All published articles of this journal are available on ScienceDirect.
Micromorphological Features, DNA Barcoding and Nutraceutical Compounds of Two Edible Flowers: Petunia × Hybrida and Verbena Bonariensis
Abstract
Introduction
Petunia × hybrida and Verbena bonariensis are two herbaceous taxa with edible flowers originating from South America. Despite the well-known ornamental value of these taxa, their potential as a food source remains underexplored.
Methods
Therefore, in our study, the flowers of P. × hybrida and V. bonariensis obtained from plants grown organically in a greenhouse were identified via DNA barcoding analysis and investigated to characterize the micromorphological features, composition in nutrients, nutraceutical compounds, and spontaneous emission of volatile compounds. P. × hybrida flowers exhibited greater levels of crude proteins, carotenoids, and antioxidant activity. In contrast, V. bonariensis had a higher content of total soluble sugars, total polyphenols, total flavonoids, and ascorbic acid. The DNA sequences obtained from both taxa returned maximum identity and were submitted to GenBank.
Results
Both taxa displayed characteristic anthocyanin patterns and taxon-specific glandular trichomes, rich in secondary compounds such as terpenoids, mucilages, and polyphenols. The fragrance and flavor detected for these edible flowers were also dependent on taxon-specific VOCs patterns: P. × hybrida VOCs were rich in non-terpene compounds, predominantly comprising esters (61.6%), especially methyl nonadecanoate (21.7 ± 0.3%) and methyl benzoate (12.7 ± 0.3%); V. bonariensis was characterized by terpenoids, particularly the monoterpene hydrocarbon (E)-β-Ocimene (47.6 ± 2.0%).
Conclusion
Our interdisciplinary study provides the first data for the authentication of these taxa. These data underline the high nutraceutical value of these edible flowers and will also be useful in the case of commercial exploitation.
1. INTRODUCTION
Petunia × hybrida E. Vilm. (Solanaceae) and Verbena bonariensis L. (Verbenaceae) are two ornamental herbaceous plants, both originating from South America, each possessing distinct characteristics. Both petunia and purpletop vervain flowers are edible and can delicately garnish dishes and salads. They were included in the Antea project, a European cross-border cooperation initiative between France and Italy (INTERREG ALCOTRA N° 1139), which focused on exploring edible flowers as a source of nutrients and nutraceutical compounds [1].
P. × hybrida, commonly known as petunia, is an interspecific hybrid between P. axillaris (Lam.) Britton, Sterns & Poggenb. (large, white flowers) and P. integrifolia (Hook.) Schinz& Thell. (small, purple-reddish flowers) [2]. Petunia produces a copious flowering with bright colors when cultivated in full sunlight. Generally, petunias are used in flower baskets and pots to beautify balconies and terraces. Its genome was sequenced in 2016 [3], and as a plant model system, it has been employed in various studies regarding plant genetics, plant biology, and biochemistry [4-7], mainly focusing on the influence of light on the biosynthesis of flavonoids in pink/purple flowers varieties [8] or carotenoids in yellow/red flowers varieties [9]. Floral scent production and volatile emissions have been widely investigated [7, 10, 11]. Petunia synthesizes a wide diversity of phytochemical compounds possessing biological functions, namely carotenoids, anthocyanins, and polyphenols [2], which have a role in plant defense and have medicinal, antioxidant, and nutraceutical properties useful for human health.
V. bonariensis, commonly known as purpletop vervain, is a fast-growing perennial plant. Its tall, upright branching stems hold clusters of magenta-purple flowers that attract bees and butterflies. In recent years, V. bonariensis has been incorporated into herbaceous borders of gardens in association with ornamental grasses to add structure or a poetic note [12]. Contrary to petunia, purpletop vervain is scarcely studied. Recent papers have investigated its tolerance to drought and cold stress [13, 14], but there is limited information regarding the nutraceutical properties of its flowers and their content of secondary metabolites [15].
The main sites of synthesis and release of essential oils are spread on the epidermis of stems, leaves, and flowers of both petunia and vervain [16, 17]. Glandular trichomes are involved in releasing volatile organic compounds (VOCs), which contribute to the essential oils’ fragrance and flavor in leaves and flowers [18]. However, information about the abundance and the localization of the localiszation of secondary metabolites production sites is currently limited for both taxa.
This work aims to enlarge the knowledge about the edible flowers of the two ornamental plants P. × hybrida and V. bonariensis, through the characterization of the micromorphological features, composition in nutrients nutraceutical compounds, and spontaneous emission of volatile organic compounds (VOCs). Furthermore, our aim was also to identify the taxa using the DNA barcoding method with floral tissues to contribute to the database of known taxa and to provide useful data for the identification of doubtful samples in mixtures or commer- cial products.
2. MATERIALS AND METHODS
2.1. Plant Material and Plant Culture
The cultivation methods varied according to the taxon: P. × hybrida was propagated using commercial seeds obtained from Sgaravatti & C. Sementi S.p.a., Arezzo, Italy, while V. bonariensis plants were sourced from the plant nursery “L’Erbaio della Gorra” (Casalborgone, TO, Italy).
Petunia seeds were sown in seedbeds containing a mixture of peaty and quartz sand (80:20, v/v). V. bona- riensis cuttings were grown in alveolate containers with a mixture of perlite and peaty (70:30, v/v). Once the roots and shoots were adequately developed, the plants were transplanted into 30 cm diameter pots with a volume of 9 L. These pots were filled with a substrate mixture of peaty and pumice (70% and 30%, respectively) (Hochmoor Vulcan– Terflor, Capriolo, BS, Italy) and included slow-release fertilizer (Nitrophoska, Eurochem Agro, Cesano Maderno, MB, Italy). All plants were cultivated at the CREA Institute (Sanremo, IM, Italy, GPS: 43.816887, 7.758900) in a greenhouse with an anti-insect net. Weekly fertilization was done using a nutrient solution (Ferti 3, Planta-Dȕngemittel, Regenstauf, Germany).
The plants were grown organically, avoiding pesticide use, as reported by Najar et al. [19]. Biological control agents like Adalia bipunctata, Aphidius colemani, Amblyseius swirskii, Chrysoperla carnea, and Phytoseiulus persimilis (Koppert Italia Srl., Bussolengo, VR, Italy) were employed. Additionally, Bacillus thuringensis subsp. kurstaki (Serbios Srl, Badia Polesine, RO, Italy) was used to manage aphids, thrips, and caterpillars.
Mature fresh flowers at full bloom were harvested between 8:00 and 10:00 a.m., stored at -80°C for later analysis, or used fresh or freeze-dried, depending on the specific analysis.
2.2. DNA Extraction and DNA Barcoding Analysis
Samples of P. × hybrida and V. bonariensis were collected from the CREA Institute. DNA extraction from young leaves was performed using the DNeasy Plant Kit (QIAGEN, Milan, Italy) following the manufacturer’s instructions. The resulting purified gDNA was then assessed for concentration and purity by using a Qubit 2 Fluorometer and Qubit dsDNA HS Assay Kit (Invitrogen, Carlsbad, CA, USA). The Internal Transcribed Spacer region (ITS2) region was chosen for species identification following the protocol described by Cheng et al. [20]. PCR amplification was performed using Wonder taq Polymerase (EuroClone S.p.A., Milan, Italy) in a 25 μL reaction volume, as per the manufacturer’s guidelines, containing 1 μL 10mM of each primer and 3 μL od DNA template (25 ng/ μL) and employing the primers described by Cheng et al. [20].
The PCR cycling conditions included an initial denaturation at 95°C for 5 min, followed by 35 cycles of denaturation (45 seconds at 95°C), annealing (45 s at 55°C), extension (1 min at 72°C), and a final extension at 72°C for 7 min. The amplified products were sequenced at Eurofins Genomics (Ebersberg, Germany). The sequences underwent manual editing, primer removal, and pairwise alignment. Species identification was carried out using a standard comparison approach against the GenBank database with BLASTn (https://blast.ncbi.nlm.nih.gov/ Blast.cgi accessed on 1 May 2023). Taxonomic assignment of each barcode sequence was performed according to the method outlined by Frigerio et al. [21]. Finally, all sequences were submitted to the GeneBank database (https://www.ncbi.nlm.nih.gov).
2.3. Flower Parameters
The length and width of fresh petunia and purpletop vervain flowers were analyzed using twenty-five and fifty flowers, respectively. The same petunia flowers were used to determine the fresh weight. For V. bonariensis, due to the small size of the flowers, twenty groups of fifty flowers were weighed, and the average weight of fifty fresh flowers was calculated.
To estimate flower water content, fresh flowers from both species at full maturity were collected, weighed, packed in paper bags, and stored at -80°C. They were then freeze-dried at -50°C under vacuum for 48 h (Labconco, Kansas City, USA) and weighed again. The water content percentage of the flower was calculated as follows:
Water content (%) = {[(fresh weight of flowers) – (freeze dry weight of flowers)] / (fresh weight of flowers)} x 100.
This analysis was performed in triplicate. The paper bags containing freeze-dried flowers were stored in plastic bags with silica gel (1 - 3 mm) (VWR Chemicals) as a dehydrating agent.
2.4. Biochemical Analysis
Freshly picked flowers were collected in the morning and stored at -80°C until further analyses. Freeze-dried samples were used to determine the total nitrogen content, while frozen flowers were used for all other analyses outlined below.
Soluble sugars were quantified following the methods reported by Najar et al. [19]. Total soluble sugars were measured spectrophotometrically using the anthrone-sulphuric acid assay [22], while sucrose, D-fructose, and D-glucose were quantified employing the Sucrose/D-Fructose/D-Glucose Assay Kit (Megazyme International Ireland, Co. Wicklow, Ireland) according to the manu- facturer’s instructions. Total nitrogen content was determined using the Kjeldhal method [23], and the percentage of crude proteins was calculated by multiplying the percentage of nitrogen by 6.25, as detailed in Marchioni et al. [1]. Three biological replicas were used for each analysis.
Total carotenoid content was measured according to the method described by Lichtenthaler [24] prior to methanolic extraction. Total polyphenolic content (TPC) was determined using the Folin-Ciocalteu method, as described by Singleton and Rossi [25]. Total flavonoid content (TFC), total anthocyanins content (TAnth), total ascorbate (AsATOT), and reduced ascorbate (AsA) were quantified as detailed by Najar et al. [19]. Radical scavenging activity was assessed by the DPPH assay [26], with results expressed as IC50 (mg/mL) [27]. All measure- ments were performed with an ultraviolet (UV)-1800 spectrophotometer (Shimadzu Corp., Kyoto, Japan). Each analysis was performed in triplicate.
2.5. Microscopy
The micromorphological characteristics of the flowers were investigated using both light microscopy (LM) and scanning electron microscopy (SEM). For LM analysis, epidermal peels or sections of the calyx and corolla were immediately examined using transmitted light or epifluorescence with a Leica DM 2000 microscope. To detect the autofluorescence of polyphenols, flavonoids, or tannins, small untreated portions were directly observed under a UV filter (340-380 nm). Lipids were visulised using Fluorol Yellow 088 [28], and observations were carried out under the UV filter. Additionlly, histochemical reactions were performed: Toluidine Blue O (TBO) for polyphenols and tannins detection, Sudan III for total lipids, and Ruthenium Red for non-cellulosic poly- saccharides.
For SEM analyses, samples were immersed overnight in FineFIX working solution (Milestone s.r.l., Bergamo, Italy), supplemented with 70% ethanol, and then refrigerated overnight at 4°C, following the protocol by Chieco et al. [29]. Specimens were then dehydrated in an ascending series of ethanol and critically dried using a K850CPD 2M processor (Strumenti S.r.l., Roma, Italy).
Subsequently, samples were mounted on aluminum stubs and coated with a 10 nm layer of gold. Observations were made with a VEGA3-Tescan-type LMU microscope (Tescan Orsay Holding, a.s., Brno, Czech Republic) at a voltage of 20kV.
2.6. HS-SPME Analysis
The investigation into spontaneous floral VOC emissions followed the methodology detailed in our previous study [1]. Briefly, fresh flowers (0.1 to 0.5g) from each plant were individually sealed in 20 mL glass conical flasks with aluminum foil for 30 minutes to establish equilibrium. Volatile organic compounds were analyzed using a conditioned 100 μm polydimethylsiloxanes (PDMS) fiber from Supelco Ltd. The fiber was exposed to the headspace of the flower samples for 15 minutes at 23°C and then transferred to the gas chromatograph injector at 250°C. The compounds desorbed from the solid-phase microextraction (SPME) fiber were subsequently analyzed using gas chromatography-mass spectrometry (GC-MS). Identification relied on comparing retention times with authentic samples, considering linear retention indices relative to a series of n-hydrocarbons. Computer matching was also conducted against mass spectra libraries derived from pure substances, known oils, and literature data on mass spectrometry [30-33].
3. RESULTS AND DISCUSSION
3.1. DNA Barcoding
Good DNA extraction yield (ranging from 20–40 ng/µL) was obtained from the samples. Each barcode sequence was taxonomically assigned by using BLASTn analysis, matching plant taxa with the nearest identities (maximum identity >99% and query coverage of 100%). All samples returned 100% maximum identity with 100% query coverage. The obtained sequences were submitted to GenBank (https://www.ncbi.nlm.nih.gov). Results are summarized in Table 1. DNA barcoding successfully identified Verbena bonariensis at the species level. However, for Petunia × hybrida, despite a 100% match being found for the declared taxon, the analyzed sequence also gave a 100% match for Petunia axillaris, Petunia integrifolia, and Petunia integrifolia subsp. depauperata (R.E.Fr.) Stehmann (syn.= Petunia littoralis (Reitz) L.B.Sm & Downs). This result is quite common in the presence of hybrids [34]. Thus, while DNA barcoding is efficient for identifying Verbena bonariensis, alternative identification methods are necessary for Petunia x hybrida.
3.2. Fresh Flower Parameters
Petunia flowers measured 40 ± 3 mm in length and 61 ± 1 mm in width, with an average weight of 0.37 ± 0.04 g per flower. On the contrary, a single flower of V. bonariensis measured 9 ± 1 mm in length and 5 ± 1 mm in width, while an average weight 0.122 ± 0.005 g (N= 50 Flowers). The water content percentage for petunia and purpletop vervain flowers was recorded at 86.62 ± 3.09% and 79.57 ± 1.18%, respectively.
3.3. Biochemical Analysis
Table 2 reports data on metabolite content and antioxidant activity in the flowers of P. × hybrida and V. bonariensis. The purple V. bonariensis showed greater levels of total soluble sugars, total polyphenols, total flavonoids, and reduced Ascorbic Acid (Table 2). In contrast, the pink-colored P. × hybrida flowers had higher crude proteins and carotenoid content, along with the greater antioxidant activity.
| Declared Species | Resulted Species | Origin | Collection Year | Accession Number |
|---|---|---|---|---|
| Petunia (×) hybrida | Petunia (×) hybrida | Italy | 2019 | OR146196 |
| Verbena bonariensis | Verbena bonariensis | Italy | 2019 | OR146197 |
| Parameters | P. × hybrida | V. bonariensis |
|---|---|---|
| Primary metabolites | - | - |
| Total soluble sugars (mg/g FW) | 34.72 ± 2.91 | 83.99 ± 2.73 |
| D-glucose (mg/g FW) | 2.38 ± 0.16 | 8.26 ± 0.33 |
| D-fructose (mg/g FW) | 2.03 ± 0.21 | 4.85 ± 0.23 |
| Sucrose (mg/g FW) | 0.80 ± 0.21 | 1.30 ± 0.20 |
| Total crude proteins (% DW) | 10.03 ± 0.41 | 7.69 ± 0.13 |
| Secondary metabolites | - | - |
| TCar (µg/g FW) | 18.37 ± 2.86 | 12.64 ± 0.43 |
| TPC (mg GAE/g FW) | 4.53 ± 0.26 | 5.57 ± 0.20 |
| TFC (mg CE/g FW) | 1.69 ± 0.08 | 1.97 ± 0.17 |
| TAnth (mg ME/g FW) | 0.52 ± 0.03 | 0.56 ± 0.01 |
| Total ascorbic acid (AsATOT) (mg/100g FW) | 10.39 ± 0.36 | 9.12 ± 0.13 |
| Reduced ascorbic acid (AsA) (mg/100g FW) | 2.12 ± 0.04 | 3.38 ± 0.04 |
| Radical scavenging activity | - | - |
| DPPH assay (IC50 mg FW/ml) | 4.69 ± 0.27 | 1.69 ± 0.24 |

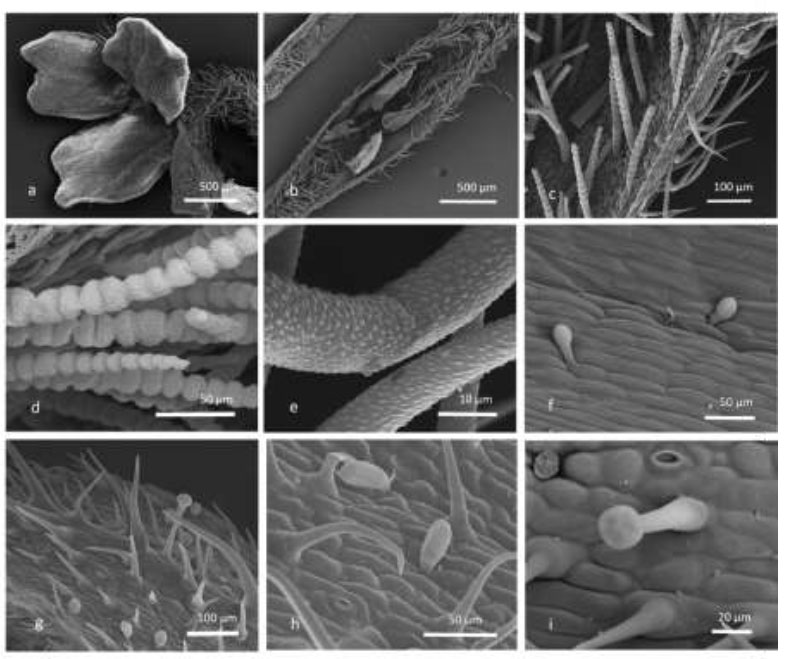
Stereomicroscope (a) and light microscope micrographs of corolla (b, c) and calyx (d-h) from V. bonariensis. (a) a single flower of the inflorescence; (b) moniliform-like non-glandular hairs spread on the internal surface of the corolla tube; (c) rough surface of a moniliform-like hair; (d) alternate distribution of non-glandular and glandular trichomes on the adaxial surface of the calyx; (e) non-glandular trichomes and a medium-sized glandular one showing a 4-celled head; (f) a medium-sized glandular hair positive to Sudan III staining revealing the lipid content in the head; (g) capitate subsessile trichome positive to TBO staining: purple violet color indicates the presence of mucilage in the glandular head; (h) a medium-sized capitate trichome with a 2 celled-head, showing positivity to Ruthenium Red dye, due to the presence of mucopolysaccharides.
In the literature, data on Verbena species are limited, mainly focusing on V. hybrida [35], which exhibited characteristic nutraceutical content. However, due to the absence of color data for this taxon, the influence of petal color in the two species remains undetermined. Recently, high soluble sugar content in V. hybrida was observed, possibly deriving from floral nectar, which serves as a nutrient source and reward for pollinators, potentially promoting insect reproduction [36].
Petunia flowers have recently been tested for their suitability as nutraceutical food [37]. Chrysargyris et al. [38] reported a biochemical composition of P. × hybrida flowers that aligns with this work (Table 2), although some metabolites differed depending on the salt treatment applied during cultivation. Conversely, the same taxon cultivated in Japan showed different secondary metabolites composition, suggesting that soil and various environmental factors may significantly impact the nutraceutical compound content.
3.4. Microscopy
3.4.1. Verbena Bonariensis
V. bonariensis flower (Figs. 1a, 2a) reveals different trichome typologies, confirming that this genus exhibits most of the trichome types typical of Verbenaceae, as reported by Mathew and Shah [39], Cantino [40], and Sanders [41]. Uniseriate, moniliform-like, non-glandular trichomes (Figs. 1b-c, 2b-d) are widely spread exclusively on the internal surface of the corolla, somewhat below the orifice of the tube. At the macroscopic level, they correspond to the pubescent/villous indumentum described by Munir [42].
This kind of trichomes were referred to as moniliform, and their presence was previously reported in species from the genera Glandularia and Verbena by Umber [43]. However, Sanders [41], following Cantino [40], stated that there are no multicellular non-glandular trichomes in Verbenaceae as a non-gynoecial synapomorphy characteristic of the family. Therefore, he called them “falsely moniliform specialized hairs”, as they appear as filiform structures with numerous crosswise constrictions. These trichomes exhibit a very rough surface (Figs. 1c and 2c-d). Non-glandular unicellular trichomes of different lengths are also present. They show radially arranged basal cells, a body with a slightly rough surface, and a curved tip. These hair densely cover the external surfaces of the corolla and the calyx (Figs. 1d-e, 2c arrow, 2g-h). Similar hair are well known for Verbenaceae and have been previously described in detail for stems and leaves of six different Verbena by Rodrìguez Morcelle et al. [16].
Two kinds of short subsessile glandular trichomes were found: the first has a 1-celled spherical head (not shown), while the other has an oblong-clavate bicellular head. They are scattered on both the calyx and the corolla (Fig. 2f-h). According to Cantino [40], the 1-celled head type is reported in only six genera of Verbenaceae, while the 2-celled one is more common in the family. In addition, medium-sized trichomes were observed only in the calyx, where they are alternately scattered among the uncinated non-glandular hairs (Fig. 1d). These trichomes show a monocellular stalk of variable length (about 20 to 100 µm) and a 2-4 celled head (Figs. 1e, f, 1h, 2g, 2i).
All three types of glandular trichomes in the calyx and corolla showed similar reactions to the different staining treatments, with a few exceptions. The presence of lipidic substances in the glands was confirmed by Sudan III staining (Fig. 1f). With TBO at pH 4.4, some heads appeared blue/dark violet, revealing the presence of polysaccharides, while other glands were stained in blue/green indicating phenolic, flavonol or tannin substances in glandular heads (Fig. 1g). Fluorol Yellow 088 showed the presence of terpenoid/lipid substances within the head and sometimes in the stalks of all four types of trichomes (not shown); it also stained the cell walls of the moniliform-like non-glandular hairs. This latter positivity could be correlated with the presence of cutin/suberin in the trichome cell wall. A red color, revealing the presence of mucopolysaccharides, was detected by Ruthenium Red in the head and, more rarely, in the stalk of glandular trichomes scattered on the corolla and calyx (Fig. 1h). Under UV excitation, all types of trichomes emitted blue autofluorescent signals (not shown), varying from weak to intense light blue, confirming the polyphenolic/tannin profile of secretions. Stomata are present in the corolla and calyx, showing an anomocytic apparatus, as previously observed in the Verbena spp. leaves by Rodríguez Morcelle et al. [16]. In the corolla, the epidermis appeared slightly papillose and was covered with thick, sinuous waxes. The presence of anthocyanins in the epidermal cells was confirmed by the rose color, well evident under transmission light.
3.4.2. Petunia × hybrida
Both the calyx and the corolla of Petunia bear 1-cell-headed glandular trichomes of different lengths, showing a stalk range from 2 to 7-8 cells. (Figs. 3a, 4a, b).
In agreement with data reported by Ahmad [44], elongated capitate hairs are more frequently present than smaller ones in both the calyx and corolla. On the other hand, branched trichomes with a glandular head at each tip were rarely detected in the calyx (not shown). The higher frequency of longer trichomes and the rare presence of branched ones are traits common to both Petunia and Nicotiana in Solanaceae [44, 45].
In the calyx, trichomes were frequently covered by exudates (not shown). These could correspond to the polyester acyl sugars, typical of Solanaceae, that cause the stickiness of the calyx surface, providing insecticidal effects [46].
By LM, the natural color of oil droplets inside the glandular heads appears brown/beige (Fig. 3a-b). Crystals of calcium oxalate were sometimes present within the monocellular secretory head of these trichomes (Fig. 3b, arrow). The ability to stock crystals in glandular heads is already known for Solanaceae: their possible role in herbivory avoidance was hypothesized for Solanum sp. by Shepardson [47]. Choi et al. [48] and Sarret et al [49], documented that Nicotiana tabacum showed Cd or Zn oxalate crystals instead of calcium oxalate crystals when these metals exceed toxic levels in the growth substrate.
TBO metachromatic staining revealed the presence in the glandular heads of both mucilages, appearing blue/dark violet and of phenolic/tannin substances colored light blue/green (Fig. 3c). The presence of terpenoids inside the head cells and sometimes in the stalk cells was confirmed by Fluorol Yellow 088 (not shown). Ruthenium Red staining revealed mucopolysaccharides both in the parenchymatic tissues and in the stalks and heads of glandular trichomes; the lipid content of the heads was confirmed by the positivity to Sudan III. However, considering the different staining intensities shown by trichomes, we hypothesize that this variation could be related to the degree of their development.
In the distal portion of the corolla, the epidermis is covered by waxes and composed of flattened, slightly sinuous cells with cell wall thickenings (Fig. 3f), as previously mentioned by Ahmad [44]. Approaching the central zone, epidermal cells gradually assume the shape of conical papillae (Figs. 3g, 4d, e). Epidermal cells appeared purple-red in transmission light, confirming the presence of anthocyanins (Fig. 3d, e, 3g). Anthocyanins’ presence and their biosynthetic pathways are well-known for Petunia × hybrida, as this taxon has been the model organism for several studies on the genes involved in secondary metabolites production [8, 50, 51].
Scanning electron microscopy (SEM) micrograph of V. bonariensis. Corolla (a-f) and calyx (g-i). a) in the corolla, the distribution of trichomes on the external surface is visible; (b) longitudinal section of a flower reveals the presence of moniliform-like non-glandular trichomes inside the corolla tube; (c) typical distribution of non-glandular trichomes on corolla: moniliform-like non-glandular trichomes are exclusively present inside the corolla’s tube, while non-glandular unicellular trichomes are spread on the adaxial surface; (d) particular of the very rough cell’s surface of moniliform-like non-glandular hairs; (e) detail of unicellular non-glandular trichomes showing a slightly rough surface; (f) subsessile capitate trichomes in the inner corolla showing the bicellular head and 1-celled stalk; (g) trichomes distribution on the external surface of the calyx; (h) subsessile capitate trichomes with an oblong-clavate bicellular head; (i) medium-sized glandular hair with a 4-celled head.

Light microscope micrographs of P. × hybrida. Calyx (a, b, e) and corolla (c, d, f, g). (a) capitate glandular trichomes showing stalks of different lengths; (b) detail of a medium-sized trichome showing a calcium oxalate druse within the head (arrow); (c) particular of a glandular head positive to TBO. The light blue color indicates the polyphenolic/flavonoid content; (d) the heads of glandular trichomes show strong positivity Sudan III, indicating the presence of lipidic substances; (e) the positive reaction to Ruthenium Red indicates the secretion of mucopolysaccharides in the glandular head; (f) epidermal cells showing cell walls with sinuous shape in the distal portion of the corolla; (g) epidermal papillose cells of the central zone of the corolla tube. A high anthocyanin content is well visible in both (f) and (g).
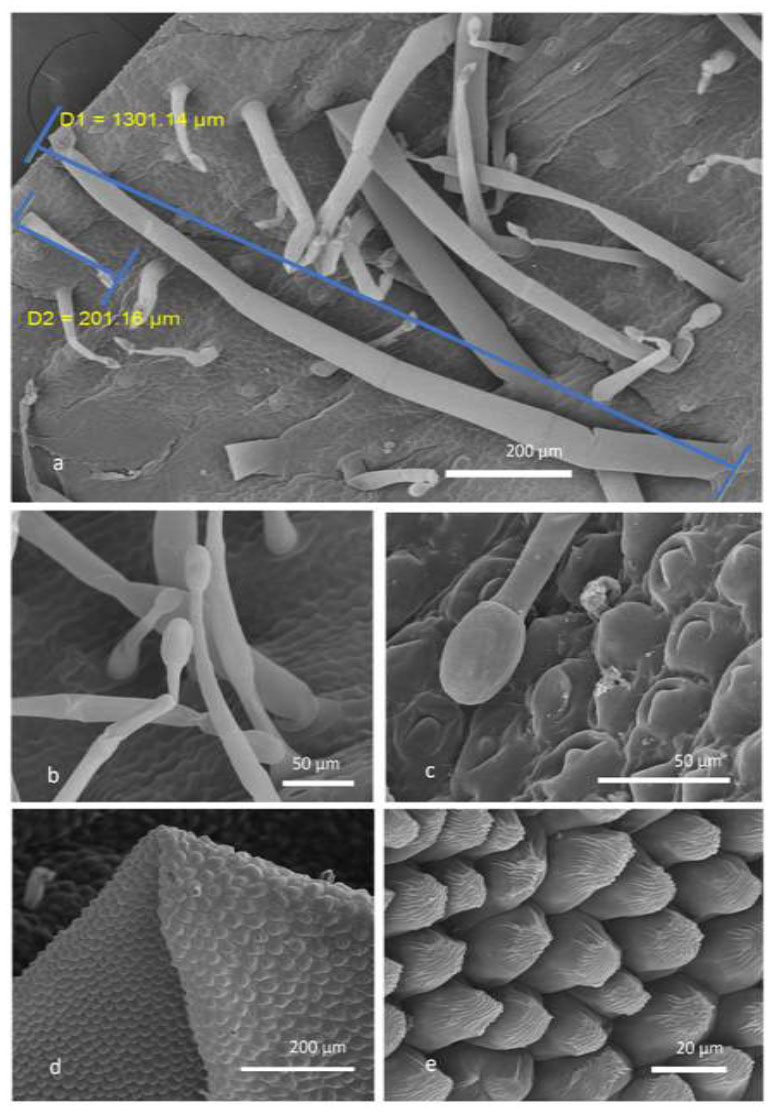
SEM micrographs of epidermal surfaces of Petunia × hybrida flower. Corolla (a, d, e) and Calyx (b). (a) Glandular trichomes with one-celled head and pluricellular stalks of different sizes in the corolla; (b) little and medium-sized glandular trichomes in the calyx; (c) detail of a glandular head; (d) morphology of the epidermal cells in the distal portion of the corolla; (e) detail of cuticular ornamentation of epidermal papillae from the central zone of the corolla tube
3.5. VOC Analyses
Petunia × hybrida VOCs were rich in non-terpene compounds, predominantly comprising esters (61.6%), followed by aldehydes and fatty acids (FAs), nearly evenly distributed (12.1% and 10.2%, respectively) (Supplementary). Esters were represented by methyl nonadecanoate (27.8%, (38)), along with lower percentages of methyl benzoate (12.7%, (7)) and dodecyl octanoate (7.4%, (35)). Aldehydes manifested in only three compounds: decanal (4.9%, (11)), nonanal (3.7%, (8)), and 5,9,13-trimethyl-4,8,12-Tetradecatrienal (3.5%, (31)). The major fatty acid (FA) compound was stearic acid (6.1%, (36)). According to a previous study, VOCs in petunia flowers are primarily composed of benzenoids and phenylpropanoids [52]. Phenylpropanoids were present in a significantly lower percentage, constituting less than 2.5% of the whole composition. In contrast, benzenoid constituents accounted for a quarter of the identified fraction in the studied species, encompassing seven compounds. Notable among these were methyl benzoate12.7%, (7)), cis-isoeugenol (4.7%, (15)), methyl salicylate (3.2%, (9)), oxime, methoxy-phenyl (2.0%, (1)), 3,5-dimethyl-2-isobutylpyrazine (1.4%, (12)), 2-methoxy-5-methylphenol (1.0%, (10)), and 2-phenoxy-Ethanole (0.2%, (13). Methyl benzoate was identified as one of the main pollen volatile organic compounds in P. secreta Stehmann & Semir (22.99%) and in P. axillaris (26.46%) [53]. However, herein, the amount of this compound is nearly half, given that our flower resulted from the crossbreeding of two species, one of which P. axillaris. Additionally, based on the same study, these species were rich in aliphatic compounds (58,66%, and 52.19%, respectively), while in the present work, aliphatic compounds constituted 66.1% of the total percentage.
On the other hand, the spontaneous emission of V. bonariensis was characterized by terpenoids, particularly monoterpene hydrocarbons, which account for 47.8% of the identified portion, while sesquiterpene hydrocarbons represented an additional 20.0%. The major compounds representing these classes were (E)-β-Ocimene (47.6%, (6)) and β-Caryophyllene (13.0%, (17)), respectively (Supplementary). Noteworthy, there was a significant content of esters (18.0%), almost exclusively represented by Methyl salicylate (16.1%, (9)). Additionally, aldehydes were present at a level of 10.3% with benzaldehyde, having a characteristic odor of bitter almond [54]. The literature on this genus primarily focused on studying its EO rather than investigating its spontaneous emission (VOC released directly from the plant). Even though EO is obtained through methods like hydrodistillation, involving the extraction of VOC, a comparison with the spontaneous emission – VOC released directedly from the plant – was conducted. Some shared compounds were identified, such as caryophyllene and (E)-9-b-ocimene [55].
CONCLUSION
Many ornamental flowers can serve as a new food source with interesting nutritional values. When grown correctly, they can be managed in small spaces such as balconies or gardens, allowing consumers to collect them as needed for use in dishes. Among these ornamental species with edible flowers, we highlight P.x hybrida and V. bonariensis, both of which are good sources of soluble sugars, carotenoids, and ascorbic acid. Further- more, the glandular trichomes characteristic of these two taxa are responsible for the production of secondary metabolites with high nutraceutical value, such as polyphenols, and are involved in the release of volatile organic compounds (VOCs), contributing to the fragrance and flavor of the flowers.
Both taxa have a delicate but distinguishable taste, as the composition of the volatile compounds they release (VOCs) differs significantly. The VOCs of P. ×hybrida are rich in non-terpene compounds, predominantly comprising esters, while those of V. bonariensis VOCs are dominated by terpenoids. Our study provides data that will allow for the identification of the taxa from the molecular, biochemical, and morphological points of view.
AUTHOR CONTRIBUTION
A.C. and M.B.: Involved in conceptualization; A.C., I.M., B.N., M.B. and J.F.: Adopted the methodology; I.M., B.N., M.B., J.F. and F.B.: Provided the formal analysis and investigation; A.C., B.N., M.B. and J.F.: Involved in writing original draft preparation; L.P. and B.N.: Contributed to writing review and editing; B.P.: Acquired the fund; B.R., L.P., L.C. and L.P.: Provided the resources and supervision.
LIST OF ABBREVIATIONS
| VOCs | = Volatile organic compounds |
| TPC | = Total polyphenolic content |
| TFC | = Total flavonoid content |
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
This research was funded by INTERREG-ALCOTRA UE 2014-2020 project: “ANTEA” - Attività innovative per lo sviluppo della filiera transfrontaliera del fiore edule (n. 1139), grant number: CUP C12F17000080003; “ANTES” - Fiori eduli e piante aromatiche: attività capitalizzazione dei progetti ANTEA ed ESSICA (n. 8336), grant number: CUP C23B22000050006; and by the Ministry of Agriculture, Food Sovereignty and Forestry (MASAF) within the RGV-FAO Program (grant D.M._n._50045/2023).


