All published articles of this journal are available on ScienceDirect.
Response of Seeds Quality of Sunflower to Inoculation with Single and Mixed Species of Indigenous Arbuscular Mycorrhizal Fungi
Abstract
Background:
The sunflower seeds which are popular in Syria and Mediterranean countries as a roasted or salted snack, supply a large number of nutritious components including protein and minerals. A pot experiment was conducted to determine the impact of indigenous Arbuscular Mycorrhizal Fungi (AMF) on phosphorus (P), nitrogen (N), and protein content in seeds of confection-type hybrid sunflower (Helianthus annuus L.). The indigenous AMF including Glomus intraradices, Glomus mosseae, and Glomus viscosum, were isolated from an agricultural field in Syria. The most abundant species (G. viscosum) was multiplied in monospecific culture.
Method:
Sunflower plants were inoculated with the mixture of three AMF species or with G. viscosum. The plants were harvested at full physiological maturity stage. Total N was determined using the Kjeldhal method and the detection of phosphorus was based on the colorimetric method. The rate of AMF-root colonization was determined microscopically by the gridline intersect method.
Result:
Seeds N and P content were enhanced by the inoculation of sunflower with single and mixture of AMF compared with non-AM plants. Higher content of N and P was observed in AMF mixture inoculation compared to individual inoculation with G. viscosum. The maximum protein concentration was found in the treatment of mixture AMF followed by individual inoculation with G. viscosum. The results indicated that mixed species of AMF were more species, and could be considered as a good inoculum for improving the seeds quality of sunflower grown under sustainable agriculture conditions.
1. INTRODUCTION
The sunflower seeds are popular in Syria and some Mediterranean countries as freshly roasted or salted snack. Yields of non-oil or confection-type sunflowers are closely tied to the quality of the seeds [1]. The sunflower seed contains a large number of nutritious components including minerals and protein [2]. On the other hand, seed proteins provide the sulfur (S) and nitrogen (N) needed for seedling development after germination [3]. Seeds productivity and quality are greatly affected by genotype and environmental factors. According to Nel and Loubser [4], seeds quality of sunflower is influenced by environmental more than by genetic effects. In conventional agriculture, improving seeds yield and quality can be conducted through the addition of chemical fertilizers [5] but the production of ‘nutrient-rich high-quality seeds’ in sustainable comportment demands the use of natural microflora of the soil rhizosphere [6].
Arbuscular Mycorrhizal Fungi (AMF) are an important component of the useful microbial community in the soil. AMF play an essential role in the nutrients uptake of the majority of plants, including many important crop species [7]. The extraradical mycelium spreads around the root and enhances the root surface area by which the AMF absorb and transport nutrients to the host plant [8]. Positive responses of sunflower to colonization by AMF were reported for growth and seed yield [9-11]. AMF can provide the dominant route for phosphorus supply to the plant [12] and strongly impact the distribution of nitrogen in seeds, increasing protein content [13]. The native species of AMF can function better in agricultural soils from which they are isolated [14]. However, there is a high intraspecific diversity between different isolates of one AMF species [15]. The diversity of AMF species in the roots was found to be correlated positively with phosphorus and nitrogen concentrations in plant shoot [16]. AMF species can generate different amounts of extraradical mycelium and can have different efficiencies in phosphate uptake [17-19], consequently, AMF species could influence plant growth differently [20]. The growth response can be highly positive when plant roots are colonized by AMF species that are complementary in nutrients uptake from soil pools. Thus, the AMF mixture may be more beneficial for the plant growth than any of the species separately [21, 22]. We hypothesized that indigenous species of AMF influence differentially the quality of seeds of sunflower grown in soil from which AMF is isolated. To test the hypothesis, sunflower plants were separately inoculated with single, or mixture of AMF species under sustainable agriculture conditions, to reveal the impact of the two types of inocula on the N, P and protein contents in seeds.
2. MATERIALS AND METHODS
2.1. Experimental Site
This experiment was conducted in plastic pots during the summer season (May to September 2015) at AECS Research Station, located within an arid region southeast of Damascus, Syria (33°21’ N, 36°28’ E) at 617 m above sea level. Sandy clay loam soil with pH 8.5, organic matter 1.8%, was air dried, sieved to pass a 3 mm screen, and pasteurized at 5 kGy of Gamma Ray (GR) with 60Co source using a gamma irradiator (ROBO, Russia).
2.2. AMF Inoculum Preparation
AMF propagules were obtained from the soil previously isolated [23] from an agricultural field in which cotton and sunflower were grown. For propagating AMF, pot-cultures were established by mixing original soil samples with sterilized sand (1:1 v/v) as a substrate for growth of onion (Allium cepa L.) as the trap plant. Pot-cultures were conducted during two successive cycles of 4 months. Plants were fertilized once a week with 20 ml Long Ashton nutrient solution [24] containing reduced P concentration. AMF isolate included Glomus intraradices (Schenck & Smith), Glomus viscosum (Nicolson), and Glomus mosseae (Nicol. & Gerd., Gerd. & Trappe). G. viscosum as the most abundant specie (based on the number of viable spores in the trap substrate) was multiplied in monospecific culture. This culture was produced in pots containing a 1:1 autoclaved soil:sand mix (vol/vol) by inoculation onion roots with the spores of G. viscosum. The whole pot’s content was air-dried and the roots were cut into 1-cm pieces and thoroughly mixed with the soil:sand substrate. The inoculum of the two AMF types consisted of fragments of onion root, mycelium and spores mixed with the substrate. The inoculum contained 580 propagules (spores, hyphae and colonized roots) per 100 g as determined using the Most Probable Number (MPN) assay [25].
2.3. Planting Procedures and Treatments
Four seeds of confection-type hybrid sunflower were sown in each pot (40 x 45 cm) containing 35 kg soil. Half of the pots received the AM inoculum (100 g per pot) by layering at 10 cm depth of the pots at the time of sowing. Two types of AMF inocula were used in this experiment: mixed species of AMF that included G. intraradices, G. viscosum, and G. mosseae, and the single specie (G. viscosum). Non-AM pots received the same amount of sterilized AMF inoculums. Seedlings were thinned to one per pot with keeping the roots of discarded plants in the soil to avoid removing the AMF inoculum. The experiment involved three treatments: control (no AMF inoculation), inoculation with AMF mixture, and inoculation with G. viscosum. The pots were arranged in a randomized complete block design with four replicates. The plants were grown under natural conditions with an average daylength 14 h, max/min temperature 36˚/17˚C, and average relative humidity 40%. No chemical fertilizers were added, and adequate soil moisture was maintained during the experiment.
2.4. Plant Sampling and Analysis
The plants were harvested at full physiological maturity stage. The seeds samples were ground and used for measuring nitrogen and phosphorus content. Total nitrogen content was determined using the Kjeldhal method [26]. The protein content was estimated by multiplying total N by nitrogen-to-protein conversion factor 6.25. The detection of phosphorus was based on the colorimetric method [26] using spectrophotometer (Thermo Spectronic, UK). The content of each nutrient (mg per plant) was obtained by multiplying nutrient concentration by seeds dry weight.
2.5. Determination of AMF Colonization
Roots samples were rinsed in tap water and cut into 1 cm fragments. The root segments, totally mixed, were cleared in 10% (w/v) KOH by heating at 90°C for 30 to 60 minutes, depending on the degree of lignification of the roots. It was then washed and stained with acid fuchsin in lactoglycerol. The rate of AMF-root colonization was determined under a binocular microscope by the gridline intersect method [27].
2.6. Statistical Analysis
The analysis of variance was done by the SAS program [28]. Means between the studied parameters were compared using Fisher’s Least Significant Difference (LSD) test at a confidence level of 5%.
3. RESULTS AND DISCUSSION
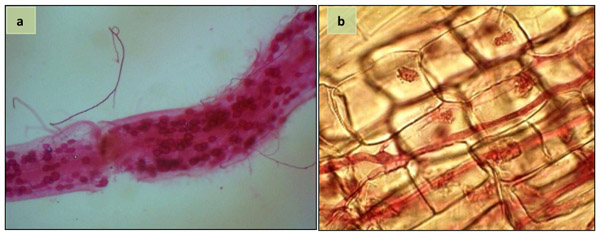
No AM colonization was noted in non-inoculated plants. AMF structures, i.e. arbuscules, vesicles and hyphae were observed in the root of inoculated plants at harvest (Fig. 1). However, no significant difference was found for the rate of AMF-root colonization of sunflower plants inoculated with single and mixed species of AMF (Table 1).
| Treatment | AM Colonization (%) |
Plant Height (m) |
Plant Dry Weight (g) |
Seeds Weight (g plant-1) |
Protein (%) |
|---|---|---|---|---|---|
| Control | 0 | 1.35±0.05 | 56.3±5.9 | 20.7±1.35c | 14.95±0.19c |
| Single AMF | 24.9±1.7a | 1.56±0.04 | 81.9±4.3 | 33.4±0.69b | 17.90±0.13b |
| mixed AMF | 27.6±2.0a | 1.71±0.06 | 96.8±8.5 | 39.5±3.64a | 18.44±0.18a |
| LSD | 3.03 | 0.99 | 12.9 | 4.5 | 0.34 |
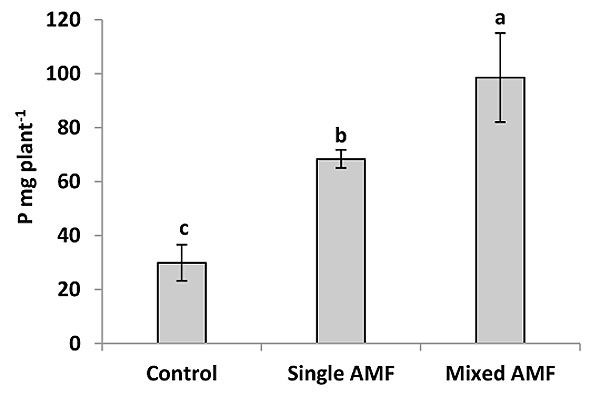
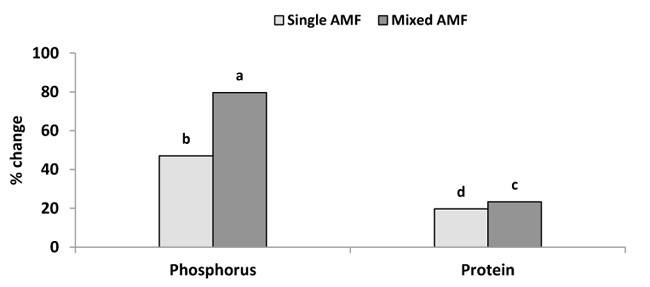
The P content was significantly greater in the seed of AM plants than the control (Fig. 2). This result was supported by Heidari and Karami [29] who showed that mycorrhiza treatment had a significant effect on P content in sunflower seeds. The inoculation with AMF enhanced P concentration in wheat grain [30] and in grain and vegetative tissue of barley [31]. In this study, the inoculation with single and mixture AMF significantly increased seed P concentration by 47 and 79.7% over the control (Fig. 4). This increase in seed P by AMF could be explained by the improvement of P uptake per unit of root length due to the enhancement of root surface area and providing an extra route for P uptake as mycorrhizal pathway [12]. The inoculation of mixed species induced higher P content in sunflower seeds compared to the individual inoculation of G. viscosum (Fig. 2). The increase in P content by AMF mixture was supported by the finding of earlier studies in cotton [32] and tomato [33]. Leek plants colonized by a mixture of G. claroideum and G. intraradices acquired more P than plants colonized with either of the two AMF separately [34]. Johnson et al. [16] reported that the P and N concentrations in the shoot were correlated positively with the diversity of AMF species in the roots. The difference in P content between the two inocula can be attributed to the different ability of AMF species to acquire resources below ground. AMF species can produce different amounts of extraradical mycelium and can have different efficiencies in phosphate uptake [17-19]. Moreover, the mycelium of AMF species uptake P from different distances from the roots [19, 35].
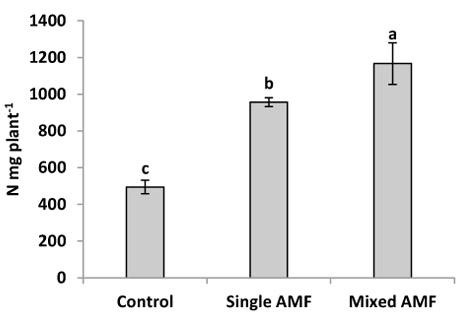
In this study, inoculation with indigenous AMF significantly increased the N content in sunflower seeds (Fig. 3). This result was supported by Criado et al. [31] who reported that AMF inoculation enhanced N concentration in grain and vegetative tissue of barley. The individual inoculation with G. viscosum gave higher N content in seeds compared to the control. However, the highest N content was observed in plants inoculated with AMF mixture (G. intraradices, G. viscosum, G. mosseae). This result is in conformity with that of Ibrahim [32] who reported that the N concentration in cotton was found to be higher in plant inoculated with the same mixture of AMF. Ramakrishnan and Selvakumar [33] showed higher N concentration in tomato seedling inoculated with AMF mixture (G. fasciculatum and G. intraradices) in comparison with single inoculation and control. Enhanced N content by AMF mixture can be explained by the suggestion that mixed AMF species which colonized roots are complementary in their uptake of nutrients from different soil pools, thus, they may be more beneficial for the plant as a mixture than any of the species separately [21, 22]. In addition, the enhanced nutrient uptake by the AMF mixture could be a consequence of greater aggregate stabilization, suggesting that a community of AMF was more efficient than single isolate [36].
The inoculation with single and mixed species of AMF gave higher protein concentration in seeds compared to control (Table 1). Single and mixture inoculation significantly increased protein concentration by 19.7 and 23.4% over the control (Fig. 4). Differences between seed protein concentrations were small but were significant for the two treatments of AMF inoculation (Table 1). The maximum protein concentration was found in the treatment of mixture AMF followed by individual inoculation and minimum protein concentration was recorded in the treatment of control (Table 1). This result of sunflower seed protein increase is in accordance with the results obtained by Kumar et al. [37] who showed increased protein content in grains of AM wheat. Sarah and Burni [38] reported that AMF had shown promising effects on crude protein in mycorrhizal sorghum. However, AMF species could influence protein content differently. Silva et al. [39] found that crude protein levels in seeds were not significantly affected by inoculation of sunflower with Glomus fasciculatum. On contrary, protein content was enhanced in seeds of soya inoculated with Glomus mosseae [40], maize inoculated with AMF mixture (Glomus intraradices, Glomus aggregatum, Glomus viscosum, Glomus etunicatum, and Glomus claroideum) [41], and wheat inoculated with G. intraradices and G. mosseae [30]. The results of sunflower seed protein increase are supported by new evidence on enhanced nitrogen nutrition in AM plants [8] and the nitrogen distribution in seeds [13]. The enhanced N acquisition increases the amino acids synthesis in the leaves, and this stimulates the accumulation of protein in the seed [42]. Moreover, protein contents are highly correlated with P nutrition, generally ameliorated in AM plants [43], but even more improved when different AMF are involved [44].
The enhanced accumulation of nutritious components in AM sunflower seeds was a reflection of the improved plant growth. Higher plant height and dry weight were observed in AMF inoculation in comparison with control (Table 1). The positive effect of AMF on growth and biomass was reported in sunflower [10, 11]. The highest plant height and weight observed in the AMF mixture inoculation could be due to the enhancement of nutrients uptake [45, 46] by the different species of AMF which can have different amounts of extraradical mycelium and different efficiencies in nutrients uptake from the soil [47].
CONCLUSION
The undertaken study shows the importance of indigenous AMF as a biofertilizer for improving seed quality of confection-type sunflower crop grown under semiarid conditions. Indigenous AMF were successful in colonizing sunflower roots and resulted in better plant growth compared to non-AMF treatment. The results indicate that the mixture of indigenous AMF (G. viscosum, G. intraradices and G. mosseae) gives higher values of N, P and protein content in sunflower seeds compared to the single species (G. viscosum). Therefore, the higher efficiency of AMF mixture than single species, suggests the preference of inoculation with mixed AMF inocula for the production of ‘nutrient-rich high-quality seeds’ of sunflower in sustainable comportment under semiarid conditions.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The author Acknowledges the Atomic Energy Commission of Syria for encouragement and technical support.


