All published articles of this journal are available on ScienceDirect.
Metabolic and Reproductive Parameters in Young Female Rats Subjected to Feed Restriction and/or Ginger Oil Administration
Abstract
Background
It is well known that the medicinal plant ginger (Zingiber officinale) has anti-oxidative properties that shield tissues and organs against oxidative damage.
Aims
Forty-eight, 9-week-old Sprague Dawley female rats (159±13 g) were used to evaluate the effects of feed restriction and/or ginger oil supplementation on growth, reproductive parameters, and blood metabolites.
Methods
Animals were randomly divided into 4 treatment groups (12 rats/treatment) in 2 × 2 factorial arrangements: ad libitum feed (CON), 25% feed restriction (G2), ad libitum feeding with 0.2 mL/rat ginger oil (G3), and 25% feed restriction plus 0.2 mL/rat ginger oil (G4). Animals in groups G2 and G4 received 75% of their pre-treatment feed intake (25% restriction). Six rats from each group were sacrificed at the end of treatments for blood and tissue collection, while the remaining rats were mated with mature males.
Results
No ginger-by-feed restriction interactions were detected in any of the tested parameters. Body size and BW increased as the experiment advanced (P < 0.01). However, G2 had the lowest BW towards the end of the treatment. Glucose was greater (P < 0.05) in feed-restricted animals, while urea nitrogen was greater in animals on ad libitum feeding. Combined ovarian weights and reproductive tract weights were greater in ginger-supplemented than non-supplemented animals. Similarly, rats on ad libitum feeding had greater ovarian weights than those on restricted feeding.
Conclusion
Ginger oil supplementation appears to have favorable effects on reproductive tract development and ovarian weight regardless of feed restriction.
1. INTRODUCTION
The metabolic status of animals affects reproductive performance. Numerous studies demonstrated the effects of nutrition on many aspects of reproduction in several species [1, 2]. Undernutrition reduces body weight and body condition scores [3]. Such effects of undernutrition are manifested in reduced gonadotropin secretion, delayed puberty, reduced estrus expression, impaired follicular development, and delayed postpartum return to estrus. These effects of nutrition are to ensure that reproduction is supported by a plentiful food supply that will meet the high nutritional demands of gestation and lactation [4].
More research addresses the nutrition-reproduction interaction in females than in males. This is primarily because the consequences of undernutrition not only affect the female herself but also her offspring’s growth and development. Thus, the female’s “nutritional investment in reproduction” is much greater than that of the male [4]. This can be seen when maternal malnutrition during gestation influences the prenatal development of her offspring, resulting in lower fetal weights and delayed ossification [5]. In addition to the effects above, undernutrition can affect the level of tissue oxidation. Feed supplements (or additives) can be used to counteract the oxidative effects. A study by Guo et al. [6] reported that feed restriction during the luteal phase of the estrous cycle altered energy metabolism and increased oxidative stress of ovarian tissues in sheep. In the same study, arginine supplementation was effective in reducing the negative effects of feed restriction through altering gene expression, increasing resistance to oxidative stress, and reducing apoptosis.
Ginger (Zingiber officinale Roscoe), a medicinal plant anciently used in Asia, Europe, and the Middle East, is known to have anti-oxidative effects [7]. Ginger has been shown to protect tissues and organs against oxidative stress. It was able to reduce the induced oxidative effects of several stressors on various tissues [8-10]. Besides acting as an antioxidant, ginger supplementation appears to improve male reproductive parameters; rats [11] and mice [12] supplemented with ginger experienced increased sperm concentration, viability, motility, and serum total testosterone. Herbal medicines with antioxidant properties, such as ginger, can neutralize intermediate free radicals, halt oxidation chain reactions, and ultimately enhance specific sperm fertility indicators, thereby improving the fertility potential of sperm in roosters [13, 14]. Male fertility can be improved by using medicinal herbs with antioxidant properties. These herbs help reduce oxidative stress, enhance gonadal hormone levels, boost spermatogenesis, and ultimately increase male reproductive efficiency [14]. In terms of direct effects on reproduction, we could not find any literature on the effects of ginger on pubertal female rats. For this reason, this study was conducted to evaluate whether ginger oil supplementation can reduce the negative effects of feed restriction on metabolic and reproductive parameters in young female rats.
2. MATERIALS AND METHODS
2.1. Animal Care
This experiment was conducted at the Animal House, Faculty of Veterinary Medicine, Jordan University of Science and Technology (JUST). All experimental procedures were approved by the JUST Animal Care and Use Committee. Forty-eight, 9-week-old, albino Sprague Dawley female rats obtained from the Animal House, Faculty of Veterinary Medicine (159±13 g body weight) were used in this study [15]. Rats were caged individually and allowed 10 days of adaptation to the experimental conditions, during which the amounts of feed offered and refused were recorded for each animal to evaluate voluntary feed intake. Cages were located in rooms with constant temperature (22 ºC) and photoperiod (12 h light/12 h dark).
2.2. Experimental Treatments
Following adaptation and intake assessment, rats were randomly assigned to one of four treatment groups (n=12) in 2 × 2 factorial arrangements: ad libitum feeding with no ginger oil supplementation (CON, G1), 25% feed restriction (based on the assessed intake; G2), ad libitum feeding with 0.2 mL/animal/d ginger oil (Hemani®, Pakistan; G3), and 25% feed restriction plus 0.2 mL/animal/d ginger oil (G4). The offered feed was a commercially pelleted mixture containing 16% crude protein. The treatments above lasted for 30 days, during which body weight and size (nose to tail) were recorded weekly for each rat. Rats in G3 and G4 received ginger oil daily (gavage), while G1 and G2 were gavaged with water. Drinking water was offered as a free choice to all animals.
At the end of the experimental period, 6 rats from each group were randomly selected to be bred with sexually mature males. Females were marked for identification and placed in pairs with one male for 10 days in a breeding cage. Litter data (birth date, size, and weight) were recorded. The remaining females were used for blood and tissue collection. Rats were anesthetized using intra- peritoneal injection of Ketamine - Xylazine combination (using 1 ml syringe, 75‐90 mg/kg ketamine, and 5‐10 mg/kg xylazine in the same syringe). Blood was collected via cardiac puncture. Animals were later killed using an anesthetic overdose, after which tissues were harvested and weighed. All rats were dissected, and their internal organs (heart, liver, spleen, pituitary gland, kidneys, and whole reproductive tract with ovaries) were removed and weighed. Blood was collected into tubes, allowed to clot at room temperature, and then centrifuged (1500 x g) for 15 minutes. Serum was harvested and stored in vials at -20 °C until assayed for cortisol or metabolites.
2.3. Blood Analysis
Serum cortisol concentration was quantified by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's specifications (Monobind, Inc., Lake Forest, CA, USA). All samples were run in duplicates in a single assay (sensitivity = 0.25 µg/dL; CV = 3.85%). Triglycerides (BioSystems S.A., Barcelona, Spain), glucose, cholesterols, and urea (IVD, Arcomex, Amman, Jordan) were each run in a single assay in duplicates according to the manufacturers’ procedures. All of the previous assays were based on measuring the absorbance of samples against that of a blank (provided).
2.4. Statistical Analysis
Data were analyzed by analysis of variance for a completely randomized design having a 2 × 2 factorial arrangement of treatments. The effects of feed restriction and ginger oil on body weight, cortisol concentration, blood metabolites, tissue weights, and birth data were tested. The interactions between feed restriction and ginger oil on the various parameters were evaluated and found to be non-significant. Body weight was analyzed using variance analysis for repeated measures. A P-value < 0.05 was considered significant for all variables. Unless otherwise stated, data are presented as means±SE. All analyses were conducted using SigmaPlot 11.0.
3. RESULTS
3.1. Body Weight and Size
Fig. (1A) shows body weight change in all groups. There was a significant sampling week effect (P < 0.05) whereby rats gained weight as the experiment advanced. However, rats in the feed-restricted group showed a modest increase in body weight during the first two weeks before reaching a plateau; they gained less weight (P < 0.05) than those in the remaining groups. There was a treatment x sampling day interaction (P < 0.05) with respect to body size. Feed-restricted rats (G2) started out numerically longer but had reduced growth in size (Fig. 1B) as the experiment advanced, accompanying the decline in body weight.
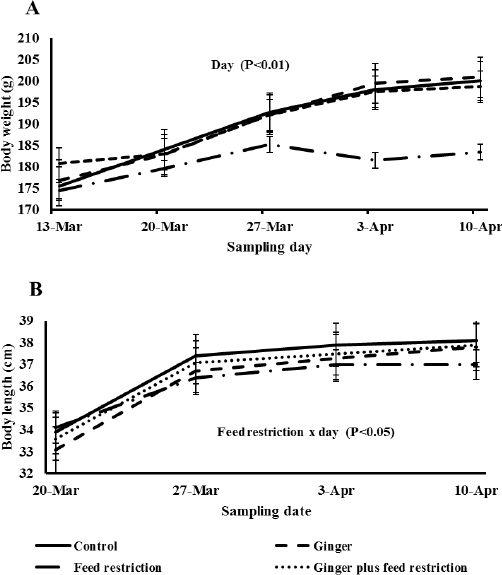
Body weight (A) and length (B) in young female rats subjected to 25% feed restriction and/or ginger oil supplementation for 30 days.
3.2. Cortisol and Blood Metabolites
Cortisol concentration was not affected by treatment (Table 1). Similarly, serum cholesterol and triglycerides did not differ among groups (Table 1). Serum glucose (Fig. 2A) and urea nitrogen (Fig. 2B) were significantly influenced (P < 0.05) by feed restriction but not by ginger supplementation. Feed restriction resulted in increased serum glucose and reduced urea nitrogen in rats.
| Blood Parameter | Treatment | |||
|---|---|---|---|---|
|
Control (n=6) |
Feed Restriction (n=6) |
Ginger (n=6) |
Ginger Plus feed Restriction (n=6) |
|
| Cholesterol (mg/dL) | 55.3±5.2 | 56.9±4.8 | 57.5±4.8 | 61.5±4.8 |
| Triglycerides (mg/dL) | 117.4±18.6 | 97.0±16.6 | 98.7±16.6 | 101.6±16.6 |
| Cortisol (µg/dL) | 4.25±0.87 | 5.06±0.87 | 4.37±0.87 | 4.92±0.87 |

Blood glucose and urea nitrogen in young female rats subjected to 25% feed restriction and/or ginger oil supplementation for 30 days1.
* denotes significant difference between means (p < 0.05).
3.3. Organ Weights
Several organs were collected and weighed. The right and left ovaries and kidneys for each animal were weighed separately. There was no feed restriction by ginger supplementation interactions with respect to any of the organs measured. Kidneys, heart, liver, spleen, and pituitary gland were unaffected by treatment (Table 2). Right ovaries were affected (P < 0.05) by feed restriction and tended to be influenced (P < 0.10) by ginger supplementation but not their interaction (Fig. 3A and C). Left ovaries, however, were not significantly affected by either treatment (Fig. 3B and D), although they were numerically heavier in the ad libitum-fed rats and in rats receiving ginger oil. The right ovaries of rats in the feed-restricted groups weighed less than those of animals receiving ad libitum feeding. Similarly, ginger supplementation tended to increase right ovarian weights compared to animals not receiving ginger regardless of feed restriction (Fig. 3B). Reproductive tract weights were also influenced by ginger supplementation (P < 0.05). Rats in the ginger-supplemented groups had heavier reproductive tracts than their non-supplemented counterparts (Fig. 4).
| Organ Weights | Treatment | |||
|---|---|---|---|---|
|
Control (n=6) |
Feed Restriction (n=6) |
Ginger (n=6) |
Ginger Plus Feed Restriction (n=6) |
|
| Left kidney (g) | 0.85±0.05 | 0.83±0.05 | 0.84±0.05 | 0.90±0.05 |
| Right kidney (g) | 0.89±0.06 | 0.93±0.06 | 0.85±0.06 | 0.98±0.06 |
| Heart (g) | 0.77±0.04 | 0.78±0.04 | 0.83±0.04 | 0.74±0.04 |
| Liver (g) | 8.29±0.36 | 7.65±0.36 | 8.49±0.36 | 8.29±0.36 |
| Spleen (g) | 0.61±0.03 | 0.65±0.03 | 0.57±0.03 | 0.61±0.03 |
| Pituitary gland (mg) | 9.9±6 | 19.6±6 | 19.4±6 | 10.5±6 |

Right and left ovarian weights in young female rats subjected to 25% feed restriction and/or ginger oil supplementation for 30 days1.
* denotes significant difference (p < 0.05), while † denotes a tendency for means to differ (p < 0.10).

Reproductive tract weights in young female rats subjected to 25% feed restriction and/or ginger oil supplementation for 30 days1.
* denotes significant difference between means (p < 0.05).
| Parameter | Treatment | |||
|---|---|---|---|---|
|
Control (n=6) |
Feed Restriction (n=6) |
Ginger (n=6) |
Ginger Plus Feed Restriction (n=6) |
|
| Dam birth weight (g) | 237.3±6.6 | 234.6±6.6 | 235.4±7.4 | 224.6±6.1 |
| Rats giving birth (Ratio) | 5/6 | 5/6 | 4/6 | 6/6 |
| Litter size (No.) | 11.5±0.7 | 11.8±0.7 | 12.8±0.7 | 12.2±0.7 |
| Litter weight (g) | 61.7±9.1 | 78.5±9.1 | 75.2±9.1 | 70.5±9.1 |
| Individual pup weight (g) | 6.3±0.6 | 6.6±0.6 | 6.0±0.6 | 5.8±0.6 |
| Days from male introduction to birth (d) | 24.2±0.7 | 25.4±0.8 | 24.0±0.7 | 24.6±0.8 |
3.4. Litter Data
Dam birth weight, parturition percentage, litter size, litter weight, individual pup weight, and the time from male introduction until parturition were unaffected by treatment (Table 3).
4. DISCUSSION
The strong link between nutrition and reproduction ensures that the female is able to sustain her own body before supporting another life (or lives). A low plane of nutrition can negatively influence reproduction; however, the magnitude of this depends on severity and duration. Feed restriction can increase the level of tissue oxidation, leading to apoptosis [6]. Ginger, an antioxidant, has been shown to protect against oxidative stress induced by many factors [16, 17]. This study evaluated the effects of supplementing ginger oil to feed-restricted female rats on metabolic and reproductive parameters.
Rats in the feed restriction group (G2) experienced a reduction in growth (body weight and size). This was expected as these animals received 75% of the estimated intake for 30 days. The other feed restriction group (G4) did not experience any effects on body weight and size. Rats receiving a lower plane of nutrition experience reduced body weights. Chapin et al. [18] reported lower body weights in Sprague-Dawley male and female rats exposed to feed restriction. Ginger oil supplementation to G4 rats appears to be the main factor preventing the effects of the low plane of nutrition on growth. This may be due to the energy content in ginger oil; various varieties of ginger oil provide from 375 to 396 kcal/100g [19].
Rats receiving ginger oil appeared to be more docile and less stressed during the daily handling for treatment administration. Ginger supplementation has been reported to modulate stress response in fish [20]. The authors observed a lesser increase in cortisol concentrations following exposure to hypoxic conditions in the ginger-supplemented group compared with the controls. In this study, however, we detected similar cortisol concentrations among the four treatment groups.
In previous studies, ginger extract administration lowered blood cholesterol and triglycerides in rats [21] and humans [22]. In the present study, serum cholesterol and triglycerides were unaffected by ginger oil administration. Similar results were reported by Jeena et al. [23], whereby ginger oil administration did not induce a significant decrease in blood cholesterol and triglycerides. The discrepancy in findings may be due to the type of supplement (extract vs oil) and dose of ginger used in the studies.
Ginger supplementation had no effect on serum urea nitrogen and glucose. Serum urea nitrogen was lower in the feed-restricted groups, while serum glucose was greater in the feed-restricted groups compared to those receiving ad-lib feeding. The lower feed intake is the primary reason for such a decrease. Reduced total feed intake is associated with lower crude protein intake, which partially accounts for the decline in urea nitrogen. Additionally, the body’s requirement for glucose may have activated gluco- neogenesis, resulting in the utilization of amino acids for the production of glucose. This can also explain the greater glucose concentration in the feed-restricted rats. One additional factor contributing to this justification is the numerical increase in cortisol concentration in the feed-restricted groups (Table 1). Cortisol is a metabolic hormone known to induce glucose production through gluco- neogenesis. Despite its effects on body weight, feed restriction did not affect organ weights, with the exception of the right ovaries, which weighed less in the feed-restricted rats than in those receiving ad libitum feeding. The lower plane of nutrition is probably the cause of reduced ovarian weights in feed-restricted rats, which also relates to the retarded growth observed in these animals. The current study might not have shown that a reduction in body weight has been severe enough to yield a measurable reduction in organ weight. Chapin et al. [18] reported lower liver, kidneys, and right ovarian weights in Sprague-Dawley female rats subjected to feed restriction, especially when those animals reached 70% of the control rats’ body weights. The severity and duration of feed restriction in our project did not match that of the aforementioned study. Similar to feed restriction, ginger supplementation did not affect the non-reproductive organ weights. Similar findings were reported when ginger powder [22] or oil [23] was supplemented in rats. In either of the previous studies, organ weights were comparable among control and ginger-treated rats regardless of form or the administered dose.
The current study found positive effects of ginger oil supplementation on reproductive development without affecting litter data (litter size, pup weight, etc.). Ginger oil-supplemented rats had heavier reproductive tracts and tended to have heavier right ovaries than their non-supplemented counterparts did. Left ovaries, although numerically heavier, were unaffected by treatment. To our knowledge, no previous studies investigated the effects of ginger oil supplementation on reproductive development in feed-restricted female rats. Our results are similar to previous studies that found that ginger supplementation increased ovarian weight in rabbits [24]. The notable increase in ovarian weight observed in the experimental group compared to the control group suggests that the dose might be an optimal level for enhancing ovarian function [24]. Feed restriction has recently been reported to alter energy metabolism and increase oxidative stress of ovarian tissue in sheep [6]. The positive effects of ginger oil reported in the current study may be due to its anti-oxidative properties. In previous studies, rats exposed to oxidative stress (treated with isoproterenol) expressed reduced tissue antioxidant levels [namely catalase, superoxide dismutase (SOD) and glutathione (GSH) [10]. Treating these rats with ginger extract reversed the oxidative effects and returned the aforementioned antioxidants to their normal (catalase and SOD) or above-normal (GSH) levels. Devine et al. [25] reviewed the effects of oxidative stress on ovarian function. They concluded that depletion of GSH stimulates apoptosis in antral follicles and granulosa cells, while antioxidant supplementation inhibits such effects.
CONCLUSION
In summary, the current study evaluated the effects of feed restriction and ginger oil supplementation on growth, blood metabolites, and reproductive development in young female rats. There was no interaction between the two treatments with respect to any of the measured variables. Feed restriction resulted in a significant reduction in body weight accompanied by a reduction in the weight of the right ovary. On the other hand, ginger oil supplementation resulted in increased reproductive tract and right ovarian weights. Such effects may have occurred due to the anti-oxidative properties of ginger oil. Future research should address longer-term, more severe feed restrictions with ginger oil supplementation. Additionally, histological assessment of ovaries should shed some light on the impact of supplementation on follicular development.
AUTHORS’ CONTRIBUTIONS
HSS, BSO, RTK, SMJ,and MDO contributed to the study’s concept and design, HSS, RIQ, LBO, BSH, AI, WAL, and MM collected the data, HSS, BSO, and RTK performed the analysis and interpretation of the results, and HSS and RTK drafted the manuscript. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| SOD | = Superoxide dismutase |
| GSH | = Glutathione |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All experimental procedures were approved by the JUST Animal Care and Use Committee (Approval No.225/03/04/16B).
HUMAN AND ANIMAL RIGHTS
This study adheres to internationally accepted standards for animal research, following the 3Rs principle. The ARRIVE guidelines were employed to report experiments involving live animals and promote ethical research practices.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available upon reasonable request from the corresponding author [H.S.S].


