All published articles of this journal are available on ScienceDirect.
Dispersing Rice-Associated Arthropods Ignore a Phantom Ultrasonic Insect Chorus
Abstract
Introduction
The acoustic environment can provide fitness-enhancing information to dispersing animals. Tropical irrigated rice ecosystems host an exceptionally rich assemblage of sound-producing animals, including meadow katydids (Tettigoniidae, Conocephalinae, Conocephalini) which produce ultrasonic choruses. We aimed to test whether aerial arthropods were attracted to, deterred by, or indifferent to an experimental ultrasonic katydid chorus.
Methods
A 100-speaker array installed in a newly planted Philippine rice paddy served as a “phantom chorus” and was turned on and off hourly for 2-4 hours on 30 nights during the first half of the growing cycle which is naturally katydid-free. Aerial arthropods were sampled hourly using three passive intercept traps: one nested within the speaker array paddy, one in a paddy with poles and lines but no speakers, and one in an empty paddy. Arthropods were subsequently identified and sorted by functional guild and family.
Results
We captured 2078 arthropods representing 158 species. Detritivores comprised 34% of captured arthropods and decreased significantly in abundance with days after planting. Alternatively, (aquatic and general) predators and herbivores both increased over time and represented 48% and 8% of captures, respectively. None of the analyzed arthropod functional guilds or taxonomic families exhibited a statistically significant response to the phantom chorus.
Discussion
Our results suggest that meadow katydid choruses may neither attract nor deter arthropods characteristic of early-stage rice. We recommend further experiments deploying a more robust ultrasonic playback system at sites and during rice stages with more herbivorous rice pests.
Conclusion
Ultrasonic noise treatments applied during early rice growth stages may have a neutral effect on non-pest species; however, we encourage further studies to test whether ultrasonic katydid choruses serve as natural pest repellents in tropical irrigated rice.
1. INTRODUCTION
Dominant and persistent acoustic landscape features, such as rushing water, crashing surf anthropogenic noise (e.g., traffic noise), and chorusing animals can structure animal assemblages by providing fitness-enhancing habitat cues [1, 7]. Some soundscapes announce opportunities; for example, chorusing frogs serve as acoustic beacons guiding the dispersal of frogs and newts to breeding ponds, and female meadow katydids use mixed-species insect choruses to locate aggregations of males [8-11]. Alternatively, a soundscape may signal heightened predation risk [12], reduced foraging efficiency due to masked prey cues or distraction [12-14]. For example, pallid bats are slower to localize prey-generated sounds, regardless of whether environmental noise overlaps spectrally with prey cues [14].
Tropical irrigated rice paddies, as cultivated wet grasslands, are species-rich and often noisy habitats harboring vocalizing anurans birds and insects whose auditory presence can indicate various stages of the rice growth cycle. For example, anuran mating choruses mark the flooding of paddies and a crescendo of vocalizing insects signals rice maturation and canopy closure [15-21]. In addition to animal-generated sounds audible to humans, ultrasonic sounds are also an integral component of the rice acoustic environment (Fig. 1a). Sonar pulses emitted by insectivorous echolocating bats are a prominent feature of the soundscape, especially over newly planted rice paddies [22]. As the rice matures, aggregations of meadow katydid males (Conocephalini, Conocephalus spp.) broadcast an intense, broadband, ultrasonic call (> 90 dB at 1 m, SPL; 30-90 kHz to conspecific females [11,26]. Individual males produce a call sequence comprising several short-duration (< 20 ms) ticks similar to bat sonar pulses, followed by a rapid series of ticks or “buzzes”. An aggregation of calling males produces a chorus so ubiquitous and intense in late growth stages that it appears to deter foraging bats whose sonar overlaps with it in frequency [23]. However, despite this, and despite the fact that these ultrasonic choruses are a prominent and predictable acoustic feature of one of the oldest (9000 mya) and most extensive crops in tropical Asia (48 million hectares; https://www.irri.org/), it is unknown whether rice-associated arthropods attend to the chorus.
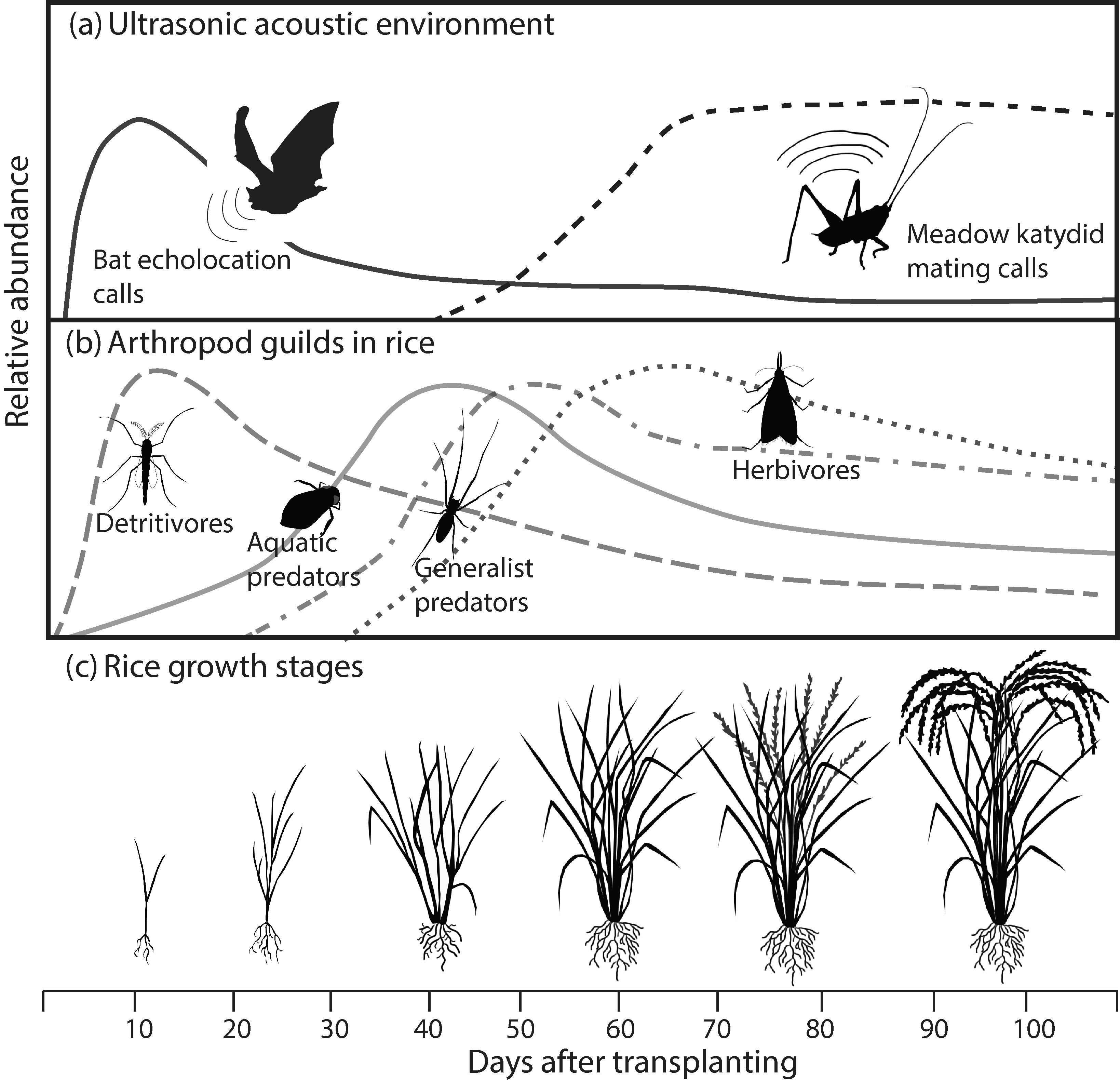
Relationship among ultrasonic acoustic environment within irrigated rice paddies (a), arrival of arthropod guilds (b), and rice growth stage (c). Generalized stage-specific abundance curves of bat activity are based on passive acoustic monitoring data [22]; katydid chorus sound levels are predicted from stage-specific calibrated recordings [23]; and arthropod guild abundances are based on data from this study as well as Li et al. [24] for detritivores, Ohba et al. [25] for aquatic predators, and Settle et al. [20] for generalist predators and herbivores.
The rice-associated arthropod assemblage represents a complex food web comprising detritivores, herbivores, predators, and parasitoids that accumulate in a predictable fashion over the growing season [20,27–29]; (Fig. 1b ,c). These include species with a known ability to detect and respond to sounds in their environment (e.g., eared moths, crickets, katydids [30, 31], and many more whose auditory capacity has yet to be tested. Arthropods indifferent to the chorus may be unable to detect the sound stimulus, either because they lack hearing organs, have a high auditory response threshold, or are insensitive to ultrasound. Others may be capable of detecting the chorus but may ignore it as irrelevant [32]. Alternatively, the ultrasonic chorus may attract arthropods that associate it with food (e.g., herbivores and predators of herbivores), host plants for egg-laying, or safety [23, 33]. Conversely, the ultrasonic chorus may provide a warning of suboptimal habitat, especially since meadow katydids not only produce a noisy backdrop that can mask important signals or cues (e.g., ultrasonic moth courtship calls [34]) but are also predators of insect eggs [35]. Moreover, it is possible that insects with sensitive anti-bat predator hearing (e.g., noctuid moths [36]) mistake the ultrasonic ticks of meadow katydids for bat sonar pulses and are repelled by the chorus. In addition to these potential direct effects, the ultrasonic katydid chorus may elicit indirect effects on dispersing rice-associated arthropods through their predators. For example, Sedlock et al. demonstrated that the chorus deterred aerial-hawking bats whose echolocation calls overlapped in spectral frequency with the katydids’ [23]. This enemy-reduced space may result in more dispersing arthropods irrespective of their hearing ability. Alternatively, the chorus may attract gleaning bats that eavesdrop on katydid calls but but are also capable of capturing aerial prey.
We used an aggregation of 100 ultrasonic speakers to mimic a meadow katydid chorus in a newly planted tropical irrigated rice field that was free of naturally calling katydids. This “phantom chorus” modified soundscape primarily aimed to test whether aerial insectivorous bats were deterred by the ultrasonic chorus ([23]). However, systematic sampling of aerial arthropods documenting bat prey availability offered an opportunity to document the accumulation of dispersing arthropods over maturing rice—adding to the existing literature that reports primarily on canopy-sampled arthropods—and to test for responses of rice-associated arthropods to the ultrasonic insect chorus. Our study is primarily exploratory, given that documentation of hearing in arthropods is limited, and it is plausible that the chorus may influence even non-hearing taxa indirectly through bat (or other) predators. Therefore, we first pooled all arthropods within each rice stage-associated guild to test whether arthropod responses to the phantom chorus were guild-specific. Then, to better detect potential behavioral responses of taxa with members that have confirmed ultrasonic hearing, we reanalyzed the data by taxonomic family. We hypothesized that if the chorus serves as a habitat beacon, guilds that would normally arrive during later (noisier) growth stages, such as predators and herbivores, would be attracted to newly planted paddies, resulting in more captures during chorus-on versus chorus-off time blocks and in the playback paddy versus the two control paddies. Alternatively, if the chorus is associated with high predation risk or is simply distracting to a particular guild or taxonomic family, we predicted fewer captures during chorus-on versus chorus-off time blocks and in the playback paddy compared to the control paddies.
2. MATERIALS AND METHODS
2.1. Phantom Katydid Chorus
We installed our experimental chorus within the International Rice Research Institute’s (IRRI) 200-hectare experimental farm in Laguna Province, Luzon Island, Philippines (14.167774 E, 121.254547 N) during the dry-season planting (Fig. 2a, b); see Sedlock et al. 2021). Three 0.25 ha plots were hand-planted with rice seedlings, received equivalent pest management, and matured over the course of the study (reaching ~ 1 m in height at the experiment’s completion). In one of the three plots, we ran our 100-speaker array to simulate a katydid chorus each night for 30 nights from 7 February – 29 March 2019, soon after rice planting and before katydid colonization. The absence of a natural katydid chorus was confirmed using passive ultrasonic detectors placed in each of the sampling plots. Logistical challenges prohibited us from replicating the playback and control plots. Specifically, silently powering the amplifiers driving the speaker array required the installation of an AC power supply adjacent to the rice paddy, and the labour required to install the array and “mock array” in the control plots was prohibitively labour-intensive to replicate given our available resources. Instead, we employed a modified BACI (before-after-control-impact [37]) design (i.e., assessing the impact of a treatment or disturbance before and after its application to an ecosystem) by broadcasting the katydid chorus during four, one-hour intervals nightly, alternating between chorus-on and chorus-off (i.e., amplifier gain at zero) between 1800–2200 h. To account for temporal variation in bat activity, we alternated the treatment order nightly, such that we started with a chorus-on or chorus-off broadcast every other night. On 11 of the 30 sampling nights, playback occurred, and insects were sampled only between 1800-2000 h. The second plot, in which we installed the poles and support lines but no speaker array, and the third plot, without poles or a speaker array, served as spatial replicates during silent blocks and contributed to estimating the effects of covariates. The three plots were within 100 m of one another and exposed to a similar pool of arthropods dispersing from adjacent habitats; however, the history of pesticide use in each plot was not known. This resulted in a total of 96 insect samples in each of the non-playback paddies, and 49 and 47 samples during non-playback and playback in the treatment paddy, respectively.
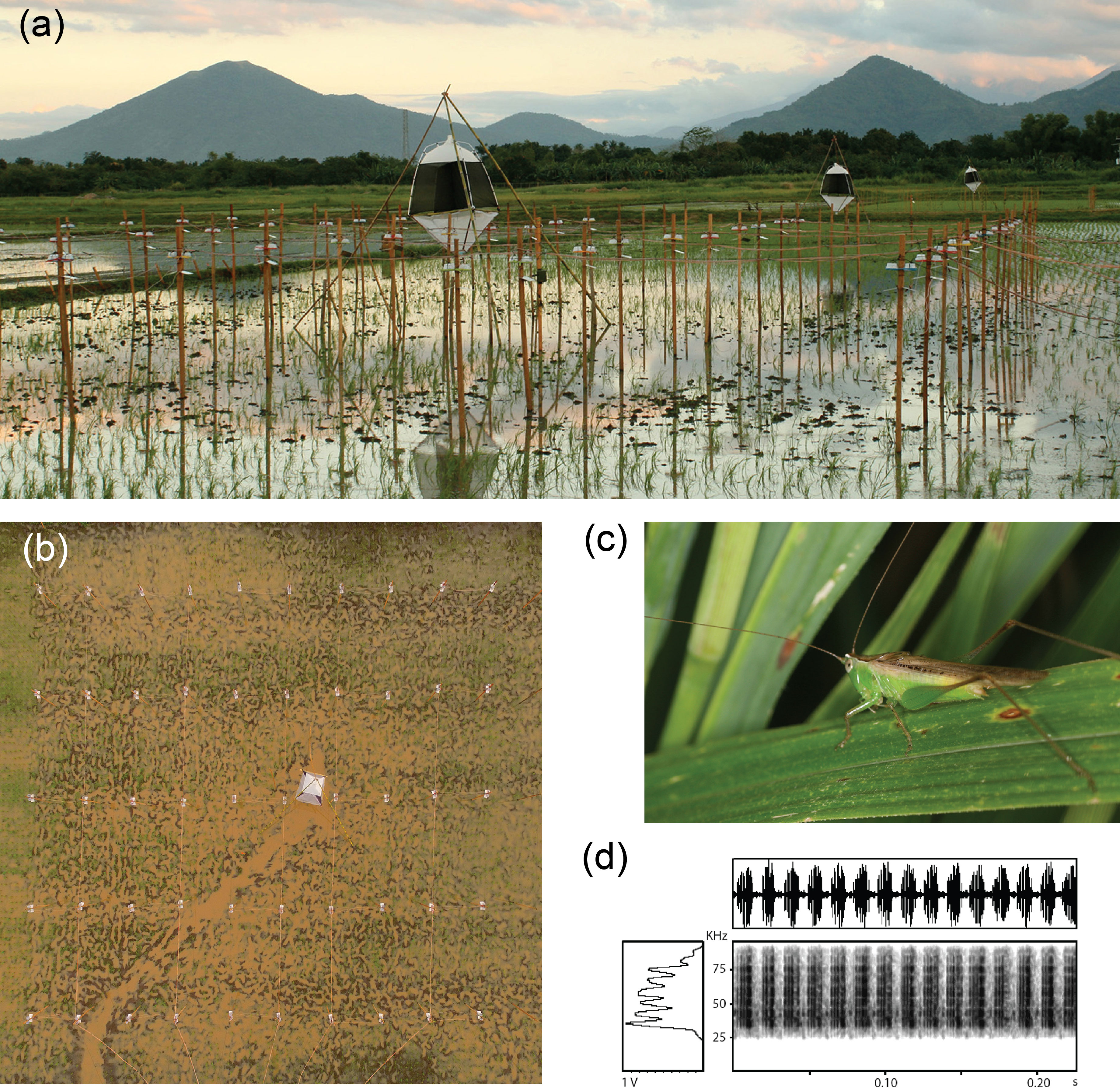
Experimental setup within the International Rice Research Institute’s farm, Los Banos, Philippines. Experimental plot with speaker array and arthropod intercept trap in foreground and two non-playback plots in the background (a), drone photo of the experimental plot (b), a representative male of the common rice paddy katydid, Concocephalus longipennis (c), and a representative portion of the playback katydid chorus file, including the waveform, power spectrum and spectrogram (d). Photo credits: J. Sedlock (a & c), IRRI (b)
We powered 100 tweeter speakers (Tymphany— Wanchai, Hong Kong, XT25SC90-04) with two AC circuits (120V, 20A). Our noise files were driven by 25 Parts Express (Springboro, IA, USA, LP-2020TI) 2-channel x 20W, 4–8 ohm, Class D amplifiers and Sony (Tokyo, Japan) NWA45L players (WAV, 192 kHz). We weatherproofed speakers using small plastic containers and mounted two units per pole, facing downward. An angled metal plate hung from each speaker to reflect the sound upward without changing the sound quality ( Fig. 2a, Fig. S1 [23]). Fifty poles, connected with rope and speaker wires, supporting the 2-speaker units, formed a 10 x 5 grid (Fig. 2a, b). This pole configuration was replicated in the second 0.25 ha plot (without the speaker array).
2.1.1. Playback File
We created the playback by combining five nocturnal katydid choruses recorded at ~1m (with similar amplitude and bandwidth) from various locations on the IRRI farm collected using a UltrasoundGate 116Hm and CM16/ CMPA condenser microphone (Avisoft Bioacoustics, Inc., Berlin, Germany, ± 3 dB(Z), 20 - 140 kHz) (Fig. 2c, d). We maximized the digital amplitude, without clipping (to avoid audio distortion), of the final 5.36-minute file, then applied a 25 kHz high pass FIR (finite impulse response) time domain filter (number of taps = 128; window = FlatTop) to remove low frequency sounds (i.e., crickets, frogs, and wind noise were removed from the playback file). The recordings include multiple meadow katydid species; however, the dominant species on the farm is Conocephalus longipennis (Fig. 2c) [35]. We maximized the amplifier and player gains without overloading, resulting in 45.7–69.4 dB (rms over 1 s at 1 m, median = 58.3 dB) directly above and 26.1–34.7 dB rms between speaker units. Sound levels over speakers were similar, but more spatially heterogeneous, compared to levels achieved by the actual katydid chorus (rms over 1 s at 1 m: 65–69 dB; [23]). We estimated katydid playback from recordings made with an UltrasoundGate 116Hm and CM16/CMPA condenser microphone (Avisoft Bioacoustics, Inc., Berlin, Germany, ± 3 dB(Z), 20–140 kHz) held one meter from each speaker in the array. The natural chorus was recorded in the experimental fields after the rice matured by simultaneously deploying three passive ultrasonic recorders (SM4Bat-FS with a SMM-U2 microphone (Wildlife Acoustics, Inc., Maynard, MA, USA, ± 5 dB(Z), 20–75 kHz) with downward facing microphones attached to poles 4 meters above the rice canopy. After 10 minutes of recording (to capture the chorus after we left the paddy), we moved the recorders systematically across the field to document the spatial variation in sound levels. These audio files were calibrated in SASLab Pro, version 5.2.13 (Avisoft Bioacoustics, Inc., Berlin, Germany) with an Avisoft Bioacoustics calibration tone recorded in the field. This tone was calibrated using a Brüel & Kjaer sound level meter 2610 measuring amplifier with B&K ¼” microphone type 4939-A-011 (grid off) in an anechoic, foam-lined room at Boise State University.
2.2. Arthropod Collection and Identification
Aerial arthropods were collected using an intercept trap (BioQuip Products Inc., Rancho Dominguez, CA, USA) with both a top and a bottom collecting bottle placed in the centre of each plot (Fig. 1a, b). At the end of each 1-hr period, we immediately placed arthropods in 70% ethanol. Arthropods were later identified by M. L. P. Almazan to the lowest taxonomic level possible and categorized into functional guilds (e.g., detritivores, predators, herbivores) using Barrion and Litsinger [38]. We acknowledge that our guild designations for taxonomic groups (e.g., family or genus) are based on current knowledge of their primary function in rice ecosystems and may not align with that in other habitats.
2.3. Statistical Analysis
All statistical analyses were completed in R v. 4.4.1 [39]. We fit generalized linear models to evaluate the response of arthropod abundance for four guilds and six families that had a sufficient sample size for analysis with either negative binomial or Poisson distributions and log link functions with the package `glmmTMB` [40]. We used the Akaike information criterion (AIC) to choose between a linear or quadratic negative binomial or Poisson distribution [41]. Subsequently, we assessed the model fit using QQ residual plots and checked for multicollinearity using the ‘DHARMa’ package [42]. For the predictors in each model, we used playback (speakers on or off), site (three 0.25 ha plots), date (days after transplanting rice), relative humidity, and temperature (daily minimum) as fixed effects. We originally included site as a random variable in a mixed effects model; however, the random-effects variance approached zero (or nearly zero), effectively causing site to drop out of the model. Therefore, we chose to use a generalized linear model, which accounts for potential baseline differences across sites.
3. RESULTS
Across 30 sampling nights and 288 insect samples, we captured a total of 2078 individual arthropods representing 158 species (Table 1) [43-58]. General predators were the most speciose (72 taxa) and abundant (45% of total numbers), and were composed primarily of ants (Formicidae, 35% of all general predators), ground beetles (Carabidae, 26%), and spiders (Araneae, 19%). Detritivores were the next most abundant guild (34% of total), comprised almost entirely of non-biting midges (Chironomidae, 94%). Aquatic predators accounted for 13% of total captures, with most of these being water boatmen (Corixidae, 54%). Herbivores were the least abundant arthropod guild, representing only 8% of total captures.
| Guild | Order | Family | Genera | No. of Taxa | Total Individuals | Hearing | Sound Production |
|---|---|---|---|---|---|---|---|
| Aquatic Predators | Coleoptera | Dytiscidae | 3 | 7 | |||
| Coleoptera | Hydraenidae | 1 | 3 | ||||
| Coleoptera | Hydrophilidae | 2 | 6 | yes1,2 | yes1,2 | ||
| Coleoptera | Noteridae | 1 | 3 | ||||
| Hemiptera | Corixidae | Micronecta | 1 | 154 | yes1,3 | yes1,3 | |
| Hemiptera | Mesoveliidae | 1 | 2 | ||||
| Hemiptera | Notonectidae | Anisops | 1 | 58 | yes4,5 | yes4,5 | |
| Hemiptera | Veliidae | Microvelia | 1 | 37 | yes5,6 | yes5,6 | |
| Total | 11 | 270 | |||||
| Detritivores | Blattodea | Ectobiidae | Blattella | 2 | 6 | ||
| unknown | |||||||
| Coleoptera | Latridiidae | 1 | 1 | ||||
| Coleoptera | Mycetophagidae | 2 | 8 | ||||
| Collembola | Entomobryidae | 1 | 1 | ||||
| Diptera | Chironomidae | 8 | 655 | yes1,7 | |||
| Diptera | Culicidae | 1 | 1 | yes1,8 | |||
| Diptera | Ephydridae | Notiphila | 4 | 7 | |||
| Paralimna | |||||||
| unknown | |||||||
| Diptera | Psychodidae | 3 | 9 | ||||
| Diptera | Sciaridae | 1 | 7 | yes9 | |||
| Diptera | Tipulidae | 3 | 4 | ||||
| Ephemeroptera | 2 | 9 | |||||
| Total | 28 | 708 | |||||
| General Predators | Araneae | Araneidae | 6 | 28 | |||
| Araneae | Clubionidae |
Clubiona unknown |
2 | 2 | |||
| Araneae | Linyphiidae |
Atypena Callitrichia |
3 | 19 | |||
| Araneae | Lycosidae |
Pardosa unknown |
3 | 3 | |||
| Araneae | 2 | 3 | |||||
| Araneae | Oonopidae | 1 | 1 | ||||
| Araneae | Salticidae | 1 | 1 | yes10 | |||
| Araneae | Tetragnathidae | Tetragnatha | 1 | 67 | |||
| Araneae | Theridiidae |
Theridion unknown |
8 | 47 | |||
| Coleoptera | Anthicidae | 3 | 9 | ||||
| Coleoptera | Carabidae | 2 | 239 | ||||
| Coleoptera | Coccinellidae | 1 | 3 | ||||
| Coleoptera | Staphylinidae | 10 | 75 | ||||
| Diptera | Ceratopogonidae |
Forcipomyia Nilobezzia Unknown |
8 | 91 | |||
| Diptera | Ephydridae | Ochthera | 1 | 2 | |||
| Hemiptera | Anthocoridae | 1 | 2 | ||||
| Hemiptera | Reduviidae | 1 | 1 | ||||
| Hemiptera | Saldidae | 1 | 1 | ||||
| Hymenoptera | Formicidae |
Camponotus Odontoponera Tetramorium unknown |
10 | 321 | |||
| Neuroptera | Chrysopidae | 1 | 1 | ||||
| Orthoptera | Gryllidae | 5 | 7 | yes11 | yes | ||
| Orthoptera | Tettigoniidae | 1 | 1 | yes11 | yes | ||
| Total | 72 | 924 | |||||
| Herbivores | Coleoptera | Bostrichidae | 4 | 7 | |||
| Coleoptera | Cerambycidae | 2 | 2 | ||||
| Coleoptera | Chrysomelidae | 7 | 11 | ||||
| Coleoptera | Curculionidae | 1 | 2 | ||||
| Coleoptera | Phalacridae | 6 | 34 | ||||
| Coleoptera | Scarabidae | 1 | 5 | yes11,16,17 | |||
| Diptera | Cecidomyiidae | 5 | 11 | ||||
| Hemiptera | Aphididae | 2 | 38 | ||||
| Hemiptera | Cicadellidae |
Recilia dorsalis unknown |
4 | 4 | |||
| Hemiptera | Cixiidae | 13 | |||||
| Hemiptera | Delphacidae | Nilaparvata lugens | 1 | 18 | |||
| Hemiptera | Meenoplidae | 1 | 1 | ||||
| Hemiptera | Lygaeidae | 3 | 8 | yes12,13 | yes12,13 | ||
| Hemiptera | unknown | 1 | 2 | ||||
| Isoptera | Termitidae | 1 | 1 | ||||
| Lepidoptera | unknown | 4 | 4 | ||||
| Lepidoptera | Gelechiidae | 1 | 1 | ||||
| Lepidoptera | Noctuidae | 1 | 1 | yes15 | yes | ||
| Lepidoptera | Pyralidae | 1 | 1 | yes | |||
| Thysanoptera | Thripidae | 1 | 12 | ||||
| Total | 47 | 176 | |||||
| Grand Total | 158 | 2078 | |||||
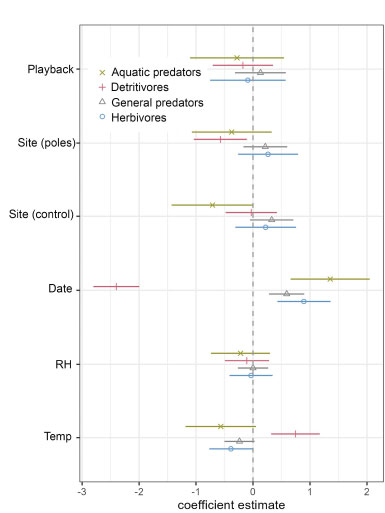
Coefficient estimates for fixed effects in the arthropod guild models. Coefficient estimates from generalized linear models with 95% confidence intervals surrounding the effect of ultrasonic katydid chorus playback (Playback), differences between the speaker array site and the pole-only site (Site_pole) or control site (Site_control), rice growth stage (Date), relative humidity (RH), and daily minimum temperature (Temp) on arthropod guild abundance.
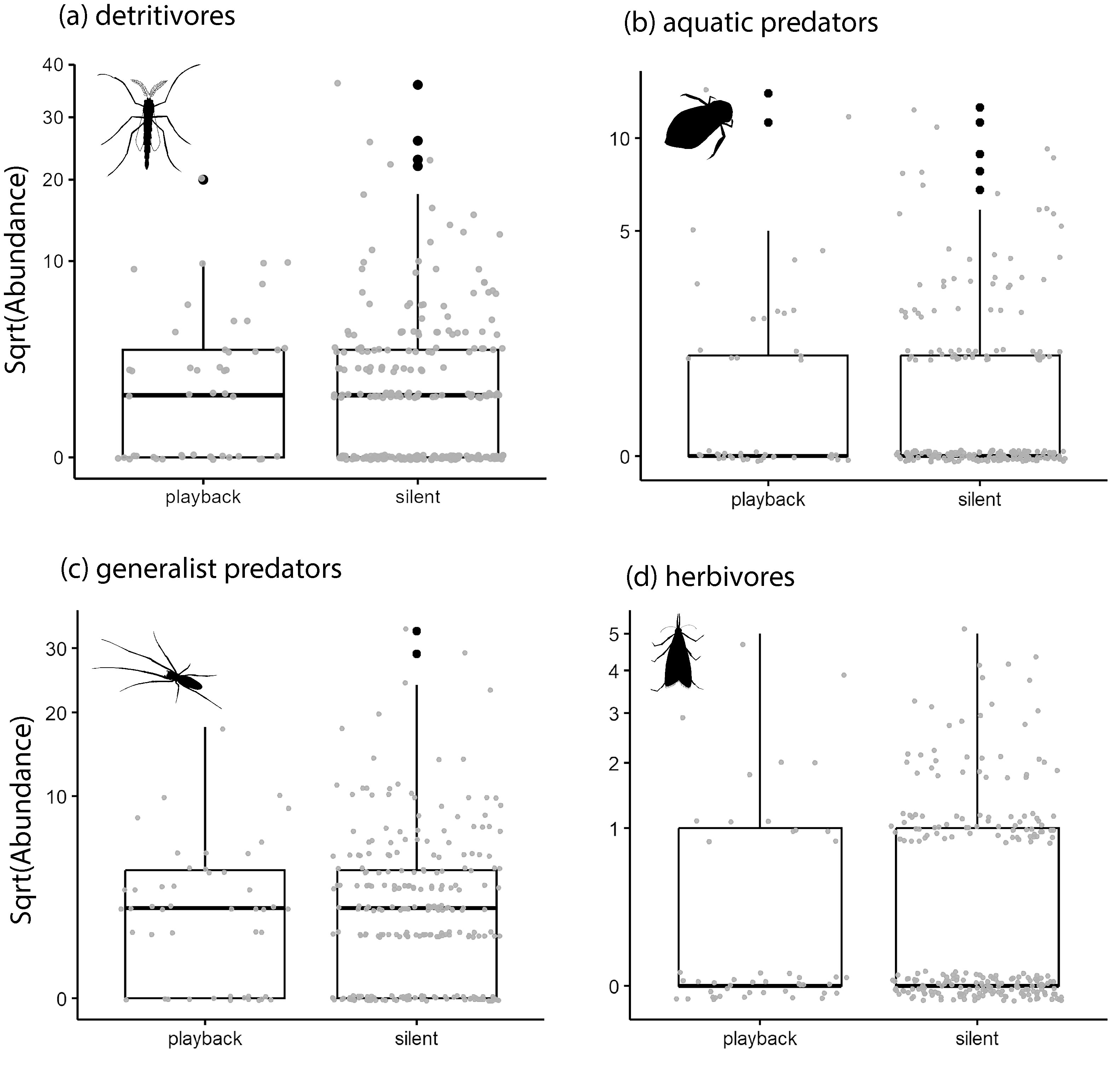
Arthropod abundance of detritivores (a), aquatic predators (b), general predators (c), and herbivores (d) during silent and playback one-hour sampling periods in rice paddies. Y-axes are square root transformed to improve the visualization of data.
Arthropod guilds did not exhibit a statistically significant response to the phantom chorus playback (Fig. 3, Fig. 4a-d, and Table S1). The strongest predictor of guild abundance was days since transplanting (Date)—detritivores decreased, and both predator guilds and herbivores increased in abundance with rice growth (Fig. 3, Fig. 5a-d), and Table S1). There were fewer aquatic predators captured in the paddy with no speaker array or poles (Site_control) and fewer detritivores in the paddy with poles but no speaker array (Site_poles) compared to the paddy with the speaker array. Minimum daily temperature was significantly and positively related to detritivore abundance, and negatively related to herbivore abundance. Relative humidity did not influence guild abundance (Supplemental Table S2).
Individual taxonomic families with sufficient sample sizes for analysis also did not respond significantly to the phantom katydid chorus (Fig. 6, Fig. 7a-h, and Table S2). Water boatmen (Corixidae) were less abundant in the control paddy (Site_control) compared to the playback site. The abundance of non-biting midges (Chironomidae) decreased, while all other families, except ground beetles (Carabidae) and biting midges (Ceratopogonidae), increased with rice growth (Date). Minimum daily temperature (temp) was positively associated with non-biting and biting midges, and negatively associated with ground beetles and orb-weavers (Tetragnathidae). Orb-weavers were more abundant with higher relative humidity (RH) (Table S2).
4. DISCUSSION
None of the arthropod guilds or taxonomic families analyzed responded to the phantom chorus either negatively or positively. Our results suggest that the ultrasonic noise we produced in an attempt to mimic a meadow katydid chorus—characteristic of the late vegetative stage and mature rice is an irrelevant acoustic backdrop in the lives of early growth stage arthropods,
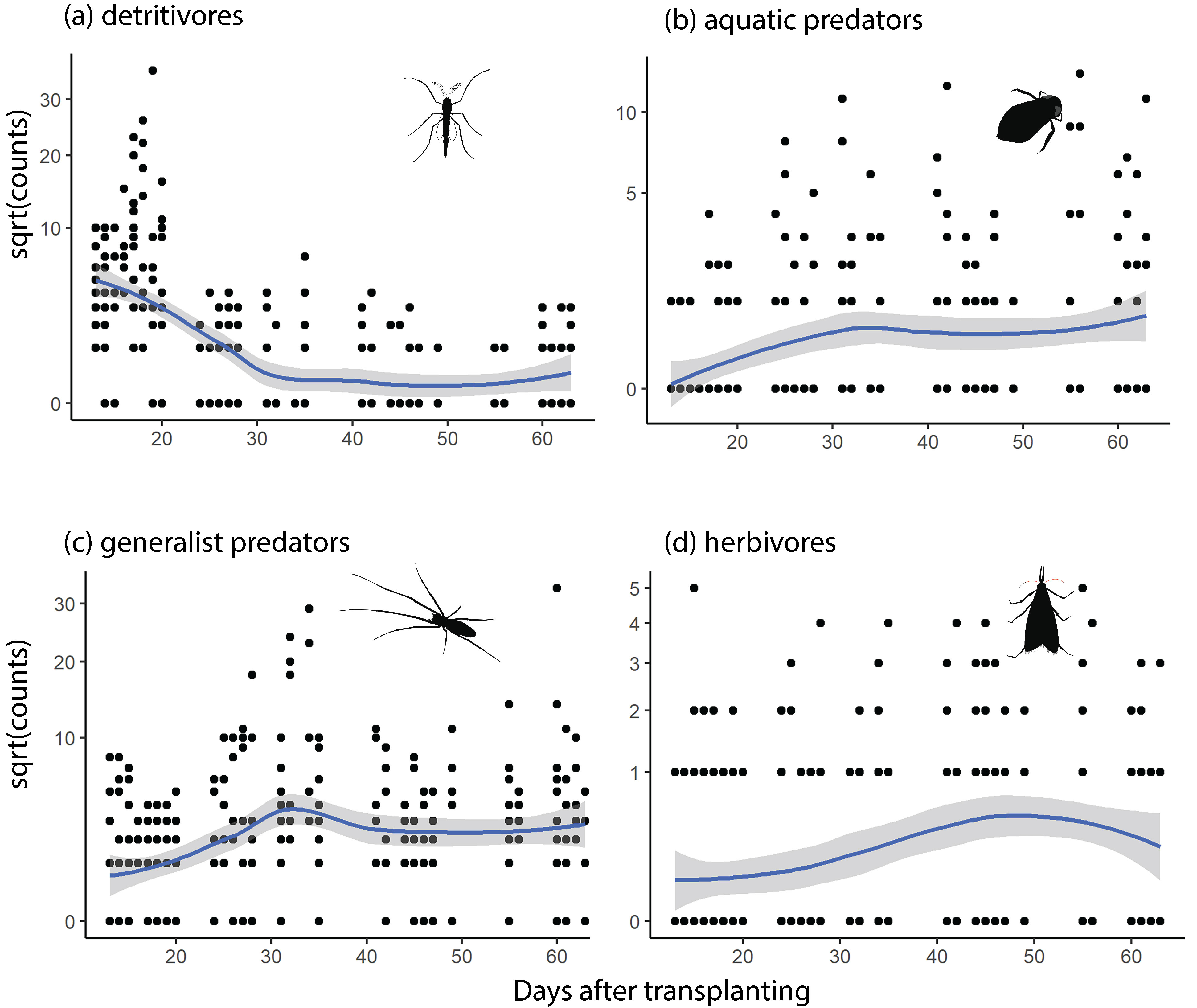
Arthropod abundance of detritivores (a), aquatic predators (b), general predators (c), and herbivores (d) by rice growth stage (days after transplanting). Y-axes are square root transformed to improve the visualization of data.
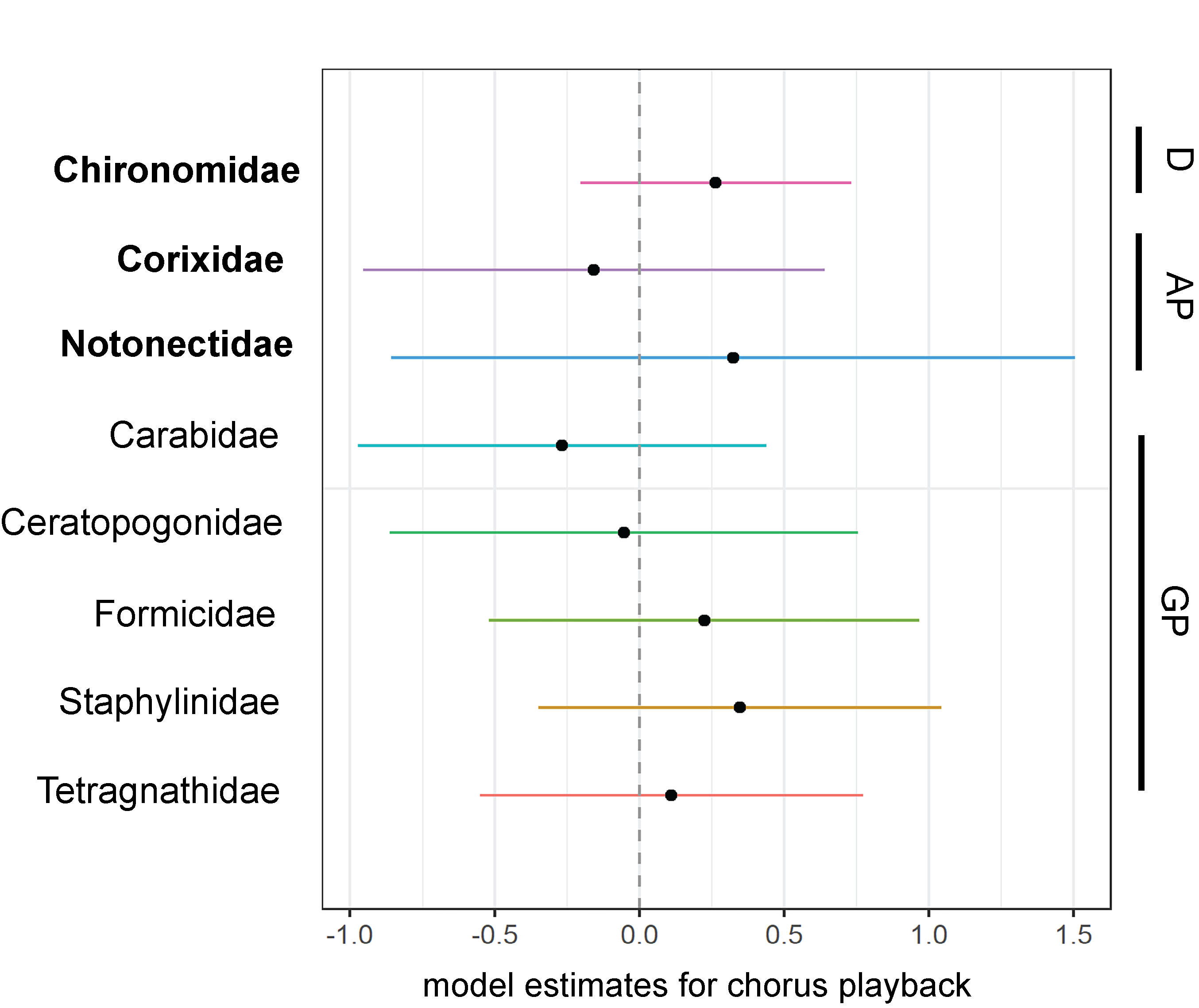
Estimates of abundance of arthropod families from generalized linear models with 95% confidence intervals surrounding the effect of ultrasonic katydid chorus playback. Arthropod families containing taxa known to possess hearing organs are in bold. Families are organized by guild indicated on the right. Sample sizes of individual herbivore families were insufficient for analysis. D = detritivore, AP = aquatic predator, GP = generalist predator.
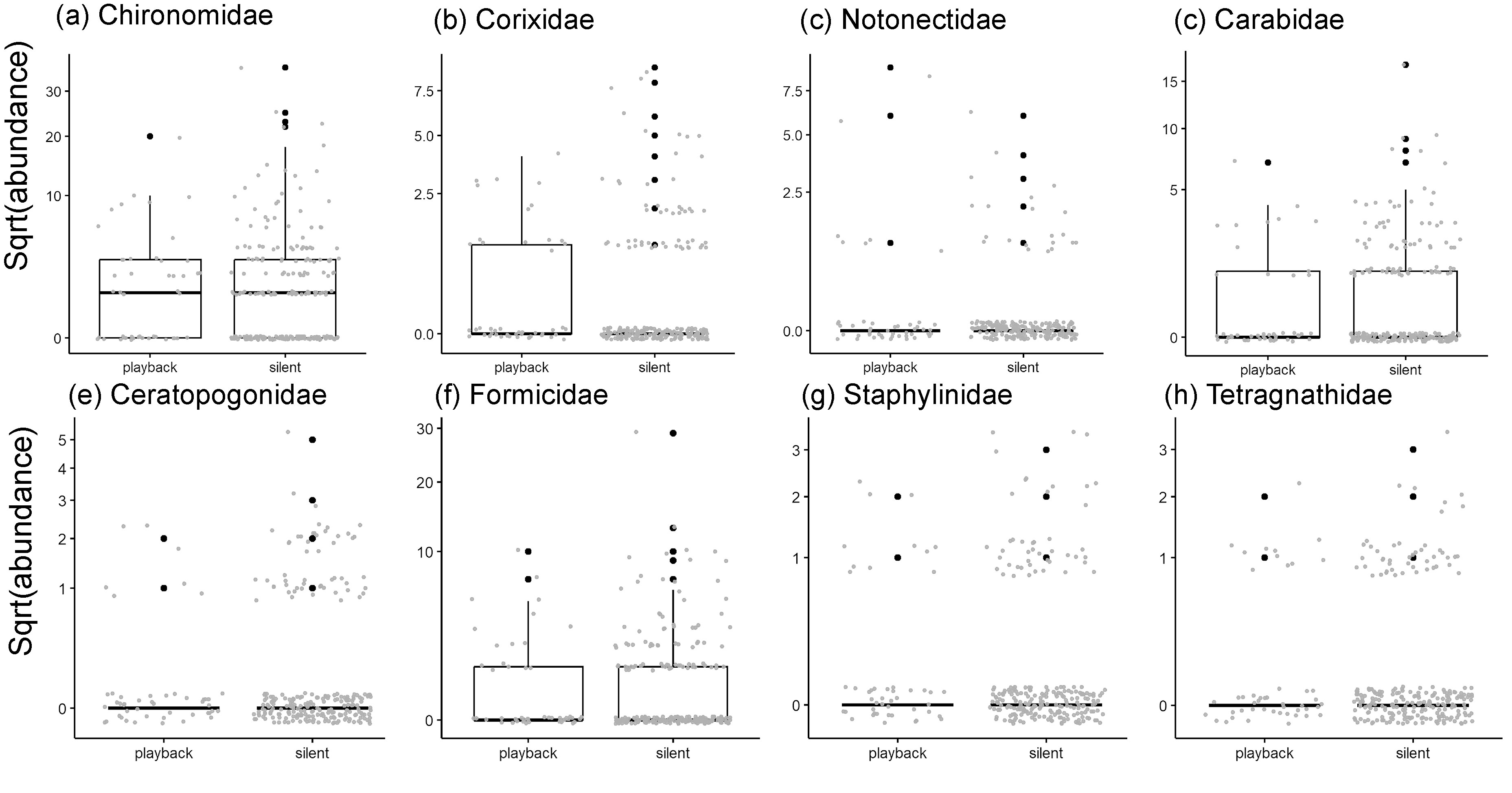
Abundance of Chironomidae (a), Corixidae (b), Notonectidae (c), Carabidae (d), Ceratopogonidae (e), Formicidae (f), Staphylinidae (g), Tetragnathidae (h), by katydid chorus playback. Insects were captured in passive intercept traps at the end of alternating 1-hr intervals of playback or silence in early-stage (0-60 day) irrigated rice at the International Rice Research Institute’s experimental farm, Philippines. Y-axes are square root transformed to improve the visualization of data.
and that later rice stage arthropods with known ultrasonic hearing (e.g., armyworm and stem borer moths) may not be attracted to the chorus as a habitat beacon. There are two broad interpretations of these results: either arthropods represented in our samples do not elicit a biological response to natural ultrasonic choruses, or we did not reproduce the natural chorus accurately enough to elicit a biological response. As we documented in Sedlock et al. [23], our 100-speaker array produced a soundscape more spatially heterogeneous in sound level than the natural chorus. This resulted from the playback reaching natural sound levels (i.e., ~69 dB at 1 m) immediately over the speaker but falling off rapidly in the spaces between sound sources (which were 2 m apart), thus producing around 50% less acoustic coverage than the natural chorus (yet is still deterred some bats [23]). Therefore, it is possible that despite their lack of response to our phantom chorus, some arthropod taxa may respond to the natural, more extensive chorus characteristic of late-stage rice. Recent work employing playback of bat-like ultrasound broadcast from powerful and rotating speakers or “ultrasonic repellers” (to cover more area) in vegetable fields demonstrated the deterrent effects of ultrasound on eared moth pests [59], and suggests our experiment could benefit from a similarly robust playback system.
Alternatively, the apparent inattention to the playback may have resulted from an inability to perceive airborne sound, frequency filtering (i.e., the chorus is outside their hearing range), or from the possibility that arthropods are detecting but ignoring the chorus as irrelevant to respond to (e.g., [32]). Among the 158 arthropod taxa we captured, 28 are known to detect airborne sounds and comprised 45% of the total individuals captured. The majority of these (70%) were detritivores, specifically non-biting midges (Chironomidae) which are attracted to tones similar in frequency to the wingbeat frequencies of conspecific mates (~ 240 Hz); however, they can only detect sound at close range and attraction outside their target frequency range is minimal [49, 60]. Similarly, aquatic predators did not respond to the chorus despite most taxa possessing tympana. Water boatman (Corixidae: Micronecta) and other hemipteran predators such as Veliids and Notonecta produce exceptionally loud stridulatory sounds underwater to attract mates; however, these bursts of sound are most intense at 15 kHz, below the frequency of the katydid chorus [45, 47, 61]. Despite possessing tympana to hear these mating songs, it is unclear whether they are sensitive to higher frequencies, for example, to evade an approaching bat when in flight. The generalist predators captured were diverse, including 27 spider taxa, a large proportion of ground beetles (Carabidae), biting midges (Ceratopogonidae), and flying ants (Formicidae)—all incapable, as far as we know, of detecting airborne sound [62] (but see [63]). Herbivores were least abundant and comprised primarily of flower beetles (Phalacridae), and aphids (Aphididae), which lacked documented ability to detect airborne sound, and included only 15 individuals with known ultrasonic hearing (i.e., Scarabidae, Lygaeidae, Noctuidae, Pyralidae). Therefore, the lack of a playback effect exhibited by herbivorous rice pests may be partly due to their scarcity and the resulting low statistical power relative to other guilds (8% of the total captured), and the absence of ultrasonic-hearing taxa. Overall, it appears that the taxa present in our study use non-auditory sensory modes to assess habitat quality [64]. For example, plant volatiles play an important role in habitat assessment for predators and parasitoids seeking herbivore prey and hosts [65], as well as herbivores selecting host plants [66].
Aerial arthropod guilds responded most strongly to days after transplanting (Date), with detritivore abundance peaking early and declining over the study, and all other guilds accumulating over time. This trophic succession echoes the results of other studies on arthropod biodiversity in tropical irrigated rice (e.g., [20, 28, 67, 68]). However, our study is unique in that we sampled aerial arthropods rather than sessile animals within the rice canopy or within floodwaters. Unlike canopy arthropod data, our data document flying or wind-dispersed arthropods that are potential prey for bats, important generalist predators in agricultural ecosystems [69]. Settle et al. [20] emphasized the importance of a healthy detritivore fauna in supporting the early arrival of generalist arthropod predators (e.g., spiders) prior to the accumulation of herbivore predators. Given that bat activity immediately above rice is highest prior to canopy closure [22], our data suggest that emerging adult detritivores may also attract bats which can subsequently consume dispersing insect pests and pest predators (see [70]).
Future work should further test these hypotheses, which have direct application to emerging pest management practices involving acoustic deterrents [59, 71, 72]. These systems deter insect pests by exploiting their documented anti-bat responses to ultrasonic pulses, or by broadcasting other sounds that disrupt or mask acoustic communication signals. While the temporal pattern of our meadow katydid playback is different from bat echolocation pulses (i.e., higher duty cycle), the spectral bandwidth is similar and may elicit a similar response. As a result, our data suggest that ultrasonic repellers, if deployed during the early stages of rice growth, may not deter detritivores or their predators. Nevertheless, the temporal attributes of playback can influence eared moths’ response (e.g., [59]); therefore, these predictions require subsequent testing with pulsed ultrasound. Moreover, given the documented success of ultrasonic (pulsed) repellers in deterring crop pests, we recommend a follow-up study deploying a more robust acoustic system and at sites and growth stages with higher abundances of ultrasonic-hearing crop pests, such as armyworms and stem borers which were virtually absent in our study, to test whether meadow katydids serve as “natural ultrasonic repellers” in irrigated tropical rice.
CONCLUSION
Our study documented a rich diversity of aerial arthropods dispersing over irrigated rice during the first half of the growth cycle, comprised primarily of detritivores (e.g., chironomids) and generalist predators (e.g., ants, ground beetles, and spiders). However, the arthropods represented in our samples were neither attracted to nor repelled by our phantom chorus, whether analyzed by guild or family. These results cautiously suggest that ultrasonic noise treatments (e.g., repellers) applied in early rice growth stages may have a neutral effect on non-pest species. However, we strongly encourage subsequent studies employing more robust ultrasonic playback in areas with ultrasonic hearing pests to test whether natural katydid choruses serve as natural repellents.
STUDY LIMITATIONS
We acknowledge that our inability to perfectly replicate the ultrasonic meadow katydid chorus and to replicate our experimental design spatially are limitations of our study. Specifically, while we were able to replicate the sound levels of a natural chorus across a large area (0.25 ha), our phantom chorus was more heterogeneous than the natural chorus. Powerful ultrasonic repellers that rotate and can create a more continuous soundscape may provide a more accurate representation of the natural chorus. Moreover, given that the arthropod assemblage varies with surrounding habitat composition, season, and history of pesticide and herbicide use in paddies, subsequent studies should replicate the phantom chorus to account for these and other sources of environmental variation. Finally, the virtual absence of specific crop pest species with documented ultrasonic hearing (e.g., armyworms and stem borers) prevented us from testing the potential repellent effect of ultrasonic choruses during later growth stages.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contributions to the manuscript as follows: J.S. and J.R.B.: Conceived and designed the study; M.L.P.A.: Conducted the data collection; B.A.R.H. and D.G.E.G.: Performed the analysis and interpreted the results;. All authors reviewed the findings and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| AIC | = Akaike Information Criterion |
| BACI | = Before-After-Control-Impact |
| RMS | = Root Mean Square |
| dB | = Decibels |
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
Permission to conduct research was granted by the Department of the Environment and Natural Resources, Philippines Biodiversity Management Bureau, and the International Rice Research Institute, Philippines. Approval Number: R4A-WGP-06-2015-LAG-003. Boise State University’s Institutional Animal Care and Use Committee approved the project methods (AC15-021).
HUMAN AND ANIMAL RIGHTS
Insect collection methods aligned with recommendations of the American Veterinary Medical Association (AVMA).
AVAILABILITY OF DATA AND MATERIAL
The data supporting the findings of the article and R code are available on Zendo at https://doi.org/10.5281/ zenodo.14072316.
FUNDING
This research was funded by the National Science Foundation (1721072 to J.L.S. and 1556177 to J.R.B.), Lawrence University (Leveraging Faculty Initiatives Fund to J.L.S.) and the National Geographic Society (C.R.E. to J.R.B.).
ACKNOWLEDGEMENTS
We thank James Alvarez, David Baldwin, Lemon Beckham, Wayne Krueger, Juliette Rubin, Zhiru Wang, Sarah Woody and Joey Zimmer who assisted with the speaker array development. We are grateful to Alexander Stuart, Teodoro Correa, Albert Naredo, and numerous IRRI staff who assisted with on-site planning and logistics. We are grateful to Roman Fornesa and Jonas Llamas, who assisted in data collection, and anonymous reviewers who provided comments on the manuscript. The Philippine Department of the Environment and Natural Resources permitted us to conduct wildlife research.


