All published articles of this journal are available on ScienceDirect.
Resistance of Black Pepper Seedlings to Phytophthora capsici-induced Basal Stem Rot Using Arbuscular Mycorrhizal Fungi and Monopotassium Phosphate Fertilizer
Abstract
Introduction
The disease caused by Phytophthora capsici is a major constraint in black pepper cultivation, with infections beginning as early as the seedling stage. Enhancing seedling resistance is crucial to mitigating losses, especially for replanting on infected land. This study aimed to evaluate the effectiveness of Arbuscular Mycorrhizal Fungi (AMF) and monopotassium phosphate (KP) fertilizer in suppressing foot rot disease in black pepper seedlings.
Methods
A split-plot randomized complete block design (RCBD) was employed. The main plot comprised two levels of AMF application (with and without), while the subplot consisted of five KP fertilizer doses (0, 1.g, 2.g, 3.g, and 5.0 g per seedling or polybag). Each treatment was replicated three times, with 10 seedlings per replicate. Observed variables included disease severity, disease incidence, disease infection rate, AUDPC, incubation period, and shoot length increment to evaluate seedling resistance to P. capsici.
Results
The AMF application enhanced black pepper seedling resistance to P. capsici, reducing disease severity and incidence, latent period, and AUDPC value, while promoting shoot growth. The application of KP fertilizer, up to a dose of 5.0 g per polybag on non-mycorrhizal seedlings, effectively induced resistance, as reflected by lower AUDPC values, slower disease progression, and delayed symptom onset.
Discussion
Black pepper seedling resistance to P. capsici is related to K nutrient uptake and AMF-induced resistance. Mycorrhizal fungi assist plants in K uptake. Evidence of the seedling resistance developed in land affected by basal stem rot is needed.
Conclusion
The combined use of AMF and KP fertilizer during the seedling stage offers a promising strategy to strengthen black pepper resistance against the disease, while also potentially reducing dependence on chemical inputs.
1. INTRODUCTION
Black pepper (Piper nigrum L.) is a plantation crop commodity used as a spice and in the food, beverage, and pharmaceutical industries, with increased demand not only from the domestic market but also for export. In Indonesia, most black pepper cultivation is managed by smallholder farmers. According to data from Badan Pusat Statistik (BPS), there has been a decline in production from 2021 to 2023. Indonesia's black pepper production reached 77.40 thousand tons in 2021, decreased to 75.20 thousand tons in 2022, and fell to 70.20 thousand tons in 2023 [1]. This decline has also affected pepper exports, both black and white pepper, which continued to decrease from 2019 to 2023.
A decline in black pepper production has also occurred in West Kalimantan. In 2023, production reached 4,631 tons, a decrease compared to 6,328 tons in 2022 [2]. This decline significantly affects community welfare, as black pepper remains one of the main income-generating commodities for local populations. Extensive black pepper cultivation is found in Sambas, Bengkayang, Sanggau, and Sintang, particularly in areas near the Malaysian border. The cultivation is generally still traditional, with limited input for plant nutrition or disease control. The cultivation practices used also contribute to the incidence of P. capsici infection, both in the field and during the seedling stage.
Black pepper production fluctuates due to multiple factors, such as agroclimatic conditions, the variety of black pepper cultivated, soil type, cultivation practices, and land conditions. One of the major limiting factors in production and quality decline is the high incidence of P. capsici (known as P. tropicalis) infection which remains inadequately controlled [3].
Poor growth performance of black pepper plants is often associated with increased incidence of basal stem rot disease. In the ultisol and inceptisol soils of Kerala, South India, black pepper production is influenced by acidic soil conditions, organic carbon content, low nitrogen (N) availability, moderate potassium (K) availability, and high phosphorus (P) availability, which vary seasonally [4].
The disease caused by P. capsici is a major concern, as it can infect all parts of the black pepper plant, including roots, stems, and leaves, at all growth stages. The most severe infections occur when the pathogen attacks the roots and stem base, leading to sudden death, wilting, and rapid plant mortality. Pathogens can also infect leaves, causing black spots and causing leaves to fall off [5].
P. capsici is a major constraint in pepper production worldwide because of its resistance to effective control measures. In Indonesia, it can reduce yields by 30–40% in affected plantations, with some cases causing total crop loss [6]. In west coast India, the average plant mortality rate reached 9.64%, resulting in losses of USD 902.04/ha, equivalent to 56% of annual net returns [7]. Meanwhile, in Vietnam, the disease reduces 2% of the total planted area every year [8].
Several integrated control strategies have been implemented, such as enhancing plant vigor through recommended cultivation practices, suppressing P. capsici populations by applying biological agents, including Trichoderma spp [9], endophytic yeasts [6], and antagonistic bacteria [10]. Additionally, a mixture of areca nut extract, gambier, betel leaf, and slaked lime at 0.01% concentration has been shown to inhibit disease development with efficacy comparable to 0.2% propineb [11].
Control of black pepper basal stem rot disease can also involve the use of mycorrhizal fungi, which are expected to enhance plant growth performance. In the rhizosphere of black pepper seedlings, several genera of mycorrhizal fungi have been identified, including Glomus, Gigaspora, Acaulospora, and Entropospora; while in mature plants, Glomus, Gigaspora, Scutellospora, and Entropospora have been reported [12]. Mycorrhizal plants are able to regulate phosphorus uptake, resulting in relatively stable absorption even under increased phosphorus fertilizer application. However, excessive phosphorus application may actually reduce its uptake in calcareous soils [13]. Phosphorus plays a role in the development of plant defenses against pathogens, although its effectiveness is closely linked to nitrogen availability. Potassium application, on the other hand, has been shown to enhance plant resistance to fungal pathogens [14].
The application of arbuscular mycorrhizal fungi (AMF) and KP fertilizer containing P2O5 and K2O can stimulate the development of plant defenses against fungal pathogens. Applying these two treatments at the seedling stage of black pepper is expected to produce seedlings with greater resistance to P. capsici infection after being transplanted to the field. This study aims to evaluate the resistance of black pepper seedlings to P. capsici infection.
The application of arbuscular mycorrhizal fungi (AMF) and KP fertilizer containing P2O5 and K2O can stimulate the development of plant defenses against fungal pathogens. Applying these two treatments at the seedling stage of black pepper is expected to produce seedlings with greater resistance to P. capsici infection after being transplanted to the field. We hypothesized that KP fertilizer would further enhance the resistance of AMF-inoculated black pepper seedlings to P. capsici. This study aims to evaluate the interactive effects of AMF and KP fertilizer on disease resistance.
2. MATERIALS AND METHODS
2.1. Experimental Design and Data Analysis
The experiment was conducted at the UPSBP experimental field in West Kalimantan Province and at the Plant Pathology Laboratory, Faculty of Agriculture, Tanjungpura University. The design used was a split-plot RCBD. The main plot factor was the application of mycorrhizal fungi, both without and with mycorrhizal fungi. The subplot factor was the dosage of KP fertilizer (without KP fertilizer; 1.5 g per polybag; 2.5 g per polybag; 3.5 g per polybag; 5.0 g per polybag). All treatments were replicated 3 times, with 10 sample plants for each replicate. Data were analyzed using analysis of variance (ANOVA) for the main factor (AMF), the subplot factor (KP fertilizer dosage), and their interaction. Treatment means were compared using Tukey’s test at α = 0.05. Data not meeting ANOVA assumptions were transformed to achieve homogeneity of variances.
2.2. Preparation of P. capsici Inoculum Source
The P. capsici inoculum used was soil from black pepper nurseries that had been repeatedly planted and showed basal stem rot symptoms. The presence of the pathogen was confirmed using a baiting technique with black pepper leaves and tendrils, followed by microscopic observation to obtain sporangia or oospores of the pathogen (Fig. 1). This soil had been used in pathogenicity tests in another study [15].
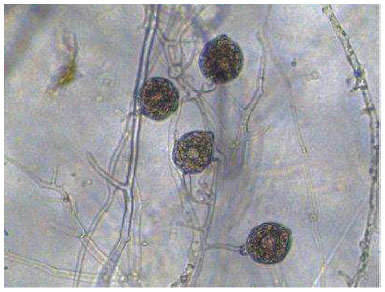
P. capsici: the causal agent of basal stem rot disease in black pepper var. bengkayang.
2.3. Preparation of Black Pepper Seedlings
Black pepper seedlings used were 2 months old, grown in a nursery under a shade house. The growing medium consisted of a mixture of soil, burnt rice husk, and cow manure in a 2:1:1 (v/v/v) ratio. Black pepper cuttings of the Bengkayang variety were prepared using one-node segments. The planting medium was divided into two groups: one added with AMF and one without AMF inoculum. The AMF inoculum was soil containing 31.5 spores of mixed Glomus sp. and Acaulospora sp. per 10 g. The spores were previously multiplied by planting them on maize cobs.
Black pepper seedlings were planted in 330 mL plastic cups. For treatments involving AMF, 20 g of inoculum was placed at the bottom of the planting hole (Fig. 2). The seedlings were kept in a shade house covered with a paranet, and humidity inside the structure was maintained by misting water with a sprayer. Seedlings remained in the shade house for two months until root formation. Every two weeks, root colonization by AMF was checked using the root staining method on three randomly selected black pepper seedlings [16]. Once 100% of the AMF-treated seedlings showed colonization, all seedlings were transferred to polybags filled with soil that had been contaminated with P. capsici.
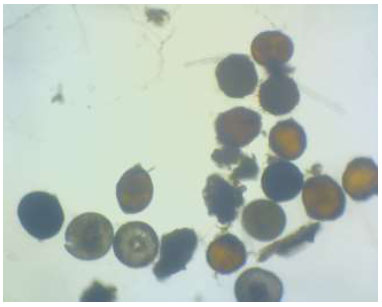
Mixed mycorrhizal fungi spores in soil used as an inoculum source.
2.4. KP Fertilization
KP fertilization was carried out according to the treatment, 3 days after transplanting to polybags. The fertilizer used contains P (P2O5 52%) and K (K2O 34%). Fertilizer was applied to both mycorrhizal and non-mycorrhizal black pepper seedlings. The fertilizer was spread around the base of the stem when the soil was moist.
2.5. Observation Variables
Observation variables included primary variables, such as disease severity, disease incidence at 1, 2, 3, 4, 5, 6, 7, and 8 WAP (weeks after planting), incubation period, disease infection rate, and area under the disease progress curve (AUDPC). Disease severity (DS) was calculated using the following formula:
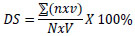 |
Where DS = disease severity (%), ∑(n × v) = number of seedlings showing symptoms at a certain score, N = total number of seedlings used, and V = highest score used. Disease severity was determined using a 4-category scoring key: 0 = healthy plant, 1 = many yellowing leaves, 2 = many yellowing and wilting leaves, 3 = blackened stem base, total wilting, and plant death [6]. Disease incidence was calculated using the following formula:
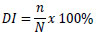 |
Where, DI = disease incidence (%), n = number of plants showing symptoms, and N = number of plants observed. The incubation period was determined from transplanting to the polybag until the appearance of initial symptoms. Observations of disease severity, disease incidence, and incubation period were conducted weekly from the onset of symptoms. AUDPC was calculated using the following formula [17]:
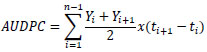 |
Where, yi = assessment of disease (ordinal score) at the ith observation, t = time (in weeks) at the ith observation, and n = total number of observations. The infection rate was measured based on the increase in the number of infected plants each week.
Supporting variables observed included shoot length increment, plant dry weight, potassium content in the shoot tissue, and salicylic acid content at the Research and Biochemistry Laboratory, Faculty of Mathematics and Natural Sciences, Universitas Tanjungpura. Measurements of salicylic acid content were carried out using a spectrophotometric method [18]. Potassium content was measured using the Morgan-Wolf method [19].
3. RESULTS
Black pepper seedlings treated with AMF inoculum and KP fertilizer showed resistance to P. capsici infection. This was evident from the lower disease severity and incidence compared to the control, i.e., seedlings not treated with AMF and the fertilizer. Seedling resistance was also observed in the relatively longer latent period, lower infection rate, and smaller AUDPC values. However, resistance of black pepper seedlings to basal stem rot disease did not significantly affect growth. This indicates that resistance induction has occurred, especially due to AMF and the provision of P and K elements through fertilizer. The growth influenced by the application of AMF and KP fertilizer was limited to shoot length increment, with no effect on shoot dry weight or fresh weight of black pepper seedlings.
The P. capsici attack on black pepper seedlings, initially with incidence and then increasing disease severity, continued especially in the control treatment. In contrast, in seedlings treated with AMF, and particularly those given high doses of KP fertilizer, the disease incidence was relatively low (Fig. 3). Disease progression appeared relatively slow, indicating that the pathogen attack was suppressed. The black pepper seedlings were relatively resistant to P. capsici infection.
The resistance of black pepper seedlings treated with AMF and KP fertilizer to P. capsici infection showed differences among treatment combinations, as determined by Tukey’s test.
As mentioned in Table 1, disease severity decreased when black pepper seedlings were inoculated with AMF, while KP fertilizer alone did not significantly suppress severity. This contrasts with disease incidence, which was reduced by KP fertilizer, especially in AMF-inoculated seedlings. Increasing the fertilizer dose did not enhance resistance, regardless of whether seedlings were inoculated with AMF. Seedling resistance was based on disease severity, disease incidence, latent period, AUDPC values, and low infection rate. The application of KP fertilizer only increased seedling resistance in non-mycorrhizal plants at the highest dose (5 g per polybag). This was demonstrated by the lack of significant differences compared to seedlings inoculated with AMF. Seedling growth of black pepper did not show differences among the treatments as a combination of AMF inoculation and KP fertilizer. Differences were only observed in non-mycorrhizal seedlings that were given KP fertilizer at a lower dose of 5 g per polybag Table 2.
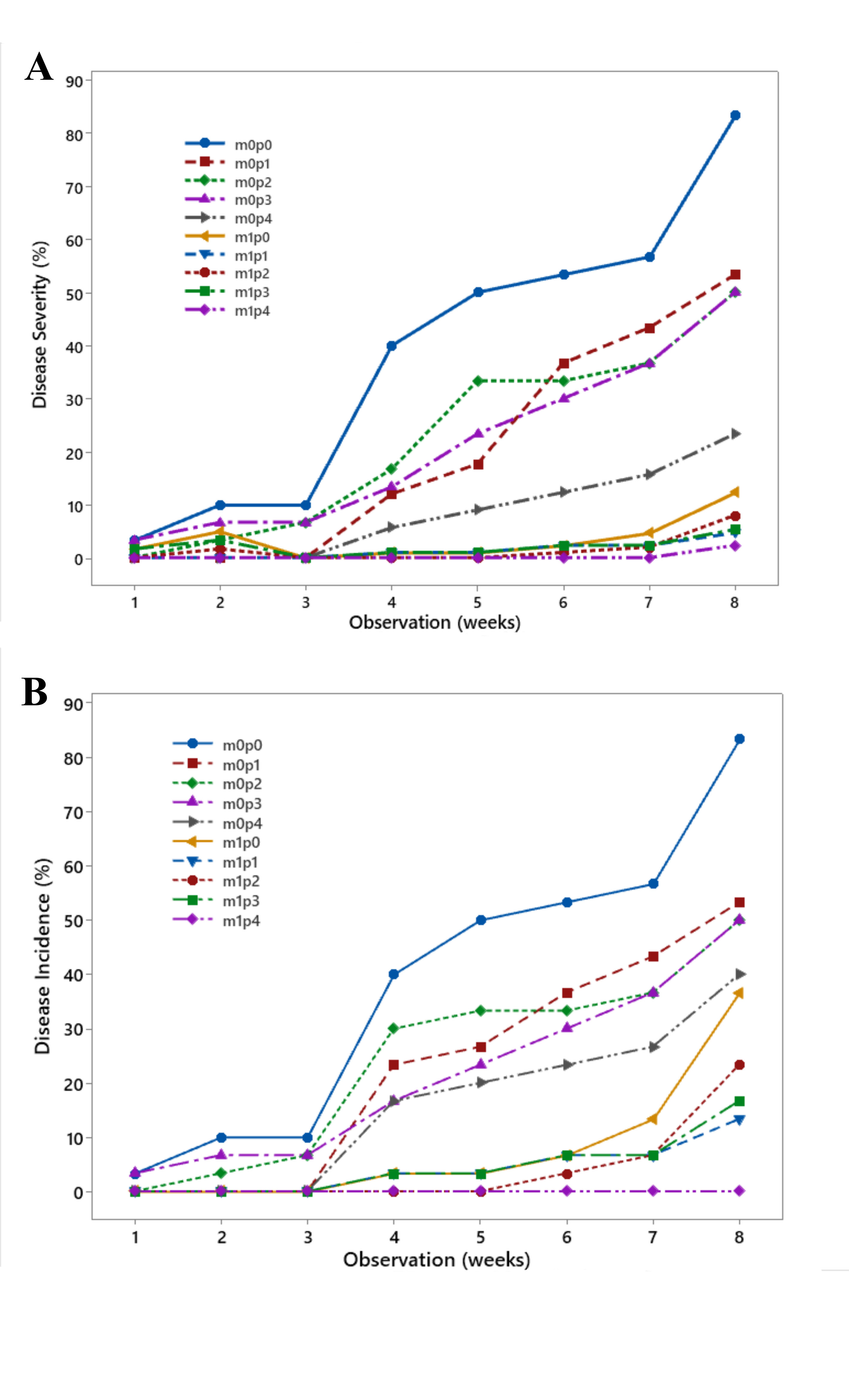
Disease progression of P. capsici infection on black pepper seedlings treated with AMF (m) and KP fertilizer (p). (A) Disease Severity (%); and (B) Disease Incidence (%). m = AMF application (0 = no, 1 = given); p = dosage of KP fertilizer application in each polybag (0 = no fertlizer, 1 = 1.5 g, 2 = 2.5 g, 3 = 3.5 g, 4 = 5 g).
| Combination of Treatments | Disease Severity (%) | Disease Incidence (%) | Latent Period (days) | AUDPC value |
|---|---|---|---|---|
| m0p0 | 83.33a | 83.33a | 38.37d | 198.3a |
| m0p1 | 53.33ab | 53.33ab | 47.27cd | 151.67ab |
| m0p2 | 50.00ab | 50.00ab | 48.53bc | 128.33ab |
| m0p3 | 50.00ab | 50.00abc | 47.53cd | 128.33ab |
| m0p4 | 23.33bc | 40.00abc | 50.70abc | 54.83bc |
| m1p0 | 12.33bc | 31.67abc | 55.87abc | 16.33c |
| m1p1 | 4.67c | 13.31cd | 57.70ab | 8.17c |
| m1p2 | 8.00 c | 23.33bc | 58.17ab | 7.00c |
| m1p3 | 5.33 c | 16.67bcd | 57.40ab | 18.17c |
| m1p4 | 2.33 c | 0.00 d | 59.50 a | 0.00c |
| Combination of Treatments | Increase of Shoot Length (cm) | Dry Weight of Shoot (g) | Infection Rate (plants/week) | K Content of Shoot (%) |
|---|---|---|---|---|
| m0p0 | 6.01b | 3.32 | 3.87 a | 1.20 |
| m0p1 | 0.03 a | 4.79 | 1.62 ab | 1.83 |
| m0p2 | 0.37 a | 6.32 | 1.49 abc | 1.69 |
| m0p3 | 1.87 a | 2.90 | 1.77 ab | 2.21 |
| m0p4 | 3.97 a | 3.93 | 1.09bc | 2.10 |
| m1p0 | 3.55 a | 5.32 | 0.99bc | 2.93 |
| m1p1 | 4.87 a | 7.26 | 0.31bc | 2.22 |
| m1p2 | 5.17 a | 3.71 | 0.56bc | 2.69 |
| m1p3 | 3.93 a | 8.41 | 0.4bc | 2.51 |
| m1p4 | 4.83 a | 7.14 | 0.00c | 2.65 |
The inhibition of disease development due to AMF and KP fertilizer application did not result in increased growth of black pepper seedlings. In mycorrhizal seedlings treated with KP fertilizer, resistance was observed, with fewer plants showing infection symptoms compared to non-mycorrhizal ones. Applying KP fertilizer at a dose of up to 5 g per polybag significantly reduced the rate of infected plants. In contrast, in mycorrhizal plants, increasing the dose of KP fertilizer did not reduce the infection rate of P. capsici in Bengkayang black pepper seedlings.
In black pepper seedlings showing healthy or mildly diseased symptoms, the detected salicylic acid levels were lower than in those exhibiting more severe symptoms. Mycorrhizal fungi may aid in the production of salicylic acid Table 3.
| Combination of Treatments | Salicylic Acid Content | Explanation |
|---|---|---|
| m0p0 | Not detected | Healthy, no symptoms |
| m1p0 | 1.16 ± 0.21 | Healthy, no symptoms |
| m1p1 | 1.44 ± 0.23 | Mildly infected; some leaves were yellowing |
| m1p1 | 2.00 ± 0.53 | Moderately infected; some leaves were yellowing and wilting |
| m1p3 | 2.43 ± 0.20 | Moderately infected; some leaves were yellowing and wilting |
| m1p3 | 2.50 ± 0.26 | Severely infected; yellowing leaves and blackening branches |
4. DISCUSSION
The suppression of P. capsici infection in black pepper seedlings may also be related to the potassium content in the plant tissue. Mycorrhizal plants showed higher K content compared to non-mycorrhizal plants. Potassium analysis was carried out on plants that did not show infection symptoms. The K content in diseased plants was only 1.05%. Mycorrhizal fungi can enhance potassium uptake even when it is sufficiently available in the soil but not readily absorbed by plants [20].
Some black pepper seedlings grown in P. capsici-contaminated soil showed greater resistance. This was closely related to the K content and the application of mycorrhizal fungi (AMF). Vanilla plants treated with KCl fertilizer and AMF showed better growth and root development in terms of weight and length [21]. P. capsici infects through the roots, causing damage and even rot. Plants with well-developed roots tend to be more resistant to pathogen infection. In this study, disease progression was slower, with fewer infected black pepper seedlings as indicated by a lower infection rate.
Mycorrhizal black pepper seedlings can absorb water and nutrients more efficiently through arbuscular mycorrhizal fungal hyphae, which expand the plant’s absorption area and are known as the mycorrhizal pathway [22]. AMF facilitate the uptake and transport of potassium to the host plant, as indicated by the high accumulation of potassium in AMF structures and plant tissues [20]. The application of AMF together with KP fertilizer can increase the availability of phosphorus and potassium nutrients for black pepper seedlings, while also enhancing the plants’ resistance to P. capsici infection.
Potassium (K) influences physiological and biochemical processes, such as enzyme activation, solute transport, and balance control, all of which are associated with plant resistance to fungal and bacterial infections. However, the level of resistance varies depending on the type of pathogen and its interaction with the host plant. The effect of K nutrients in forming resistance to pathogen infection and plant growth depends on its content in the tissue and the ratio with other nutrients. [23]. The plant’s ability to absorb K depends on its availability in the soil and soil conditions. Under limited K availability, absorption can be enhanced through mycorrhizal fungi.
The use of mycorrhizal fungi significantly enhanced the resistance of black pepper seedlings to P. capsici infection. Several indicators of pathogen attack, such as disease severity, disease incidence, infection rate, and AUDPC values, were lower in mycorrhizal-treated seedlings. The resistance observed in mycorrhizal plants is not only associated with improved nutrient balance and rapid replacement of damaged tissues, but also with the expression of genes responsible for the synthesis of defense-related proteins, competition with other soil microbes, including Phytophthora spp., and increased levels of the phytohormone salicylic acid, which plays a key role in induced resistance [24, 25], as well as the production of phytoalexins [26].
Salicylic acid accumulation in plant tissues can serve as an indicator of a plant’s defensive capacity. Mycorrhizal fungi can act as a trigger for plants to synthesize salicylic acid, which has the ability to activate various antioxidant enzymes, act as an elicitor for PR-protein genes with antimicrobial properties, induce programmed cell death [27], regulate the formation of reactive oxygen species (ROS), and contribute to the establishment of induced resistance or systemic acquired resistance (SAR) [28]. The accumulation of salicylic acid in plant tissues aligns with the findings of a previous study [29], which reported higher levels of salicylic acid in infected plant tissues.
STUDY LIMITATION
It is recommended that seedlings showing resistance be planted in land infected with Phytophthora to evaluate whether the developed resistance remains effective under field conditions.
CONCLUSION
Based on the research results, it can be concluded that applying AMF and KP fertilizers to black pepper seedlings can increase resistance to P. capsici attacks. This resistance is based on lower disease severity, disease incidence, infection rate, and AUDPC values, as well as a longer latency period. Increasing the K content of KP fertilizer resulted in increased resistance, but in mycorrhizal seedlings, the K application did not increase plant resistance to P. capsici infection.
The induced resistance response in plants due to the application of AMF and KP fertilizer, while effective against the pathogen, did not result in increased growth of black pepper seedlings, shoot dry weight, or shoot length. This suggests a potential trade-off in resource allocation between defense and growth in the seedlings.
AUTHORS’ CONTRIBUTIONS
The authors confirm contribution to the paper as follows: N.I.L. and F.R.: Conceptualization; N.I.L.: Methodology and Investigation; F.R.: Writing the original draft preparation; Formal analysis; F.R. and E.S.: Writing, reviewing, and editing; All authors have read and agreed to the published version of the manuscript.
LIST OF ABBREVIATIONS
| AMF | = Arbuscular Mycorrhizal Fungi |
| RCBD | = Randomized Complete Block Design |
| UPSBP | = Unit Pengawasan dan Sertifikasi Benih Perkebunan |
| AUDPC | = Area Under Disease Progress Curve |
| DI | = Disease Incidence |
| DS | = Disease Severity |
AVAILABILITY OF DATA AND MATERIALS
The data sets used and/or analysed during this study are available from the corresponding author [F.R.] upon request.
ACKNOWLEDGEMENTS
The authors would like to express their gratitude to the UPSBP West Kalimantan for the research facilities used during the fieldwork. They would also like to extend their gratitude to the Faculty of Agriculture's Plant Disease Laboratory for facilitating the microscopic observations.


