All published articles of this journal are available on ScienceDirect.
Phosphate-Solubilizing Rhizosphere Bacteria Enhancing Phosphorus Uptake and Maize Yield in Dyked Alluvial Soil
Abstract
Introduction
Low phosphorus (P) availability, despite adequate total P, is a major constraint to maize production in dyked alluvial soils (DAS) of the Mekong Delta, Vietnam. Conventional P fertilizers are inefficient in these soils, leading to low P use efficiency and reduced yield. Thus, the aim of this study was to identify phosphate-solubilizing rhizosphere bacteria (PSRB) from maize cultivation soil and assess the performance of selected indigenous bacteria in maize cultivation in DAS.
Methods
A total of 36 maize rhizosphere samples from DAS were collected to isolate PSRB and determine which was most beneficial for maize cultivation. Additionally, the pot experiment consisted of nine groups as follows: 100% phosphate according to the recommended fertilizer formula (P-RFF), 75% P-RFF, 50% P-RFF, 25% P-RFF, 75% P-RFF and PSRB, 50% P-RFF and PSRB, 25% P-RFF and PSRB, 0% P-RFF and PSRB, and 0% P-RFF in DAS with low phosphorus availability.
Results
The results indicated that among the 67 isolates, strains ASD-15, ASD-43, and ASD-56 exhibited the highest phosphorus concentrations, solubilizing 74.1 mg Al-P/L, 42.2 mg Ca-P/L, and 98.0 mg Fe-P/L, respectively, after five days of incubation. The strains were identified as Enterobacter asburiae by 16S rDNA sequencing. The application of PSRB increased soil-soluble P by 7.1 mg kg−1 compared with the uninoculated control, enhanced total P uptake in maize by 31%, and improved grain yield by 20.2%. Notably, combining PSRB with 75% P-RFF achieved yield and P uptake equivalent to 100% P-RFF, indicating that biofertilizer use could reduce chemical P fertilizer input by 25% without yield reduction.
Conclusion
Indigenous PSRB, E. asburiae, can improve soil P availability and maize productivity in DAS, supporting reduced reliance on chemical fertilizers. Future work should validate these findings under field conditions and explore PSRB-based biofertilizer formulations for large-scale application.
1. INTRODUCTION
Maize (Zea mays L.) is one of the world’s most important cereals and a major crop in An Giang Province, Vietnam. To sustain high yields, farmers frequently apply chemical phosphate fertilizers, but these inputs can introduce heavy metals such as cadmium and negatively affect soil health [1–3]. Phosphorus (P) is a vital macronutrient for plant growth [4]. However, in the soil, phosphorus is fixed in insoluble forms, including Al–P, Fe–P, Ca–P, and Zn–P, which plants are unable to take up from the soil [5,6], resulting in low phosphorus use efficiency [7].
Dyked alluvial soils (DAS) of the Mekong Delta are characterized by low soluble P content despite having sufficient total P, largely due to strong fixation of phosphate by Al, Fe, and Ca compounds [8]. This P unavailability severely constrains maize production in these systems. Soil management strategies, such as site-specific nutrient management (SSNM) and crop rotation, have improved nutrient balance but remain insufficient to enhance soluble P availability in DAS [8,9]. Therefore, alternative solutions are needed to overcome this constraint.
These insoluble phosphate compounds can be biologically converted into soluble forms that plants can use [10]. Phosphate-solubilizing bacteria (PSB) contribute to solubilizing phosphate in the soil and improving plant nutrition [11]. Moreover, the PSB can boost plant growth by synthesizing phytohormones, siderophores, HCN, ammonia, and hydrolyzing enzymes, thereby protecting plants from non-biological factors [12]. These PSBs are thought to be safe for sustainable agriculture and greater yields [13, 14]. In addition to enhancing plant growth and increasing the concentration of soluble phosphate in the soil, the PSB ameliorates soil fertility and reduces the amount of chemical phosphate fertilizer used, fulfilling the demand for sustainable agricultural practices [4]. Phosphate-solubilizing bacteria can contribute to plant phosphorus nutrition and also possess other functions, including N-fixation and indole-3-acetic-acid (IAA) production, which are key for evaluating the effects of PSB on crop cultivation and soil conditions [15, 16]. Moreover, the bacteria also perform other functions, as evaluated in DAS, such as N production, in the study by Khuong et al. [17]. Recent studies have demonstrated the effectiveness of PSB in maize [14], sugarcane [11], cucurbit crops [18], and wheat [19], showing their potential to reduce chemical fertilizer inputs while maintaining or improving yields. In maize, although studies on the PSB are focused on endophytic bacteria [14], the insoluble forms of phosphorus present in soil are more likely to be studied, with the colonization, diversity, and qualification of rhizosphere bacteria being more likely to be studied than endophytic bacteria [20]. Moreover, most work has focused on soils such as calcareous, saline, or acid sulfate soils [21-23].
Despite the importance of DAS in the Mekong Delta, little is known about the indigenous PSB community in these soils or their potential to improve P availability for maize cultivation. Addressing this gap is essential for developing sustainable nutrient management strategies tailored to local soil constraints. Therefore, this study was performed to (i) identify phosphate-solubilizing rhizosphere bacteria, and (ii) determine the effects of the identified strains on improving soluble phosphate contents in DAS and the performance of hybrid maize. These PSB were hypothesized to reduce chemical P input by 25%.
2. MATERIALS AND METHODS
2.1. Materials
Location: A pot experiment was performed in the greenhouse, College of Agriculture, Can Tho University (10°01′51.1″N, 105°46′08.4″E), at an elevation of 1.0 m above the sea level and a mean temperature of 37° C, from September, 2019 to March, 2022.
Source of bacteria and soil: Soil was collected from the rhizosphere of hybrid maize for bacterial isolation. The soil was collected at days 40–45 after planting at a depth of 0-15 cm in alluvial soil for maize cultivation. Plant residues were removed from the soil. Thirty-six soil samples were obtained from maize fields in An Phu district, An Giang province, Vietnam.
2.1.1. Maize Variety
The hybrid maize variety CP888 was used in this study. The CP888 maize has short ear length, firm, yellow kernels, hard stems, green leaves, stable growth, and drought tolerance. It has a high yield ranging from 10 to 12 t ha−1 and a complete growth cycle of 95–100 days.
Chemical fertilizers: The fertilizers consisted of nitrogen from urea fertilizer (46% N), phosphorus from superphosphate fertilizer (16% P2O5), and potassium from potassium chloride (60% K2O).
2.2. Methods
2.2.1. Screening for Phosphate-solubilizing Rhizosphere Bacteria from Maize Fields
2.2.1.1. Isolation of Phosphate-solubilizing Rhizosphere Bacteria
One gram of the soil sample was placed in a flask containing 99 mL of distilled water and shaken for 12 h at 200 rpm. The solution was allowed to settle for 3 h. Then, 0.1 mL of the solution was spread on a petri dish with the NBRIP medium [24]. The dish was left to dry and incubated at 30 °C. After 48 h of incubation, colonies appeared on the surface of the medium. They were inoculated into another medium until pure colonies were obtained. Purity was checked microscopically using the hanging-drop method. When the purity was obtained, the samples were stored at 4 °C for further experiments. Pure colonies in the NBRIP broth were used to determine the phosphate-solubilizing ability.
2.2.1.2. Quantification of Phosphate-solubilizing Capacity
For Al-P-solubilizing determination, the NBRIP medium was modified by adding an insoluble phosphate compound (1 g L-1 AlPO4O2H2O) to replace Ca3(PO4)2 [24]. Then, 1 mL of each bacterial culture, with OD660 adjusted to 0.5, was placed in a tube containing 9 mL NBRIP broth and shaken at 120 rpm in the dark for 48 hours. After that, 1 mL of the 48-hour culture was centrifuged at 10,000 rpm for 15 minutes. The solubilized phosphate content was quantified by the ascorbic acid method at 880 nm [25]. For the Fe-P and Ca-P, the experiment was done the same, but AlPO4O2H2O was replaced by FePO4O2H2O and Ca3(PO4)2, respectively [14]. An NBRIP broth without bacteria served as a negative control. Forty-eight bacterial strains isolated in our previous study from the rhizosphere of the maize cultivation were used for this screening.
2.2.1.3. Identification of Rhizosphere Bacteria
Based on the phosphate-solubilizing screening output, three strains of PSRB were selected. These strains were identified by 16S rDNA sequencing. In brief, 2 mL of a culture of each strain was centrifuged at 10,000 rpm for 5 min to obtain a cell pellet. Then, the DNA was extracted from the cell pellet using the Genomic DNA Prep Kit (BioFACT, Daejeon, Republic of Korea) according to the manufacturer’s instructions. The genomic DNA from the PSRB strains was visualized electrophoretically, i.e., the DNA samples were resolved on a 1.0% w/v agarose gel, and bands were observed under UV light to determine the DNA purity. Bacterial 16S rDNA sequences were amplified by the polymerase chain reaction (PCR) using the T100TM thermo cycler (BioRad, Hercules, California, the United States of America) and the following primer pair: 16S Forward Primer - 8F (5′-AGA GTT TGA TCC TGG CTC AG-3′), 16S Reverse Primer - 1492R (5′-GGT TAC CTT GTT ACG ACT T-3′) [26] and the iProof High-Fidelity PCR-Bio-Rad (BioRad, Hercules, CA). The amplification reaction was performed as follows: 95 °C predenaturation for 5 min; 95 °C denaturation for 30 s, 55 °C annealing for 30 s, and 72 °C extension for 2 min × 30 cycles; final extension at 72 °C for 10 min; and termination at room temperature. The amplicons were confirmed with a DNA marker by the electrophoresis method with a 1.0% w/v agarose gel in 1× TAE buffer and under UV light and purified by the Purification Kit of TIANquick Midi (Tiangen Biotech Ltd., Beijing, China). The purified sequences were read by an automated DNA sequencer at Macrogen DNA Sequencing Service (Macrogen, Seoul, Korea). The reads and chromatograms were analyzed using BioEdit version 7.0.5.3 and ChromasPro version 1.7, respectively, and compared with available sequences in the GenBank database at the National Center for Biotechnology Information (NCBI). Alignment was made using ClustalW. The phylogenetic tree was constructed using the neighbour-joining method MEGA version 6.06. An evolutionary distance matrix was calculated according to the Jukes–Cantor model using a 1,000-replicate bootstrap resampling method.
2.2.2. Pot Experiments
2.2.2.1. Treatment Description
Nine groups included: 100% phosphate according to the recommended fertilizer formula (P-RFF), 75% P-RFF, 50% P-RFF, 25% P-RFF, 75% P-RFF and PSRB, 50% P-RFF and PSRB, 25% P-RFF and PSRB, 0% P-RFF and PSRB, and 0% P-RFF. They were arranged in a completely randomized design with four replicates corresponding to a pot with one plant.
2.2.2.2. Soil and fertilizer preparation
Dyked alluvial soil [pHH2O = 5.75, pHKCl = 4.69, total phosphorus = 0.031%, and available phosphorus = 28.6 mg P kg−1, soil texture including clay 49.7%, silt 49.0 and sand 1.30%, total bacterial count = 2.05 × 105 CFU g-1 dry soil weight (DSW), organic matter (OM) concentration = 1.95% C] was sampled from maize fields in An Giang province. The soil was cleaned of residual materials, then mixed and left to dry in the open air. Each pot contained 10 kg of prepared soil for each replicate.
The RFF for maize was 200 N, 90 P, and 80 K kg ha−1 [9]. Based on the RFF for a ha at a depth of 20 cm the maize cultivation, a dose (g pot-1) for each pot of 10 kg was 1 N, 0.45 P, and 0.40 K.
2.2.2.3. Kernel Preparation
Maize kernels were sterilized by submerging them first in 70% ethanol for 3 min and then in 1% sodium hypochlorite for 10 min. The final rinsing was done in distilled water (DW). Then, the kernels were incubated in the dark for a day. Approximately 100 kernels were germinated. For bacterial inoculation, the kernels were soaked in 63 mL of a mixed PSRB suspension at 108 cells mL−1 each. Kernels submerged in DW served as the negative control. The mixture of kernels and the liquid bacteria was covered with aluminum foil, shaken at 60 rpm for 1 h, and dried under laminar airflow for 1 h. Finally, kernels with a bacterial density of 63 × 106 bacterial cells/kernel (6.3 × 103 cells/g DSW) and the uninoculated kernels (negative control) were grown in designated pots.
2.2.2.4. Solid Biofertilizers Preparation
The procedure followed the method of Kantha et al. [27] with slight modifications in which ash and maize leaves were mixed at a ratio of 1:4 and used as carriers.
The agronomic characteristics assessment according to Khuong et al. [14] is as follows:
- Plant height (cm): the distance between the ground and the plant apex;
- Stem diameter (cm): an average diameter derived from diameters at a plant’s top, middle, and bottom;
- Number of leaves per plant (leaves): leaves in each plant in each pot;
- Ear appearing height (cm), a segment between the ground and the first ear formation.
Yield components were evaluated according to Khuong et al. [14] and included:
- Ear length (cm): the length was measured from both ends of an ear;
- Ear diameter (cm): the diameter was measured at the middle of the ear;
- Number of rows per ear (rows): the rows were counted in each ear;
- Number of kernels per row (kernels): the kernels were counted on each row;
- 100-kernel weight (g): 100 kernels were collected randomly in each replicate and weighed electronically.
2.2.2.5. Maize Yield (g per pot)
All kernels from maize plants were collected 100 days after planting. After the fresh weights of the kernels were measured, they were dried at room temperature. The moisture of the kernels was measured to calculate the yield at 15.5% moisture.
2.2.2.6. Biomass (g per pot)
Fresh weights of kernels, stems, leaves, and roots were evaluated. Stovers were dried at 70 °C for 72 h to determine dry biomass.
2.2.2.7. Soil Analysis
According to Sparks et al. [28], soil pHKCl, pHH2O, electrical conductivity (EC), total nitrogen (Ntot), NH4+, total phosphorus, and available phosphorus (Pavail) were detected.
2.2.2.8. Plant Analysis
When the maize plants matured, stover straws and kernel samples were collected and dried at 70 °C for 72 h. The samples were crushed using a 0.5-mm net to determine the total phosphorus content [29]. The total phosphorus was measured using the ascorbic acid method. From these concentrations, the phosphorus uptake in the kernels, stems, leaves, and roots was calculated using the sum of each concentration multiplied by its biomass.
3. RESULTS
3.1.Phosphate-solubilizing Rhizosphere Bacteria to Produce Available Nutrients for the Plant
3.1.1. Selection of Acidic Resistance of Rhizosphere Bacteria
A total of 18 PSRB strains were selected for their acid tolerance capacity (Fig. 1). All of them could solubilize all three forms of insoluble phosphate compounds. Nevertheless, the amount of the soluble phosphate produced varied among the acid-tolerant PSRB strains. For the Al–P solubilization, the ASD-15 strain (74.1 mg P L−1) had the greatest capacity, and the ASD-01, ASD-44, and ASD-50 strains (16.9–17.6 mg P L−1) had the lowest capacities. They were all significantly different from one another at 5%. For the Fe–P-solubilizing capacity, the amount of soluble phosphate produced by ASD-56 was significantly greater than that of the other PSRB strains, at approximately 98.0 mg P L−1. The same trend was observed with clear differences among groups for the Al–P and Fe–P contents. However, differences among the PSRB strains in the amount of soluble phosphate produced from Ca–P were quite different, ranging from 17.2 to 42.2 mg P L−1. The ASD-43 strain had the greatest Ca–P-solubilizing capacity, which was significantly different from that of other PSRB strains, excluding the ASD-19 strains. Thus, the bacterial strains of ASD-15, ASD-43, and ASD-56 were chosen for their ability to solubilize insoluble phosphate compounds.
Values followed by no identical letters were statistically different at 5% by Duncan’s test.
16S rDNA sequencing of the selected PSRB
The potent PSRB strains of ASD-15, ASD-43, and ASD-56 were identified by the 16S rDNA gene sequences in a group within Enterobacter, where E. asburiae was the closest strain, with 100% sequence similarity for all three strains (accession numbers of ON386141, ON386142, and ON386143, respectively) (Fig. 2).
3.2. Effects of Phosphate-solubilizing Rhizosphere Bacteria
3.2.1.Dyked Alluvial Soil Fertility
The properties of DAS changed dynamically in response to phosphate fertilizer and PSRB (Table 1). The pHH2O values proportionally correlated with the reduction in the phosphate fertilizer level, from 5.89 at 100% P-RFF to 6.09 at 25% P-RFF. However, the differences in pHH2O were not significant among bacterial groups, ranging from 6.24 to 6.36. Nevertheless, the pHH2O value in the group fertilized with the PSRB alone was greater than in the control group. However, for the pHKCl value, the results did not show a clear trend, with the exception of comparing groups fertilized with 75% and 50% P-RFF and PSRB to those fertilized with 25% and 0% P-RFF and PSRB, where higher phosphate fertilizer rates resulted in higher pHKCl values. Although the total nitrogen and phosphorus in the soil did not change remarkably, the available nitrogen and soluble phosphate were significantly affected by both factors. In addition to the decline in the phosphate fertilizer level, the available nitrogen content increased from 84.0 to 90.9 mg NH4+ kg−1 when no bacteria were applied, and from 86.1 to 93.5 mg NH4+ kg−1 when the PSRB were added. However, the PSRB supplementation seemed to have a significant impact on the available nitrogen content. For the soluble phosphate content, as expected, the greater the level of phosphate fertilizer, the greater the soluble phosphate content. However, the group fertilized with 50% P-RFF and PSRB had a soluble phosphate content equivalent to that of the group fertilized with 100% P-RFF, at 71.8 and 72.3 mg P kg−1, respectively. Moreover, without chemical fertilizers, the group fertilized with PSRB outperformed the group without bacteria in terms of soluble phosphate concentration in the soil, with values of 44.8 and 41.8 mg P kg−1, respectively.
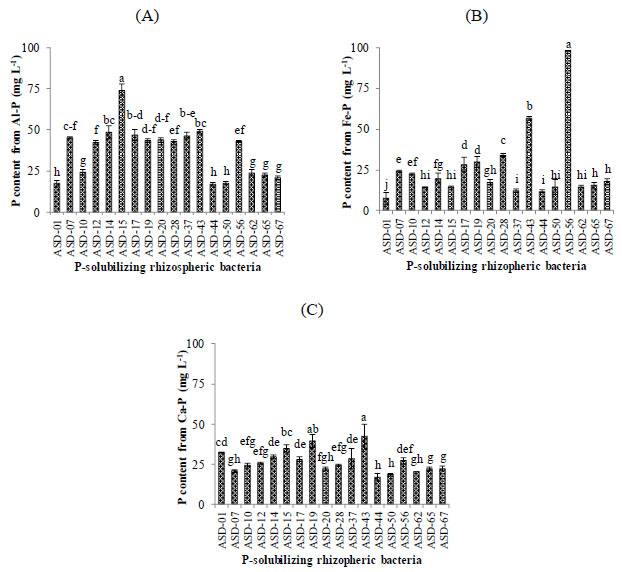
Ability of potent bacteria isolated from the rhizosphere of hybrid maize to solubilize (A) aluminum phosphate (AlPO4), (B) iron phosphate (FePO4), and (C) calcium phosphate (Ca3(PO4)2)
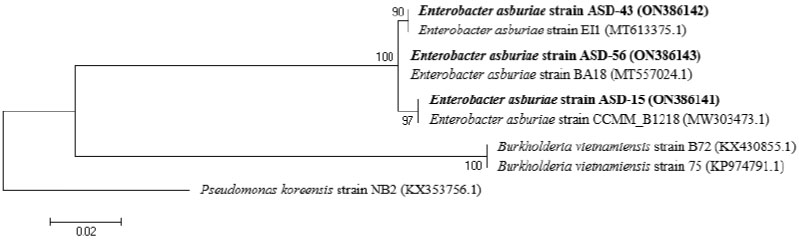
Phylogenetic tree based on the 16S rDNA sequences of the selected phosphate-solubilizing rhizosphere bacterial strains
| Group |
pHH2O (1:2.5) |
pHKCl (1:2.5) | Ntotal (%) |
Navailable (mg NH4+/kg) |
Ptotal (%) |
Psoluble (mg P/kg) |
|---|---|---|---|---|---|---|
| 100% P-RFF | 5.89f ± 0.02 | 5.13b ± 0.01 | 0.27a ± 0.03 | 84.0def ± 1.85 | 0.14c ± 0.00 | 72.3a ± 0.96 |
| 75% P-RFF | 6.20c ± 0.02 | 5.14b ± 0.02 | 0.30a ± 0.05 | 86.7cd ± 1.51 | 0.14c± 0.01 | 62.4c ± 1.91 |
| 50% P-RFF | 6.12d ± 0.09 | 5.14b ± 0.04 | 0.28a ± 0.07 | 82.7efg ± 1.16 | 0.16a ± 0.00 | 61.9c ± 1.03 |
| 25% P-RFF | 6.09d ± 0.09 | 5.01c ± 0.04 | 0.29a ± 0.07 | 90.9a ± 1.16 | 0.14c ± 0.00 | 56.4d ± 1.03 |
| 75% P-RFF + PSRB | 6.36a ± 0.02 | 5.41a ± 0.04 | 0.29a ± 0.06 | 86.1cde ± 1.74 | 0.15ab ± 0.01 | 69.5b ± 0.41 |
| 50% P-RFF + PSRB | 6.30ab ± 0.02 | 5.42a ± 0.02 | 0.22a ± 0.03 | 88.6bc ± 0.00 | 0.14c ± 0.00 | 71.8a ± 1.50 |
| 25% P-RFF + PSRB | 6.24bc ± 0.04 | 5.12b ± 0.00 | 0.23a ± 0.06 | 93.5a ± 4.61 | 0.15b ± 0.00 | 61.9c ± 2.00 |
| 0% P-RFF + PSRB | 6.30ab ± 0.07 | 5.18b ± 0.06 | 0.25a ± 0.05 | 82.1fg ± 3.54 | 0.14c ± 0.00 | 44.8e ± 2.31 |
| 0% P-RFF | 5.98e ± 0.06 | 5.13b ± 0.09 | 0.20a ± 0.04 | 79.4g ± 2.14 | 0.16a ± 0.01 | 41.8f ± 1.15 |
| F | * | * | ns | * | * | * |
| CV (%) | 2.52 | 2.60 | 21.4 | 5.55 | 5.60 | 17.6 |
3.2.2. Plant Phosphorus Uptake
Phosphorus concentration, biomass, and phosphorus uptake in stovers of maize, including kernels, stems, leaves, and roots, varied statistically among groups (Table 2). In detail, for the phosphorus concentration in leaves, the results of the groups fertilized with \PSRB outweighed those of groups fertilized with no bacteria at the same phosphate fertilizer level. Notably, the phosphorus concentration in leaves in the group fertilized with 75% P-RFF and PSRB (0.98%) was greater than that in the group fertilized with 100% P-RFF (0.59%). However, phosphorus concentrations in the kernels, stems, and roots were not significantly affected in groups with and without the PSRB at the same phosphate level. For the biomass, a clear trend was observed. In the biomass of all plant parts, the group fertilized with 75% P-RFF and PSRB was statistically equal to the group fertilized with 100% P-RFF, and the group fertilized with only PSRB had better results than the control, excluding the stem biomass result. Equivalent biomass values between groups fertilized with 75% P-RFF and the one fertilized with 100% P-RFF were found at 64.9–67.3 g biomass per pot in kernels, 10.0–10.4 g per pot in stems, 4.45–4.66 g per pot in leaves, and 3.97–4.09 g per pot in roots. The group inoculated with only PSRB showed biomass values of 18.2 g per pot in kernels, 3.62 g per pot in leaves, and 2.62 g per pot in roots, which were significantly higher than those of the unfertilized group (9.79, 2.65, and 1.57 g per pot, respectively). The phosphorus uptake in stovers showed the same trend. Excluding phosphorus uptake in the stem, the groups inoculated with only PSRB showed higher phosphorus uptake than those without fertilizer—0.15 g per pot versus 0.08 g per pot in kernels, 0.39 g per pot versus 0.19 g per pot in leaves, and 0.01 g per pot versus 0.005 g per pot in roots. Notably, the group fertilized with 75% of RFF and PSRB exhibited better results in phosphorus uptake in kernels and leaves than the group fertilized with 100% P-RFF, at 0.72 and 0.45 g per pot, compared with 0.56 and 0.26 g per pot in kernels and leaves, respectively. However, the values in the group fertilized with 75% P-RFF and the group with 100% P-RFF were equivalent in terms of the biomass of stems and roots. Thus, the total phosphorus uptake showed a similar pattern. The group fertilized with 75% P-RFF and PSRB had total phosphorus uptake values of 0.81 and 0.21 g per pot, respectively, significantly greater than those of the groups fertilized with 100% P-RFF (0.62 g per pot) and without fertilizers (0.12 g per pot).
3.2.3. Maize Growth
For maize growth, neither factor severely affected the traits (Table 3). The plant height ranged from 170.8 to 178.5 cm with chemical fertilizers alone, and from 181.5 to 172.3 cm with both chemical fertilizers and the PSRB. Consistently, the ear height ranged from 62.5 to 69.0 cm and from 67.9 to 74.7 cm, the leaf number ranged from 10.3 to 11.3 in both cases, and the stem diameter ranged from 1.11 to 1.13 cm and from 1.04 to 1.15 cm, respectively. Moreover, when no chemical phosphate fertilizer was applied, no statistical difference was observed between the group fertilized with PSRB and the group without bacteria.
| Group | Phosphorus concentration (%) | Biomass (g/pot) | Phosphorus uptake (g/pot) | Total phosphorus uptake (g/pot) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kernels | Stem | Leaves | Roots | Kernels | Stem | Leaves | Roots | Kernels | Stem | Leaves | Roots | ||
| 100% P-RFF | 0.86cde ± 0.03 | 0.11a ± 0.01 | 0.59c ± 0.08 | 0.63abc ± 0.02 | 64.9a ± 1.53 | 10.0ab ± 0.19 | 4.45a ± 0.13 | 3.97ab ± 0.11 | 0.56b ± 0.02 | 0.011a ± 0.001 | 0.26d ± 0.004 | 0.025a ± 0.000 | 0.62b ± 0.02 |
| 75% P-RFF | 0.92bc ± 0.05 | 0.09bc ± 0.01 | 0.65c ± 0.05 | 0.66ab ± 0.03 | 61.1b ± 1.00 | 9.79ab ± 0.08 | 4.05d ± 0.06 | 3.95ab ± 0.17 | 0.56b ± 0.03 | 0.009c ± 0.001 | 0.27d ± 0.002 | 0.026a ± 0.002 | 0.63b± 0.03 |
| 50% P-RFF | 0.81e ± 0.05 | 0.11a ± 0.01 | 0.60c ± 0.03 | 0.68ab ± 0.06 | 54.4d ± 3.27 | 9.45bc ± 0.87 | 4.07cd ± 0.13 | 3.88ab ± 0.05 | 0.44c ± 0.05 | 0.010abc ± 0.001 | 0.25d ± 0.002 | 0.026a ± 0.003 | 0.50c ± 0.04 |
| 25% P-RFF | 0.83e ± 0.05 | 0.11a ± 0.01 | 0.70bc ± 0.03 | 0.60bc ± 0.06 | 54.7cd ± 3.27 | 9.04c ± 0.87 | 3.95d ± 0.13 | 3.54c ± 0.05 | 0.45c ± 0.05 | 0.010bc ± 0.001 | 0.27d ± 0.002 | 0.021b ± 0.003 | 0.51c ± 0.04 |
| 75% P-RFF + PSRB | 1.07a ± 0.03 | 0.11ab ± 0.01 | 0.98a ± 0.10 | 0.68a ± 0.10 | 67.3a ± 0.41 | 10.4a ± 0.54 | 4.66a ± 0.10 | 4.09a ± 0.06 | 0.72a ± 0.02 | 0.011a ± 0.001 | 0.45a ± 0.005 | 0.028a ± 0.004 | 0.81a ± 0.02 |
| 50% P-RFF + PSRB | 0.94b ± 0.06 | 0.09bc ± 0.01 | 1.06a ± 0.08 | 0.56cd ± 0.04 | 57.5cd ± 1.28 | 10.1ab ± 0.33 | 4.40b ± 0.03 | 3.84b ± 0.16 | 0.54b ± 0.04 | 0.010abc ± 0.001 | 0.47a ± 0.004 | 0.022b ± 0.002 | 0.62b ± 0.04 |
| 25% P-RFF + PSRB | 0.90bcd ± 0.00 | 0.09c ± 0.01 | 0.82b ± 0.05 | 0.49d ± 0.03 | 57.8c ± 4.61 | 8.50c ± 0.29 | 4.21c ± 0.16 | 3.86b ± 0.18 | 0.52b ± 0.04 | 0.010bc± 0.001 | 0.34c ± 0.001 | 0.019b ± 0.002 | 0.58b ± 0.04 |
| 0% P-RFF + PSRB | 0.86de ± 0.05 | 0.10bc ± 0.01 | 1.07a ± 0.14 | 0.39e ± 0.05 | 18.2e ± 0.86 | 7.75d ± 0.55 | 3.62e ± 0.09 | 2.62d ± 0.19 | 0.15d ± 0.01 | 0.007d ± 0.001 | 0.39b ± 0.004 | 0.010c ± 0.002 | 0.21d ± 0.02 |
| 0% P-RFF | 0.81e ± 0.02 | 0.09c ± 0.01 | 0.70bc ± 0.03 | 0.35e ± 0.06 | 9.80f ± 0.16 | 7.54d ± 0.59 | 2.65f ± 0.04 | 1.57e ± 0.14 | 0.08e ± 0.01 | 0.007d ± 0.001 | 0.19e ± 0.001 | 0.005d ± 0.001 | 0.12e ± 0.01 |
| F | * | * | * | * | * | * | * | * | * | * | * | * | * |
| CV (%) | 9.53 | 11.7 | 24.5 | 23.0 | 40.2 | 11.4 | 14.3 | 23.4 | 44.4 | 16.8 | 30.3 | 38.1 | 41.2 |
| Group | Plant height (cm) | Ear appearing height (cm) | Number of leaves (leaves) | Stem diameter (cm) |
|---|---|---|---|---|
| 100% P-RFF | 178.5a ± 2.87 | 69.0a ± 5.68 | 11.3a ± 0.82 | 1.13a ± 0.03 |
| 75% P-RFF | 171.0ab ± 9.22 | 66.0a ± 6.29 | 10.8a ± 0.96 | 1.12a ± 0.09 |
| 50% P-RFF | 174.5ab ± 7.68 | 66.7a ± 3.86 | 10.5a ± 0.50 | 1.12a ± 0.04 |
| 25% P-RFF | 170.8ab ± 7.68 | 62.5a ± 3.86 | 10.3a ± 0.50 | 1.11a ± 0.04 |
| 75% P-RFF + PSRB | 181.5a ± 5.50 | 74.7a ± 7.32 | 11.3a ± 0.82 | 1.15a ± 0.09 |
| 50% P-RFF + PSRB | 173.5ab ± 3.20 | 67.9a ± 1.50 | 10.5a ± 0.96 | 1.11a ± 0.05 |
| 25% P-RFF + PSRB | 172.3ab ± 9.60 | 68.0a ± 4.35 | 10.3a ± 0.58 | 1.04a ± 0.13 |
| 0% P-RFF + PSRB | 164.7bc ± 7.80 | 61.8a ± 0.58 | 10.3a ± 1.15 | 0.83b ± 0.07 |
| 0% P-RFF | 156.0c ± 1.15 | 61.0a ± 2.00 | 9.8a ± 0.00 | 0.76b ± 0.09 |
| F | * | ns | ns | * |
| CV (%) | 5.42 | 10.3 | 7.69 | 14.6 |
| Group | Ear length (cm) | Ear diameter (cm) | Number of rows per ear (rows) | Number of kernels per row (kernels) | 100-kernels weight (g) | Yield (g/pot) |
|---|---|---|---|---|---|---|
| 100% P-RFF | 11.6ab ± 0.29 | 3.93a ± 0.05 | 10.5b ± 1.15 | 25.5a ± 2.22 | 33.9a ± 2.47 | 72.7a ± 7.66 |
| 75% P-RFF | 11.2ab ± 0.25 | 3.90ab ± 0.00 | 10.5b ± 0.00 | 23.0a ± 1.26 | 33.4a ± 2.79 | 62.3b ± 5.11 |
| 50% P-RFF | 11.0bc ± 1.28 | 3.80abc ± 0.08 | 9.75b ± 1.00 | 22.7a ± 2.36 | 33.8a ± 3.14 | 54.2b ± 13.4 |
| 25% P-RFF | 10.4cd ± 1.28 | 3.85abc ± 0.08 | 10.0b ± 1.00 | 22.0a ± 2.36 | 32.7a ± 3.14 | 55.5b ± 13.4 |
| 75% P-RFF + PSRB | 11.9a ± 0.65 | 3.93a ± 0.10 | 11.5a ± 1.15 | 25.3a ± 2.16 | 31.1a ± 2.46 | 74.9a ± 11.9 |
| 50% P-RFF + PSRB | 11.9a ± 1.06 | 3.73bc ± 0.14 | 10.0b ± 0.00 | 23.3a ± 0.82 | 33.5a ± 3.09 | 55.8b ± 4.55 |
| 25% P-RFF + PSRB | 9.85d ± 2.04 | 3.68c ± 0.43 | 10.0b ± 1.50 | 23.0a ± 6.95 | 30.6a ± 3.23 | 55.4b ± 19.1 |
| 0% P-RFF + PSRB | 5.83e ± 0.55 | 2.98d ± 0.13 | 7.38c ± 0.58 | 9.75b ± 0.50 | 29.8a ± 2.06 | 18.1c ± 1.30 |
| 0% P-RFF | 4.95f ± 0.26 | 2.95d ± 0.12 | 7.33c ± 0.58 | 8.75b ± 0.58 | 29.5a ± 3.00 | 17.8c ± 2.27 |
| F | * | * | * | * | ns | * |
| CV (%) | 25.8 | 10.7 | 15.1 | 31.6 | 9.23 | 39.9 |
3.2.4. Yield Components And Maize Kernel Yield
Among agronomic characteristics, the ear length was most significantly influenced by the PSRB supplementation (Table 4). Notably, without phosphate fertilizer, the group with the PSRB had a longer ear size (approximately 5.83 cm) than the group fertilized with no bacteria (4.95 cm). Moreover, the group with 75% P-RFF and the PSRB showed results equivalent to those of the one fertilized with 100% P-RFF, whose results were 11.9 to 11.6 cm in the ear length, 3.93 to 3.93 cm in the ear diameter, and 25.3 to 25.5 kernels in the kernel number per row. In terms of the number of rows per ear, the group with 75% P-RFF (11.5 rows) outperformed the group with 100% P-RFF (10.5 rows). However, the kernel weight remained unchanged statistically under the influence of both factors. Furthermore, the 100-kernel weight ranged from 29.5 to 33.9 g across groups. The ear length reduced in response to lower phosphate fertilizer levels—from 11.6 cm at 100% P-RFF to 10.4 cm at 25% P-RFF. Consequently, the maize yield increased according to the phosphate fertilizer level and the PSRB supplementation. It increased from 55.5 to 72.7 g per pot when the phosphate level of the RFF changed from 25% to 100%. The group with 75% P-RFF and PSRB had a maize yield of 74.9 g per pot, which was statistically comparable to the yield of the group with 100% P-RFF. As shown in Fig. (3), plants fertilized with 75% P-RFF and PSRB grew stronger than those fertilized with 75% P-RFF, and equivalent to those fertilized with 100% P-RFF. A further evaluation revealed that the ears got smaller, corresponding to a decline in the phosphate fertilizer level (Fig. 4). However, the ear size was equivalent to that of the plant fertilized with 100% P-RFF upon an application of PSRB plus 75% P-RFF. The kernels in the group fertilized with the PSRB were firmer than those in the group without fertilizers.
4. DISCUSSION
Phosphorus availability is limited in many soils, but this limitation is more pronounced in areas with dyke formation that lack soluble phosphate sources, which can subsequently affect maize yield responses [14]. Many methods have been used to ameliorate the situation [8,9]. For example, crop rotation can improve soil fertility; however, it cannot increase the available phosphorus in DAS in one year in this area [9]. A supply of organic phosphate is required to sustain maize yield, but the abuse of chemical fertilizers can lead to soil contamination [1–3, 30]. This study demonstrates that indigenous phosphate-solubilizing rhizosphere bacteria (PSRB) isolated from DAS can alleviate this limitation by increasing soil soluble P, improving maize P uptake, and sustaining yields with reduced chemical fertilizer application. The strains of ASD-15, ASD-43, and ASD-56 were selected according to their phosphate solubilization performance. These three strains were identified as E. asburiae (Fig. 2). Their effects in pot experiments are consistent with previous findings that PSRB enhance soil P availability by secreting organic acids (gluconic, oxalic, citric), siderophores, and phosphatases that release phosphate from insoluble complexes [31, 32]. Although these mechanisms were not directly measured in this study, the observed improvements in soil soluble P and plant uptake align with the expected action of these metabolic pathways. Future work should include direct measurement of organic acid production, phosphatase activity, and siderophore release to confirm the mechanisms of action.
In other studies, strains of E. asburiae were selected from maize fields to reduce the use of chemical fertilizers [14, 33]. In those studies, rhizospheric and endophytic microbes were selected for their ability to increase maize yield and replace a portion of phosphate chemical fertilizers. Thus, the selected strains of E. asburiae, ASD-15, ASD-43, and ASD-56, can be used in the field for maize cultivation by improving soil fertility, plant growth, and yield. Previous studies have focused on low phosphorus in ASS due to precipitations of insoluble compounds of AlPO4•2H2O and FePO4•2H2O, and in saline soil due to the formation of Ca3(PO4)2 [5,6]. However, generally in alluvial soils, particularly in DAS, the soluble phosphate content is low. Therefore, this is one of the first studies to select PSRB strains from DAS and then apply them to the same DAS.

Maize plants at day 55th after planting (from left to right) in the group fertilized with 75% P-RFF and the PSRB, the group fertilized with 75% P-RFF, and the group fertilized with 100% P-RFF
P-RFF: phosphate according to the recommended fertilizer formula; PSRB: phosphate-solubilizing rhizosphere bacteria.

Maize ears after harvesting under the influence of phosphate-solubilizing rhizosphere bacteria.
NT1: 100% P-RFF, NT2: 75% P-RFF, NT3: 50% P-RFF, NT4: 25% P-RFF, NT5: 75% P-RFF and PSRB, NT6: 50% P-RFF and PSRB, NT7: 25% P-RFF and PSRB, NT8: 0% P-RFF and PSRB, NT9: 0% P-RFF.
P-RFF: phosphate according to the recommended fertilizer formula; PSRB: phosphate-solubilizing rhizosphere bacteria.
Beyond DAS, PSRB have shown promise in diverse soils. For example, Bacillus sp. PIS7 increased maize P uptake in calcareous soils [34], PSB enhanced wheat growth in P-deficient loamy sand [23], and purple nonsulfur bacteria improved crop productivity in saline and acid sulfate soils [21, 22]. The results extend this evidence to DAS, a major soil type in the Mekong Delta, and highlight the adaptability of PSRB across contrasting soil systems.
Biofertilizers containing a mixture of the three PSRB strains of E. asburiae, ASD-15, ASD-43, and ASD-56, were used to improve DAS’s properties and increase plant growth, yield, and yield components in pot conditions (Tables 1–4). Based on the pH classification of Horneck et al. [35], the pHH2O values of 5.89 and 5.98 in the groups with 100% P-RFF and 0% P-RFF, respectively, were considered to be moderately acidic, whereas in other groups, the pHH2O values were >6.0 and slightly acidic. The acidity can affect the availability of phosphorus in the soil. Moreover, the group with the PSRB induced pHH2O values ranging from 6.24 to 6.36, which were greater than those in the group fertilized without bacteria (Table 1). A higher pH value resulted in higher phosphorus availability in the soil [36]. The combination of 50% P-RFF and PSRB resulted in a soluble phosphate amount of 71.8 mg P kg−1, equivalent to the amount in the group fertilized with 100% P-RFF (72.3 mg P kg−1). This may be because PSB can dissolve phosphate forms by producing siderophores, organic acids, or hydroxyl and carboxyl functional groups [31]. The bacteria used in the current study may also have followed these mechanisms. However, because insoluble phosphate forms are found in the soil, rhizobacteria perform their roles in solubilizing phosphate significantly better than microbes that live in plants, due to a greater density of rhizobacteria in the soil [19, 20]. Thus, the group supplied with PSRB obtained a greater soluble phosphate content compared with the groups with phosphate chemical fertilizer (Table 1). This is consistent with the study by Xuan et al. [18], which showed that applying PSRB increased the soluble phosphate content from 60.7 to 79.6 mg kg-1.
The phosphorus concentration in plant parts of hybrid maize, including kernels, stems, leaves, and roots, in the group fertilized with 75% P-RFF and PSRB was equivalent to that in the group fertilized with 100% P-RFF (Table 2). Rhizosphere bacteria can improve the solubility of nutrients by producing organic acids, thereby increasing biomass and phosphorus uptake in plant tissues and promoting plant growth [37, 38]. This results in increased total phosphorus uptake by the plants (Table 3). This result is consistent with those of Malboobi et al. [39], Wen et al. [40], Eida et al. [41], and Khuong et al. [14], where inoculating with PSB improved phosphorus uptake, plant growth, and the yield of different plants. The efficiencies of plant growth-promoting rhizobacteria can be affected by the nutritional conditions of soil phosphorus. In detail, bacterial inoculation has a significantly more stimulatory effect on plants grown in a phosphorus-deficient calcareous soil than in a phosphorus-rich loamy sand soil [23]. The PSB strain Bacillus sp. PIS7 is able to increase phosphorus uptake in calcareous soil after two consecutive crops of maize [34]. This showed the potential of the ASD-15, ASD-43, and ASD-56 strains in improving phosphorus availability in phosphorus-limited DAS.
The height of the maize plant in groups fertilized with 25–75% P-RFF and PSRB fluctuated from 172.3 to 181.5 cm, statistically equal to the height in the group fertilized with 100% P-RFF, at 178.5 cm (Table 3). The solubilization of insoluble phosphate compounds in the soil by the PSRB led to an increase in the soluble phosphate content (Table 1), which improved the plant growth. Ramachandran et al. [32] reported that PSRB enhances the growth of shoots and roots in plants. Additionally, PSRB are able to secrete phytohormones, including IAA and gibberellin, that support plant growth [42, 43]. Thus, it is vital to determine the plant growth-promoting substances produced by bacteria for a better understanding of their multifunctional effects.
The maize yield components, including ear size, row number per ear, and kernel number per row, statistically remained unchanged in the group fertilized with 75% P-RFF and PSRB, when compared to the group fertilized with 100% P-RFF (Table 4). Unfortunately, the group with 100% P-RFF was not designed in this experiment to evaluate the potential of PSRB to increase maize yield in cases of 100% P-RFF application. However, the maize yield in the groups supplied with the PSRB biofertilizer was greater than in groups fertilized with either chemical fertilizer or left unfertilized. Thus, although the amount of phosphate fertilizer was decreased by 25%, the yield components under the inoculation of the PSRB were maintained at the same level as those in the group fertilized with 100% P-RFF. For instance, the maize yield values in the groups fertilized with 100% P-RFF and 75% P-RFF and PSRB were identical, approximately 72.7 and 74.9 g per pot, respectively (Table 4). The result was in accordance with other studies where PSB can balance plant nutrition and provide phosphorus to plants [14, 44]. However, the absence of a 100% P-RFF + PSRB treatment prevents us from assessing whether PSRB could increase yields beyond full fertilizer application. Moreover, the present study was conducted in pots, which may not fully reflect field conditions. Field-scale validation trials are needed to confirm these findings under farmer management and across multiple seasons.
According to Khuong et al. [14], the phosphate-solubilizing endophytic bacterium, Enterobacter spp., promotes maize growth and increases its yield. In the current research, the phosphate-solubilizing rhizosphere bacteria also supported the growth and yield of maize. Moreover, in the soil, rhizosphere bacteria are present in greater populations than the endophytic bacterial group [19, 20]. Importantly, the application of PSRB allowed a 25% reduction in chemical P fertilizer without compromising maize yield. This has significant implications for sustainable agriculture, including reduced fertilizer costs for farmers, a lower risk of cadmium accumulation from phosphate fertilizers [1–3], and a decreased environmental impact from excess fertilizer use. Adoption of PSRB biofertilizers can thus contribute to both economic and ecological sustainability in maize-based systems.
CONCLUSION
This study identified eighteen strains of PSRB that were capable of living under low pH conditions and solubilizing insoluble phosphate compounds. They were selected from 67 strains of PSRB isolated from DAS. The strains of ASD-15, ASD-43, and ASD-56, selected on the basis of the highest amount of phosphate solubilized (42.2–98.0 mg L−1), were identified as E. asburiae. A biofertilizer made of the selected strains provided soluble phosphate to maize and replaced 25% of the recommended inorganic phosphate fertilizer without affecting the traits related to the yield and growth of maize. Importantly, soil fertility was also improved by the application of PSRB biofertilizer. These findings highlight the potential of PSRB as a biofertilizer to improve maize productivity while reducing dependence on chemical fertilizers in DAS and similar soil systems. Future research should validate these results under field conditions, explore the underlying biochemical mechanisms (organic acids, phosphatases, and siderophores), and develop scalable formulations suitable for farmer adoption. Integrating PSRB into nutrient management strategies can provide a sustainable pathway toward resilient maize production and improved soil health in the Mekong Delta and beyond.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: H.H.D.: Study conception and design; L.N.T.X. and L.T.M.T.: Data collection; N.Q.K., L.T.Q., and N.T.P.: Draft manuscript; All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| DAS | = Dyked alluvial soils |
| PSRB | = Phosphate-solubilizing rhizosphere bacteria |
| SSNM | = Site-specific nutrient management |
| PCR | = Polymerase chain reaction |
| (NCBI) | = National Center for Biotechnology Information |
AVAILABILITY OF DATA AND MATERIAL
The data that support the findings of this study are available from the corresponding author, N.T.P., on special request.
ACKNOWLEDGEMENTS
Declared none.


