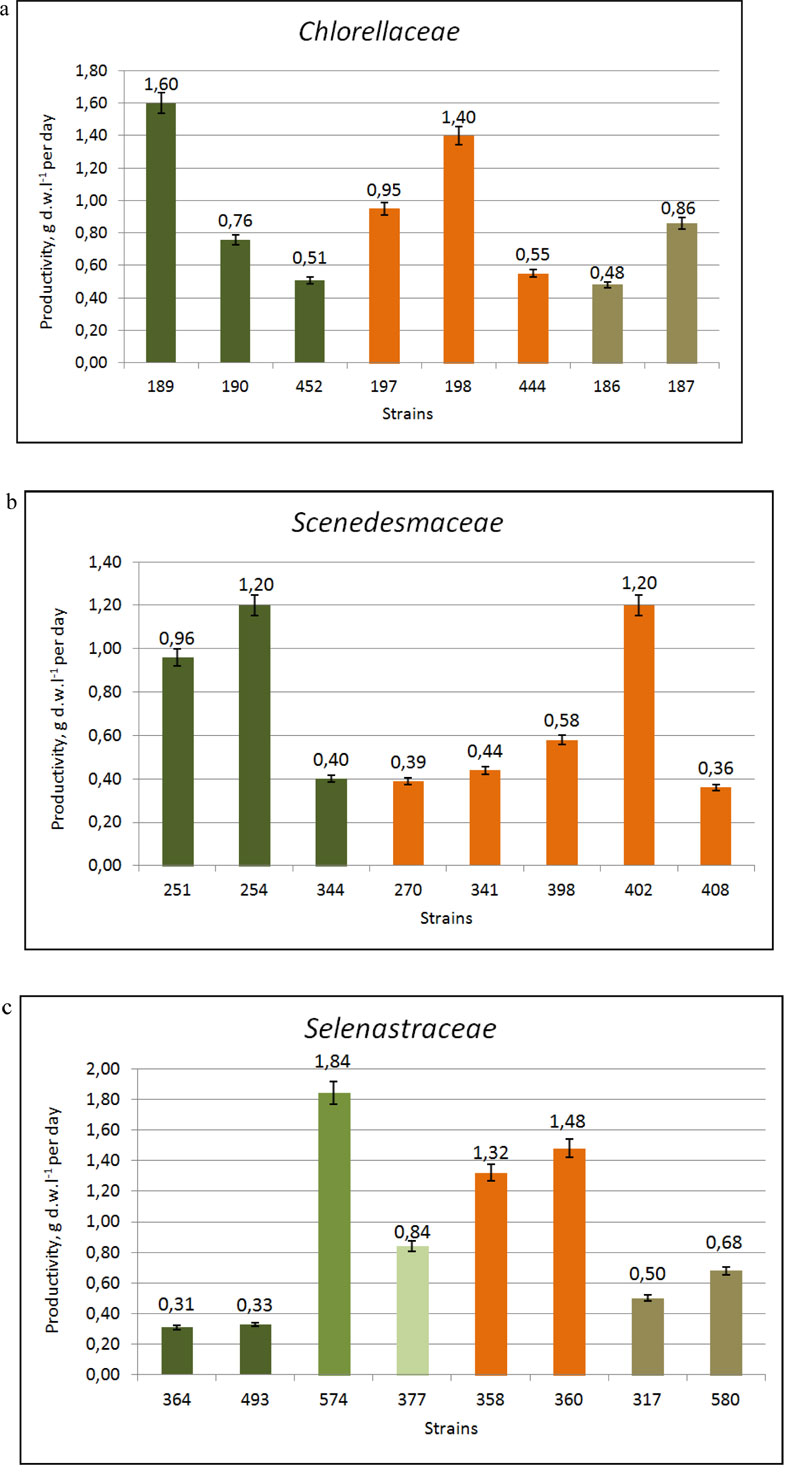
The comparative studies on the growth characteristics of selected strains showed that the majority of them demonstrated a rather active growth [32]. The maximal cell concentration (B) was shown by strains of Ch.vulgaris IBASU-A 189, 190, 192, 326, 452, Chloroidium saccharophilum IBASU-A 186, 187 and Parachlorella kessleri IBASU-A 197-201, 444 and ranged from 38·106 to 250·106 cells mL-1, whereas their specific growth rate (µ) was in the range of 0.55-1.4 day-1 and biomass productivity (P) in the range of 9.5-72.5·106 cells mL-1 day-1. A little less was the maximal cell concentration obtained for species of Desmodesmus (IBASU-A 270, 310, 341, 371, 384, 398, 401, 402, 407), and Tetradesmus (T. dimorphus IBASU-A 251, 252, 344, T. obliquus (Turpin) M.J. Wynne IBASU-A 292, 473) varying in the range of 26-84.5·106 cells mL-1, with the specific growth rate ranging from 0.35 to 1.2 day-1 and the biomass productivity from 6.4 to 29·106 cells mL-1 day-1. There was no obvious adaptation phase in cultures of all strains under study. The stationary phase was observed at the fifth or sixth day from the beginning of experiments for the majority of strains. The presence of the satellite bacteria did not influence the growth characteristics of the algal strains under study. Under optimal conditions of batch cultures, the biomass productivities in the strains of Chlorella, Chloroidium and Parachlorella species varied in the range of 0.51-1.6 g DW L-1 ·day-1 and in those of Desmodesmus and Tetradesmus species in the range of 0.34-1.2 g DW L-1·day-1. The biomass production of Botryococcus braunii IBASU-A 504 reached 1.3 g DW L-1·day-1. Monoraphidium griffithii (Berk.) Komárk.-Legner. (IBASU-A 166, 364) and Euglena viridis (O.F. Müller) Ehrenb. (IBASU-A 496, 498) species had a rather high level of productivities, up to 0.29-0.38 g DW L-1·day-1.
Eight strains, including control Ch. vulgaris IBASU-A 189 and T. dimorphus IBASU-A 254 were identified as the highest productive ones since they showed the increase of biomass in the range of 0.58 ±0.03 -1.6 ±0.26 g DW L-1·day-1. They were Desmodesmus magnus (Meyen) P. Tsarenko IBASU-A 402, D. multivariabilis var. turskensis P. Tsarenko et E. Hegew., Ch. vulgaris IBASU-A 190 (=CALU 158), P. kessleri IBASU-A 197 (UTEX 397), P. kessleri IBASU-A 198, and T. dimorphus IBASU-A 251 [33].
According to the published data, a number of promising biomass and lipid producers have been found among the representatives of families Chlorellaceae (Auxenochlorella (I. Shihira et R.W. Krauss) T. Kalina et M. Punčochárová, Chlorella, Chloroidium, Parachlorella) and Scenedesmaceae (Desmodesmus, Scenedesmus, Tetradesmus (=Acutodesmus) [12, 13, 34-37].
Mahmoud et al. [35] reported the strains of Ch. vulgaris and “Scenedesmus quadricauda” isolated from different localities of Egypt with the biomass productivity of 1.23-1.09 g DW L-1·day-1 and lipid content of 37% and 34% of DW, respectively.
Aboun-Shanab et al. [36] reported the strain “Scenedesmus obliquus” YSR01 isolated in China from a fresh water body with a growth rate of 1.68 day-1, biomass productivity of 1.57 g DW L-1·day-1 and total lipid content 58% of DW [37].
Liu et al. [12] studied 43 algal strains from the FACHB collection; China isolated and identified as members of Chlorellaceae and Scenedesmaceae families for biofuel production purposes. In the course of a wide-scale screening of Chinese algal strains, 10 promising candidates for biofuel applications were selected according to three important parameters: total lipid content, biomass productivity and lipid productivity. The biomass productivity of the tested strains ranged from 0.53 g DW L-1·day-1to 6.07 g DW L-1·day-1, with the highest biomass of 6.07 g DW L-1·day-1for S. bijugatus. The lipid content for all strains varied from 20% to 51% of DW. “Chlorella pyrenoidosa” Strain 4 was considered the best oil producers since it showed the best combination of biomass productivity and total lipid content. Under optimal conditions (BG-11 medium, bubbling air with 0.5% CO2, light intensity 150 µmol m-2 s-1) algae accumulated 3.02 g DW L-1·day-1, with total lipid 51.28% of DW.
Although research on this field is mainly focused on testing the oil-producing strain capacity in outdoor cultivation under stress conditions such as changing light intensity, pH, temperature, salinity etc, we believe that two additional criteria should be considered: resistance to stresses and biological contaminations. Many researchers use the first one in preliminary experiments for the determination of optimal growth conditions for experiments; however, the second one is often left out of consideration. Regardless of high growth parameters and lipid content, the use of some strains is challenged because of their vulnerability to invasion by other algae, bacteria or fungi. The species of Chlamydomonas have been excluded from our list of promising strains due to their high sensitivities to contamination by the small-celled green Chlorella-like and green-blue algae, which were evident under cultivation. Some strains of Botryococcus also appeared to be highly sensitive to biological contaminations.
Our experiments on the oil accumulation included the determination of total lipid content and the fatty acid profile as two very important parameters for the selection of strains for biodiesel purposes. The strains Ch. vulgaris IBASU-A 189, 190, P. kessleri IBASU-A 444 and T. dimorphus IBASU-A 344 were cultivated under normal growing conditions without nitrogen deprivation [13, 14, 16, 38], phosphate limitation [39], high salinity [14, 16, 40], high light intensities [41] and iron content of the media [42] to improve the lipid content. Only for the T. dimorphus IBASU-A 251, an additional experiment was carried out to determine the impact of nitrogen deprivation on total lipid concentration and fatty acid profiles. Accordingly, T. dimorphus IBASU-A 251 was cultivated in two stages: the first two weeks on full media and the second two weeks on media without nitrogen.
For the determination of total lipids, samples (1 g of freeze-drying algal biomass) were extracted with isopropanol, then by a mixture of isopropanol: chloroform (1:1, v/v), followed by a mixture of chloroform: methanol (1:1, v/v) repeated twice. The weight of the crude lipid obtained was determined gravimetrically. The fatty acids profile of the extracted lipids was determined as methyl esters (FAMEs). The FAME composition was determined by the gas-liquid chromatography method using a Gas Chromatography GC-16A Shimadzu (Japan) with a flame ionization detector and the GS solution software. A capillary column THERMO TR-FAME (30 mm×0.25 mm ID×0.25 mm film) with a temperature gradient from 70 to 230°C was used for a split. Helium was used as carrier gas at a flow rate of 1 mL∙ min-1. The temperature of the injector and detector was 280 and 260°C, respectively. The identification of the fatty acids was carried out by comparing the retention time of the extracted compounds with that of standards. The content of fatty acids was expressed as a percentage of total dry weight [43].
Overall, under standard growing conditions, the total lipid content was 16.9±0.7% and 17.5±1.2% of DW for Ch. vulgaris IBASU-A 189, 190, respectively; 10.53±0.5% of DW for P. kessleri IBASU-A 444 and 12.4±2.5% and 17.5±3.8% of DW for T. dimorphus IBASU-A 251 and 344, respectively (Fig. 2 a, b). The total lipid content of 19.8±2.4% of DW was obtained for algal biomass of T. dimorphus IBASU-A 251 grown under nitrogen-deprivation; thus lipid content increased up to 37.4% of DW under nitrogen deprivation with respect to standard growing conditions [39]. This observation is in line with the data from other researchers who investigated the effects of nitrogen deprivation on the lipid content of green microalgae from Chlorellaceae and Scenedesmaceae [13, 16].
The chemical characteristics of fatty acids, such as carbon chain length and unsaturation extent determine biodiesel properties. Therefore, it is necessary to identify fatty acid profiles of the most promising algal strains. Palmitic (C16:0), oleic (C18:1), linoleic (C18:2) and linolenic (C18:3) are recognized as the most common fatty acids in microalgal lipids [22]. Our results showed that all these saturated, monounsaturated and polyunsaturated fatty acids were produced by the strains under study (Fig. 3). The most abundant fatty acids found in Ch. vulgaris IBASU-A 189, 190 were C16:0 – 21.28% and 18.94%, C18:2 – 20.92% and 26.82% and C18:3 – 26.48% and 26.48%, respectively. The same fatty acids were found in P. kessleri IBASU-A 444 – C16:0 – 16.32%, C18:2 – 26.38% and C18:3 – 22.80%. Ch. vulgaris IBASU-A 189, 190 and P. kessleri IBASU-A 444 also produced palmitoleic (C16:1) and oleic (C18:1) acids but in small amounts. On the contrary, in T. dimorphus IBASU-A 251, 344 most abundant fatty acids were C18:1 – 21.80% and 20.96%, less abundant – 11.41% and 12.41%, respectively, and minor acids C18:0, C18:2 and C18:3 were also found. Under nitrogen-deprivation in the culture of T. dimorphus IBASU-A 251, the quantity of dominant acids C16:0 increased twice, C18:1 up to 34%; minor acids C18:2 – one and a half and C20:1 – in three times [43].
Thus, the screening of promising candidates for the algal-based production conducted on IBASU-A collection allowed to reveal five high-efficiency strains belonging to genera Chlorella, Parachlorella and Tetradesmus, which were capable of accumulating biomass more than 1.2 g DW L-1·day-1. While the total lipid contents for them varied from 10.53% to 19.8% of DW, the composition of fatty acids in the selected strains was mainly C16:0, C18:2 and C18:3. They were considered to have significant potential for biofuel applications, but further investigations on improvements in culture conditions to elevate their lipid productivities have to be conducted.
| Species | Strains of IBASU-A |
Growth Characteristics | ||
| Maximal Cell Concentration (B), Cells mL-1 |
Specific Growth Rate (µ), Day-1 |
Productivity (P), Cells mL-1∙Day-1 |
||
|
Monoraphidium griffithii (Berk.) Komárk.-Legner. |
364. 493 | 80-82·106 | 0.24 | 21.0-21.5·106 |
| M. minutum (Nägeli) Komárk.-Legner. | 574 | 248·106 | 1.4 | 72.5·106 |
| Monoraphidium sp. | 377 | 248·106 | 0.36 | 37.4 |
|
Raphidocelis subcapitata (Korschikov) Nygaard et al. |
358. 360 | 142-168·106 | 1.01-1.04 | 37.4-41.7·106 |
| Selenastrum gracile Reinsch | 317. 580 | 56.4-71.6·106 | 0.64-0.78 | 18.2-25.0·106 |
| Chlorela vulgaris Beijr. | 189 | 220·106 | 0.9 | 65.5·106 |

3.2. Screening of Wild Microalgae of Family Selenastraceae Adapted to Regional Environmental Conditions
During the years 2013-2018 48 strains of Chlorellaceae, Scenedesmaceae and Selenastraceae families were isolated to enrich the IBASU-A collection with Ukrainian strains from different climatic conditions. They were isolated mainly from inland shallow mesotrophic water bodies from the different physical and natural geographical regions (forest, steppe-forest and steppe zones). Among them eight strains of species were found and further investigated from Monoraphidium, Raphidocelis Hindák, Selenastrum Reinsch (Selenastraceae) genera that have currently attracted more attention of researchers worldwide [44-52].
The perspective of use and the need to study Selenastraceae-related strains have been most fully considered by Bogen et al. [44, 45]. A detailed investigation of some species from the genus Monoraphidium (M. arcuatum, M. contortum, M. dybowski, M. griffithii, M. neglectum, M. terrestre and M. tortile, all strains from SAG) was conducted in regard to biomass productivity, lipid amount and lipid profile as well as biomass degradability. The mean increase of biomass of strains was measured by the determination of dry biomass after the cultivation period, divided by the number of days of cultivation. It ranged from 0.033 g DW L-1·day-1 in M. tortile SAG 16.81 to 0.360 g DW L-1·day-1 in M. terrestre SAG 49.87. While total lipids content varied in the range of 15.9-31.5%. The strains of M. terrestre SAG 49.87 M. dybowski SAG 202-7e and M. contortum SAG 47.80 showed the highest biomass productivities; the highest total lipid content was found in M. tortile SAG 16.81.
Shrivastav et al. [51], studied a Monoraphidium sp. microalga isolated during the mass cultivation of Ettlia sp. for its biodiesel production properties. The biomass, lipid productivity and fatty acid profile of a new microalgal strain were examined under autotrophic cultivation conditions using various CO2 concentrations and mineral and organic nitrogen sources. This new strain was found as a promising feedstock for biodiesel production due to its high lipid content (28.92% of DW) and increase of C18:1 amount under cultivation with urea as a source of nitrogen.
Patidar et al. [50], also considered Monoraphidium minutum (Nägeli) Komárk.-Legner. for carbon sequestration and lipid production in response to varying growth modes.
Indeed, a previous study reported the use of Monoraphidium sp. for biodiesel production. As a matter of fact, Yu et al. [14], identified Monoraphidium sp. FXY-10 strain, isolated from Lake Fuxian in China, as a potential candidate for biodiesel production purposes. This strain had high growth and total lipid content up to 56.8% of DW under photoautotrophic conditions. Moreover, the cultivation of FXY-10 under heterotrophic conditions led to increase lipid productivity from 6.88 mg L−1 day−1 to 148.74 mg L−1 day−1 and the production of saturated and monounsaturated fatty acids up to 77.5%. Another Monoraphidiumsp strain, QLY-10, was isolated from Qilu Lake in Yunnan Plateau (China) and used for developing a two-step strategy for algal biomass and lipid production [46].
During our investigations for algal strains adapted to climatic conditions, eight strains of Selenastraceae family were isolated by conventional methods: cultivation of algae on enriched selective media followed by single-cell isolation using either a micropipette or agar plate techniques [29]. For taxa identification, both morphological and molecular-biological analyses were used. The last consisted of RAPDs (Random Amplified Polymorphic DNA) and TBP-method based on the conservatism of the exon sequences of β-tubulin genes in all eukaryotic organisms [53, 54].
The comparative studies by Nygaard et al. conducted on the growth characteristics of Monoraphidium griffithii IBASU-364, 493, M. minutum IBASU-A 574, Monoraphidium sp. IBASU-A 377, Raphidocelis subcapitata (Korschikov) Nygaard et al. IBASU-A 358, 360, Selenastrum gracile Reinsch IBASU-A 317, 580 and Ch. vulgaris IBASU-A 189 as a control, revealed that most of them possessed active growth, high specific growth rate (µ), and biomass productivity (P) (Table 1 ). There was no obvious adaptation phase in cultures of all strains under study. The stationary phase was observed at the fifth or sixth day from the beginning of experiments for the majority of strains. In comparison with Ch. vulgaris 189, the most productive strain was M. minutum 574 with increasing biomass of 1.84 g DW L-1·day-1, followed by R. subspicata 358, 360 with 1.32-1.48 g DW L-1·day-1 and Monoraphidium sp. 377 with 0.84 g DW L-1·day-1. Generally, the newly isolated Ukrainian strains demonstrated rather high growth characteristics such as the specific growth rate (0.38-1.2 day-1) and biomass productivity (0.31-1.84 g DW L-1·day-1) (Table 2, Fig. 2c). The total lipid contents of the three most productive strains M. minutum 574, Monoraphidium sp. 377 and R. subcapitata 358 were 33.65±0.87%, 29.43±1.07% and 23.14±1.25%, respectively. We note that the lipid content in the Monoraphidium spp. cultured under standard conditions was in the range of 19.9–43.5% [19].
A study of fatty acid composition of these strains showed a certain variability of dominant fatty acids in different strains (Fig. 3). The dominant fatty acids found in M. minutum 574 were C18:2 – 29.17% and C18:3 – 29.72%, in Monoraphidium sp. 377 – C16:0 – 28.32%, C18:1 – 38.86% and C18:2 – 14.63%, and in R. subcapitata 358 – C18:1 – 61.28% and C18:2 – 19.48%.
Our preliminary data about the fatty acid composition of Monoraphidium species are in accordance with those of other researchers. According to Li et al. [49], the fatty acid profile of Monoraphidium sp. QLY-1 (China) included dominant C16:0, C18:1, C18:2, and C18:3. The same dominant fatty acids were found in M. minutum isolated from freshwater lagoon from Hazira Surat (India) and their content varied under different experimental conditions [51]. The dominant fatty acids of Monoraphidium sp. FXY-10 (China) were C16:0, and C18:3 in autotrophic and C16:0 and C18:1 in heterotrophic cultures [14]. A cold-adapted strain of Monoraphidium sp. isolated from an Antarctic ice-covered lake produced a high amount of polyunsaturated fatty acids C16:4 and C18:4 under low temperature conditions [19].
As shown by Bogen et al. [44], all species of Monoraphidium (M. arcuatum, M. contortum, M. dybowski, M. griffithii, M. neglectum, M. terrestre and M. tortile) under study had the fatty acid profiles with high relative amounts of C16:0, ranging from 16.5% in M. dybowski SAG 202-7e to 27.3% in M. neglectum SAG 48.87 and high contents of C18:1 fatty acids of up to 57.6% in M. neglectum. It has been revealed that there is a link between the prevalence of C16:0 and C18:1 fatty acids and the maximum growth phase when algae are harvested. The increasing contents of these fatty acids were also observed during nitrogen starvation. Thus, it can be argued that specific nitrogen starvation conditions might enhance the levels of useful lipids.
4. RESULTS AND DISCUSSION
Our results confirmed the necessity of continuing investigations on this promising family of green microalgae. The eight Ukrainian Selenastraceae strains recently added to the IBASU-A collection had quite high active growth, biomass productivity, total lipid content and favorable fatty acids for biodiesel production purposes. The most promising strains were M. minutum IBASU-A 574, Monoraphidium sp. IBASU-A 377 and R. subcapitata IBASU-A 358 with increasing biomass 0.84-1.84 g DW L-1·day-1.
CONCLUSION
The screening of the Microalgae Culture Collection of M.G. Kholodny Institute of Botany, NAS of Ukraine (IBASU-A), the largest algal collection in Ukraine, allowed to reveal 33 strains belonging to the Chlorellaceae and Scenedesmaceae families, which partly satisfied the criteria for the selection of promising candidates for biodiesel production. They showed high growth characteristics (specific growth rate and biomass productivity). However, the lipid content in these strains did not exceed 20% of DW. Additional efforts to increase lipid productivities in these strains are therefore needed. Nevertheless, these strains could be used as dual-purpose organisms suitable for feedstock production and bioremediation.
The screening of wild microalgae of the Selenastraceae family allowed the isolation of a number of strains from Ukrainian regions under different climatic conditions. In particular, eight strains of species from Monoraphidium, Raphidocelis and Selenastrum genera had rather high active growth and biomass productivity. The most high-efficiency strains were M. minutum IBASU-A 574, Monoraphidium sp. IBASU-A 377 and R. subcapitata IBASU-A 358. They basically satisfied all the criteria for biodiesel fuel production. Their biomass productivities were recorded as 1.84, 0.84 and 1.32 g DW L-1·day-1, while the lipid contents were 33.65%, 29.43% and 23.14%, respectively. Moreover, they had favorable fatty acid compositions for biodiesel production purposes.
Thus, the special IBASU-A collection of algal strains has been supplemented with eight strains of Selenastraceae with valuable biotechnological properties. These strains will be the subject of future biotechnological and genetic engineering studies.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial and otherwise.
ACKNOWLEDGEMENTS
We are very grateful to Dr. Sc. O.O. Molodchenkova for help in the analysis of the fatty acid composition of total lipids of coccoid green algae, and M.O. Konishchuk for assistance in the technical work and design of graphic research material.
This work was carried out in accordance with the projects of targeted, comprehensive research programs of the National Academy of Sciences of Ukraine “Biomass as a raw material for biofuel” (Biofuels) and “Bioenergoconversion”.


