All published articles of this journal are available on ScienceDirect.
Fish Hydrolysates as Potential Biostimulants for Growing Legumes and Cereals to Reduce Temperature Stress
Abstract
Introduction
This study aimed to develop an optimal method for the production of fish hydrolysates and to test their effectiveness as plant biostimulants under low-temperature growing conditions.
Methods
To obtain fish hydrolysates, the waste of a rainbow trout was used. Hydrolysates were prepared by enzymatic hydrolysis using fish entrails as a source of enzymes. Differences in the methods of obtaining fish hydrolysates: samples І, ІІ, ІІІ, and IV were placed into a water bath at +37°C, and sample V was left at room temperature. Further nitric, phosphoric, and acetic acids were added to samples I, II, and III, respectively, while distilled water was added to samples IV and V. The effectiveness of hydrolysates on the germination and growth of corn and beans under low-temperature growing conditions (+15/8°C) was determined.
Results
Hydrolysate I showed a positive effect on the germination and growth of beans and corn. Hydrolysate V stimulated germination and plant growth; however, it caused the abnormal development of some seedlings. The same deformations were observed with hydrolysate II. Hydrolysate III inhibited mold but also germination and the growth of crops. The greatest effect for both crops was found with the use of hydrolysate IV. The optimal concentration for beans was 1% and for corn - 10%.
Conclusion
A positive result was obtained from the use of fish hydrolysate to stimulate the germination and growth of beans and corn at low growing temperatures. Therefore, the use of hydrolysates will allow for less yield loss in case of unforeseeable temperature drops after seed sowing.
1. INTRODUCTION
Agricultural products are important for the survival and well-being of mankind. They are the main source of food for humans and domestic animals, and the industry is a major contributor to the world economy. Along with this, weather conditions and climate in general are some of the decisive factors in the cultivation of plants. According to the Intergovernmental Panel on Climate Change (IPCC), global climate change is expected to have a significant impact on agriculture, including yield changes in many regions of the world [1]. One of the main problems associated with potential climate change is the increase in extreme factors, such as sharp changes between high and low temperatures, intense precipitation, or prolonged [2, 3]. Also, global climate change is accompanied by sharp temperature fluctuations, both sudden increases in temperature and spontaneous decreases in temperature [4, 5], which will make the climate less predictable and crop production less successful. A sharp, unpredicted drop in temperature after sowing agricultural land can lead to a decrease in germination rates, suppression of growth processes, and even crop losses. In addition, changes in temperature and precipitation patterns can lead to the spread of pests and diseases, which will reduce agricultural productivity. Sharp temperature fluctuations have a negative effect on both the host plant and the pathogens of these plants, with parasites more likely to gain an advantage over the host during sharp temperature fluctuations [6]]. In addition to plants, animals are also significantly affected by climate change. Many species face habitat loss, altered food availability, and increased vulnerability to diseases as temperatures rise. Understanding the interconnectedness of these effects is crucial for developing comprehensive strategies to mitigate climate change impacts [7, 8]. Since global climate models predict an increase in the number of climatic extremes of varying intensity and duration [4], obtaining tools that would allow plants to better adapt to unfavorable temperature conditions would allow us to be less dependent on spontaneous temperature fluctuations and have fewer losses in growing crops. One of the effective solutions to this problem is to use biostimulants. These products contain bioactive molecules, improve the ability of plants to withstand adverse environmental conditions by affecting primary or secondary metabolism, and have a stimulating effect on the growth and productivity of plants [9-11]. One type of raw material for plant biostimulants is fishery waste, which accounts for up to 50-80% of all fish products and poses an environmental threat if they are not properly disposed of [12-14]. At the same time, the production of plant biofertilizers from fish waste in world practice is obtained mainly by composting [15-18]. In this way, fish residues are decomposed only into high molecular weight compounds, and this process is quite long and takes about four months on average. In recent years, scientists from different countries have shown increasing interest in fish protein hydrolysates [19-21]. Fish hydrolysates dominated by low molecular weight protein fragments are likely to have a more effective effect on plant growth and development, and their production is much faster and takes only a few days [22-24]. Fish hydrolysates can improve plant nutrient utilization, enhance drought tolerance, stimulate bene- ficial microbial activity, and improve plant antioxidant activity [23, 24]. In some studies, better growth of vegetative mass, acceleration of flowering, and regulation of fruit ripening were observed [25]. Therefore, it is important to investigate whether fish hydrolyzates stimulate the resistance of plants to low temperatures as well. The development of high-quality plant biostimulants will increase the yield of agricultural plant products, and the rejection of inorganic fertilizers and the targeted use of fish industry waste will significantly reduce environ- mental pollution.
There are several approaches to obtaining protein hydrolysates, in particular chemical (acid or alkaline treatment) or enzymatic hydrolysis and fermentation. Enzymatic hydrolysis has a number of advantages over other methods, including high efficiency, environmental safety, and a higher content of biologically active molecules. In the literature, many pre-treatment methods are applied to fish protein substrates: high-pressure processing, ultrasound, microwave, proteolytic digestion, and thermal treatments to achieve better bioactivity and increase the number of smaller bioactive peptides [26, 27]. However, most of these methods are complex, have low raw material processing volumes, and require expensive equipment. The use of enzymatic hydrolysis with the addition of exogenous enzymes to the substrate was excellent in terms of waste digestion and high degree of hydrolysis [28-30]. However, this method is quite expensive. Borges et al. showed that both conditions for enzymatic hydrolysis are highly effective: using viscera enzymatic extract and adding alcalase [31]. Fish waste itself contains highly active enzymes such as proteases, chitinase, lipases, alkaline phosphatase, hyaluronidase, transglutaminase, acetylglycosaminidase, etc., and can be used as sources of enzymes [32]. The production of fish hydrolysates could be a good way to convert fish waste, which is underutilized and a low-value raw material, into value-added products. This approach could have economic benefits by increasing the efficiency of fish processing by redirecting by-products for further use. In addition, this will partially solve the problem of accumulation of fish processing waste.
Thus, this study aimed to develop an optimal method for the production of fish hydrolysates and test their effectiveness as plant biostimulants under low-temperature growing conditions.
2. MATERIALS AND METHODS
To obtain fish hydrolysates, waste (bones, heads, and entrails) from rainbow trout (Oncorhynchus mykiss) was used, which was provided by “Research and Production Center “FOREL.” Fish hydrolysates were prepared by enzymatic hydrolysis using fish entrails as a source of proteolytic enzymes. Five modifications to the preparation of fish hydrolysates were tested in order to select the most effective one. In the first stage, bones and heads were ground to homogeneity in a blender and then mixed with homogenized fish entrails at a ratio of 1:1 (w: w). Then, the sample No. I, II, III, and IV were placed into a water bath at +37°C, while sample No. V was left at room temperature. After 12 hours of incubation, the sample No. I, II, and III were mixed with an equal amount of 2 M acid. Nitric acid was added to sample No. I, phosphoric acid was added to the sample No. II, and acetic acid was added to the sample No. III. The samples IV and V were mixed with an equal amount of distilled water. Further, the samples No. I, II, III, and IV were placed in a water bath for 12 hours at a temperature of +37°C, while sample No. V was kept at room temperature for the same time. Afterward, all samples were boiled in the water bath for 1 hour. Then, the samples were cooled to room temperature and filtered with Whatman qualitative filter paper, Grade 1. The hydrolysates prepared using acids (No. I, II, and III) were neutralized to pH 7.0-7.5 with 1 M NaOH. In the final step, all hydrolysates were sterilized by the Tyndallization method.
Seeds of medium-ripening varieties of common bean (Phaseolus vulgaris L.), variety Igolomska, common corn (Zea mays L.), and variety Golden cob of Ukrainian production were used for the study. Seeds of the same size, color, and shape with intact seed coats were manually selected.
The effectiveness of hydrolysates I-V on the germination and growth of corn and beans was determined. Previously, in order to accelerate germination, the seeds were soaked in water for 5 hours. In each experimental group (for example, 1% hydrolysate I), 10 wells were planted with 2 bean or corn seeds in each (20 planted seeds were taken as 100% germination). The experiment was conducted in three replications (N=60, n=3). The seeds were planted in Peatfield universal soil pH 5.5-6.5 without any bio-additives. The soil was not additionally sterilized. Seeds were not treated with fungicides to avoid the possible interaction of different active ingredients. After planting, the seeds were watered once 20 ml per well with 1% or 10% solutions of hydrolysates I-V. The control group was watered with water without hydrolysates. The plants were grown in greenhouse conditions with a daytime temperature of +15.3 ± 2.4°С and a nighttime temperature of + 8.2 ± 1.8°С, which is close to the minimum conditions for planting in the ground in spring.
Additional control was also made with a daytime temperature of +25°С and a nighttime temperature of +20°С, which is close to the optimal conditions for planting seeds in the ground.
Seed germination was recorded daily.
The percentage of seed germination (Eq. 1) was determined on the 21st day of germination, when the last individual seeds germinated, later, the seeds did not germinate.
 |
The length of the seedlings was measured every 7 days from the moment of emergence of the first seedlings for 35 days after sowing the seeds.
The seedling vigor index was calculated following Srivastava (Eq. 2) [33].
 |
To determine the seedling’s energy index, the average value of the seedling length and the percentage of germination at the 21st day of germination were taken.
Statistical analysis was performed using the GraphPad Prism 8 program by multivariate ANOVA with Tukey's correction.
3. RESULTS
3.1. Effect of Fish Hydrolysates on Germination of Bean and Corn Seeds Planted in Soil under Low-Temperature Conditions
The percentage of germination of corn when sown in the soil under normal temperature conditions without treatment with hydrolysates is 76.67% and 84.21% for beans, which is almost four times higher than at low temperatures for these crops (Figs. 1, 2).
The germination of beans in most experimental groups under 15/8°С did not differ from the control; only with the addition of 1% hydrolysate IV on the 9th day, the germination was twice as high as in control, and 1% hydrolysate V on the 14th day twice stimulated seed germination compared to the control (Fig. 1). It can be seen that no seeds emerged at all under treatment with 10% hydrolysates I and IV, which can be explained, in addition to the inhibitory effect of a high concentration of these hydrolysates, by the possible negative effect on seed germination of stimulation of fungal infection, which was most observed in these groups, especially in 10% IV. The development of mold fungi appeared as a white coating on the soil surface already on the 4th-5th day after the treatment of planted seeds with hydrolysates. Over time, as the hydrolyzate was used as a substrate, the development of fungi slowed down. Hydrolysate III had a negative effect on seed germination in both concentrations, and hydrolysate V had a sharp inhibitory effect on the development of fungi, as in the case of cultivations on filter paper. Seed germination after treatment with hydrolysate II in both concentrations did not differ from that in the control (Fig. 1).
For maize, the germination results after seed treatment with hydrolysates differed from those of beans. In particular, unlike beans, the optimal concentration of hydrolysates for maize was 10% rather than 1% (Fig. 2). Fig. (2) shows that the best effect on the germination of corn seeds was the treatment with hydrolysates I 10%, II 10%, IV 10% and V 1%.
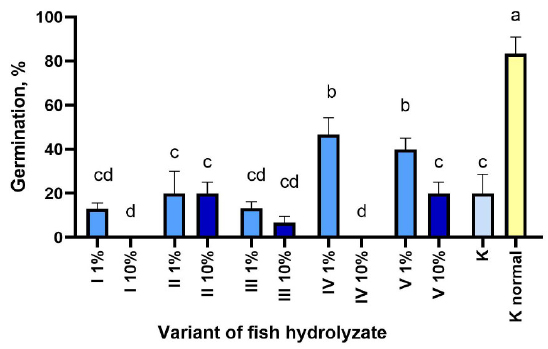
Germination of bean seeds under cold conditions after treatment with hydrolysates I-V on the 21st day of germination. K - control at 15/8°C, K normal - control at 25/20°C. (M±m). Different letters indicate significant differences at P < 0.05.
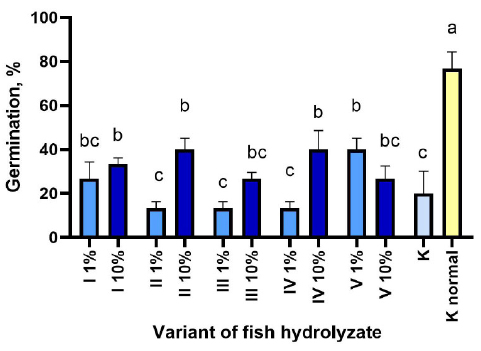
Germination of maize seeds under cold conditions after treatment with hydrolysates I-V on the 21st day of germination. K - control at 15/8°C, K normal - control at 25/20°C. (M±m). Different letters indicate significant differences at P < 0.05.
3.2. Effect of Fish Hydrolysates on the Growth of Bean and Corn Seedlings under Low-Temperature Conditions
It should be noted that under optimal growing temperature conditions (25°С), beans and corn began to germinate above the soil surface on the 4-5th day, while at a lower temperature, the first seedlings appeared only on the 9th day. Faster germination, as well as more favorable conditions, caused the height of bean seedlings on the 14th and 21st days of cultivation under warmer conditions to be almost four and five times higher, respectively, than under colder growing conditions, and corn seedlings on the 14th and 21st days under warmer conditions were almost ten times larger (Table 1).
The growth of bean seedlings at 15/8°C after watering with 1% hydrolysates for up to 21 days did not differ significantly from the control group. The treatment with hydrolysate IV gave the most effective results. At 28-35 days, all plants had the most developed leaves and the largest growth (Fig. 3). After 21 days, hydrolysate I tended to stimulate growth (Fig. 3). Whereas, treatment with hydrolysate V caused abnormal changes in bean shoots: dwarfism of shoots with a thickened stem and underdeveloped leaves was observed, or three first leaves were formed instead of two leaves, which are typical for the control (Fig. 4a).
Treatment of beans with 10% solutions of hydrolysates resulted in a strong suppression of germination and growth (Fig. 5). In the groups with hydrolysates I and IV, no seeds emerged. In contrast, in the groups with hydrolysates II and III, individual seeds began to emerge only after 21 days, compared to 9 days in the control. In the group with 10% hydrolysate V, there were both relatively strong seedlings and too-weak seedlings that stopped their development. Abnormal plant development was also observed in the groups with 10% hydrolysates II and V: thickening of the hypocotyl part of the seedling led to the rise of the root part to the soil surface, late establishment of the first two leaves, and the formation of deformed leaves (Fig. 4b).
It should be noted that irrigation with 10% hydrolysate solutions intensively stimulated the development of mold fungi on the soil surface, most of all after irrigation with hydrolysate IV, many in the groups treated with hydrolysates I and II, little in the group treated with hydrolysate III, and no fungal contamination in the group treated with hydrolysate V. Moreover, this ratio was also observed for the groups with corn. Thus, it can be said that watering with 10% hydrolysates after planting had a depressing effect on the germination and growth of beans in all groups, and in groups I, II, III, and IV, fungal infection had an additional negative effect.
| Plant | Conditions | 6 Days | 9 Days | 14 Days | 21 Days |
|---|---|---|---|---|---|
| Beans | 15°С | - | 1.5 ± 0.2 | 3.3 ± 0.5 | 4.5 ± 0.5 |
| 25°С | 3.5 ± 0.4 | 12.8 ± 0.9* | 19.3 ± 1.6* | 29.6 ± 0.6* | |
| Corn | 15°С | - | 0.5 ± 0.0 | 1.5 ± 0.2 | 3.5 ± 0.4 |
| 25°С | 2.1± 0.2 | 6.2 ± 1.1* | 19.4 ± 1.4* | 31.6 ± 1.8* |

Length of bean seedlings after irrigation with 1% hydrolysates. I-V (n=20). ~- P<0.05 - IV relative to the control group. (K). No significant differences were found between the other groups.

Bean seedlings after irrigation: a) 1% V; b) 10% II.

The length of bean seedlings after irrigation with 10% hydrolysates I-V (n=20). (K) - the control group. No significant differences were found (P<0.05).
The results obtained on corn were somewhat different. After irrigation with all hydrolysates at a concentration of 1%, there was a tendency to stimulate corn growth (Fig. 6). Corn growth was best stimulated by 1% hydrolysate V after treatment with which the plant mass was the largest, the number of plants was greater, and their height was higher. However, there were no significant differences between these groups.
A single fertilization at a concentration of 10% had a positive effect on corn growth in all hydrolysates. The best performance was shown by 10% hydrolysates V, III and I (Fig. 7). However, in terms of plant weight, the best result was given by the groups with 10% hydrolysates IV and V. It should be noted that seed germination and growth of maize plants were stimulated more intensively after treatment with 10% solutions compared to 1% solutions.
The seedling vigor index is one of the most indicative parameters, as it reflects not only the number of germinated seeds but also the quality of the seedlings themselves. This parameter, like the previous data, indicates the inhibitory effect of 10% hydrolysate solutions, with the mildest effect of hydrolysate V (Fig. 8). Hydrolysate III confirmed its negative effect on the germination and growth of beans in both studied concentrations, while hydrolysates I, II, and V at a concentration of 1% had almost no effect on these parameters of beans. Only the plants treated with 1% hydrolysate IV had a seedling vigor index four times higher than the control plants.
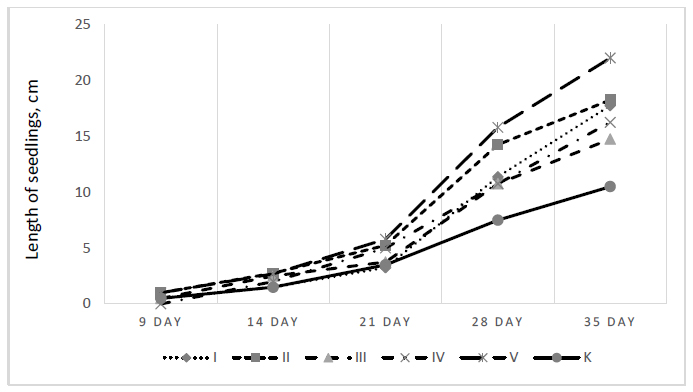
The length of corn seedlings after irrigation with 1% hydrolysates I-V (n=20). (K) - the control group. No significant differences were found (P<0.05).

The length of maize seedlings after irrigation with 10% hydrolysates I-V (n=20). * - P<0.05 - I relative to the control group; ^ - P<0.05 - III relative to the control group; » - P<0.05 - V relative to the control group (K). No significant differences were found between the other groups.

The vigor index of bean seedlings under cold conditions after treatment with hydrolysates I-V on the 21st day of germination. K - control at 15/8°С.
The vigor index of bean seedlings at 25/15°С was 2,492.6, which is more than 35 times higher than in the control under cold conditions. This confirms the inhibitory effect of cold on bean seed germination and seedling growth.
Unlike beans, treatments with 10% hydrolysate solutions proved to be more optimal for corn. Plants treated with 1% solutions almost did not differ from control plants. Only treatment with hydrolysate V 1%, as well as with all hydrolysates at a concentration of 10%, stimulated an increase in the vigor index of maize seedlings under cold conditions by approximately three times (Fig. 9).

Vigor index of maize seedlings under cold conditions after treatment with hydrolysates I-V on the 21st day of germination. K - control at 15/8°С.
The vigor index of maize seedlings at 25/15°С was 2,424.31, which is almost 50 times higher than the control at 15/8°С, indicating much more optimal growing conditions, that contribute to high germination and intensive growth.
4. DISCUSSION
Increasing the percentage of seed germination is an extremely important issue for the agricultural industry, as it allows for a higher yield per unit area and more economical use of land and planting material. One of the methods of increasing yield is to select very high-quality seeds. However, this approach is not feasible on a large industrial scale. Therefore, the use of biostimulants is reasonable for increasing viability and germination rates. One of them, in our opinion, can be stimulants based on fish hydrolysates, which can also contain bioactive peptides with different types of activity [22].
A sharp drop in temperature in spring is the most problematic when growing such heat loving, economically important agricultural crops as beans and corn in a temperate climate zone [34]. Most legumes are sensitive to cold. The optimal temperature for their cultivation is 21-27°C, and the soil temperature should be more than 16°C. If the temperature is significantly lower, the seeds can rot, which is why this crop is planted only when the soil is well warmed up. For corn, spring frosts slow down germination, can negatively affect the growth and development of the plant, cause various types of physiological damage, and significantly reduce yields. Soil fungi cause seed rot and seedling burns under such conditions [35]. Since our experiment was not conducted under sterile conditions but in greenhouse conditions, there were spores of soil fungi in the air, and in the presence of sufficient moisture and low temperatures, soil treatment with certain fish hydrolysates proved to be very favorable for the development of fungi. Hydrolysates I and IV were particularly nutritious for fungi. High doses of hydrolysates stimulated the development of the fungus more intensively, so it is likely that treatments with 10% hydrolysates I and IV had a negative effect on the germination of bean seeds with thin seed coats, while no negative effect of these hydrolysates was found on the germination and development of maize. Keeping corn plants under conditions of 10°C during the day/4°C at night for only 6 hours led to chlorosis, a decrease in photosynthesis and, accordingly, reduced biomass, increased formation of reactive oxygen species, and the destructive changes caused by them, and in some cases, plants died at low temperatures [36, 37]. Low temperatures can reduce plant growth rates, delay flowering by prolonging the vegetative phase, and reduce fruit yield [38, 39].
We obtained similar results for the negative effect of low temperatures on the germination and growth of beans and corn. In particular, the vigor index of corn and bean seedlings was, respectively, 35 and 50 times higher under temperature conditions of 25/15°С than under 15/8°С. That is, low temperatures significantly reduced seed germination and slowed the growth of seedlings. The decrease in the percentage of seed germination is due to the fact that at low temperatures, respiration processes slow down, and, accordingly, the seed is activated more slowly, as a result of which a significant number of seeds under high humidity rot before germination.
Treatment with fish hydrolysates during cultivation in cold conditions had different effects on beans and corn. For beans, in general, it is better to use lower concentrations of hydrolysates (1%), while for corn, 10% concentrations of solutions were more effective. In our opinion, this is due to the denser seed coats of corn and the longer period of root emergence. This is possibly due to the slower germination and denser seed coats of maize, which have a more gradual and gentle effect on the seed root. That is, high concentrations of hydrolysates are more likely to damage the seed and bean embryo with a thin seed coat and rapid emergence of the radicle. For beans, the best stimulants of seed germination were 1% hydrolysates IV and V, with hydrolysate V having a milder effect. Hydrolysates I and III performed the worst. The group with hydrolysate II did not differ from the control group. 1% hydrolysate V and 10% hydrolysates I, II, and IV stimulated corn germination the most. Hydrolysate III also proved to be ineffective, as well as for beans. Treatment with certain hydrolysates increased similarity in cold conditions by two times, compared to the cold control, but these indicators were half of the heat control. That is, treatment with fish hydrolysates can help to save part of the harvest in case of sharp unforeseen temperature drops, but their effect is not sufficient to use them for planned earlier sowing of seeds. In general, hydrolysates IV and V were more effective in stimulating the germination of bean and corn seeds.
Similar to the results of the effect of fish hydrolysates on seed germination, a 1% concentration was more optimal for stimulating the growth of beans, while for corn - 10% concentration. Stimulation of growth processes by hydrolysate I of beans (in low concentration) and corn (in high concentration) was observed. Hydrolysates II and V mostly did not have a significant effect on the growth of the studied plants, which is due, among other things, to the destructive effect on seedlings. Among the plants in these groups, seedlings with abnormal growth and deviations in the morphological structure of leaf blades were found. Hydrolysate III showed a certain stimulating effect only at a concentration of 10% on the growth of corn. However, this hydrolysate had almost no effect on beans. Hydrolysate IV showed a strong growth-stimulating effect at a concentration of 1% on beans and a tendency to stimulate corn at both concentrations. Thus, among the studied hydrolysates, hydrolysates I and IV are the best growth stimulants for both cultures.
If we analyze the combined data of the effect of fish hydrolysates under cold conditions on seed germination and seedling growth, which are reflected in the seedling vigor index, we can see that 1% IV hydrolysate was highly effective for beans. For corn, all hydrolysates at a concentration of 10% increased the vigor of seedlings by 3-4 times. Fish hydrolysates contain low molecular weight peptides ranging from 1000 Da to 200 Da. More than 15 amino acids have been identified, including the highest concentration of glutamic acid [26, 28, 29], which plays an important role in the metabolism of nitrogen-containing substances, possibly stimulating the development of plants, especially legumes, by activating nitrogen-fixing bacteria. These researchers also found a high antioxidant activity of hydrolysates, which may have a potential positive effect under stressful growing conditions, in particular, cold stress. Our studies have shown a positive effect of treatment with fish hydrolysates when growing under cold stress, which complements the data of other researchers on the enhancement of drought resistance in plants by hydrolysates [23, 24].
In other words, beans were more sensitive to the influence of fish hydrolysates, which, in higher concentrations, strongly inhibited plant development. Perhaps this is also related to the effect of hydrolysates on nitrogen-fixing bacteria, which are characteristic of legumes.
According to the results obtained for both studied cultures, irrigation with 10% solutions of hydrolysates stimulated the development of mold on the soil surface on the 4-5th day of germination. Most of these pathogens were found after irrigation with hydrolysate IV, the average number - in groups treated with hydrolysates I and II and almost absent fungal contamination in groups with hydrolysate III and V. Irrigation with 1% solutions stimulated the development of mold less than with 10% solutions, but the trend for hydrolysates was similar. This revealed that the fungicidal effect of hydrolysate III is probably due to the effect of acetic acid residues, which were used in the process of obtaining the hydrolysate to enhance the hydrolysis of the primary raw material. Acetic acid is known to be a very effective antifungal and antimicrobial agent and is used as a food preservative [40-42]. On the other hand, this hydrolysate also had a predominantly inhibitory effect on the germination and growth of plants, especially beans. Perhaps such an inhibitory effect is due to both the direct effect of the hydrolysate and the suppression of beneficial nitrogen-fixing nodule bacteria that enter into symbiosis with the roots of leguminous crops. The absence of stimulation of fungal contamination after treatment with hydrolysate V is possibly due to the presence of proteins of higher molecular weight in the studied sample since acids and elevated temperature were not used during its preparation to enhance hydrolysis. High molecular weight compounds cannot be used immediately by molds but require additional breakdown to lower molecular weight compounds, which takes longer [43]. Since corn has a longer germination period compared to beans, it is possible that the delayed breakdown of the protein component of the hydrolysate into low-molecular-weight compounds makes it possible for this plant to use fertilizers more efficiently, which explains the high efficiency of hydrolysate V for corn even at low concentrations. On the other hand, various biologically active substances may be present among the high molecular weight compounds of hydrolysate V, the influence of which can cause abnormal development of bean seedlings, which form earlier than those of corn. Madende & Hayes have also shown that fish hydrolysates improve plant nutrient utilization and can induce morphological changes in root architecture [25].
The conditions of obtaining hydrolysates I, II, and IV led to the formation of protein fragments of lower molecular weight than in hydrolysate V. This may explain the stronger development of molds on the soil surface after treatment with hydrolysates. They developed especially intensively after treatment with hydrolysate IV because this hydrolysate, on the one hand, contains low molecular weight compounds, and on the other hand, it does not contain acid residues that can inhibit molds. Nitric acid produced toxic nitrogen dioxide when heated, so it is possible that traces of this substance in the hydrolysate I may have somewhat inhibited the development of mold fungi after fertilization. Phosphates are more effective as stimulants of disease resistance in the plant itself; mainly, phosphites (H3PO3) are used as a fungicide, which, in addition to increasing plant resistance, also inhibit the development of certain pathogens, such as Phytophthora, Phythium and powdery mildew, in a number of crops [44]. On the other hand, nitric acid is much more active than phosphoric acid, which may have been the reason for the more intensive breakdown of the primary raw material and the resulting compounds with lower molecular weight. Therefore, hydrolysate I had a predominantly more intense positive effect on plants than hydrolysate II. Also, according to literature data, even the use of diluted HNO3 to acidify the substrate did not have a negative effect on plant growth and development [45]. Nitrogen is absorbed by the plant more than any other element, including phosphorus [46, 47], so the possible increase in nitrogen compounds in the hydrolysate due to the treatment of primary raw materials with nitric acid should not cause an overdose effect after plant treatment.
Whereas, treatment with hydrolysate II (obtained by hydrolysis with phosphoric acid) in some cases led to abnormal development of bean stems and leaves, which may have been a consequence of increased doses of phosphorus-containing compounds in hydrolysate II, compared to hydrolysates I, III, IV, and V.
Thus, hydrolysate IV 1% (as well as V 1%) showed a statistically significant increase in seed germination in beans compared to other hydrolysates and cold control. For maize, most hydrolysates stimulated seed germination. The length of bean seedlings was significantly stimulated by only 1% hydrolysate IV. For maize, hydrolysate V was generally the most effective. However, this hydrolysate caused the abnormal development of bean seedlings. Therefore, taking into account the effect on different plants, hydrolysate 4, obtained without additional hydrolysis by adding acids, proved to be more effective and versatile.
CONCLUSION
The greatest effect for both crops, corn, and beans, was found with the use of IV hydrolysate, which was obtained by enzymatic hydrolysis at 37°C and without the addition of acids and exogenous enzymes. It can be used as a pre-sowing seed treatment to stimulate germination. The application of hydrolysates to the soil during sowing differs for different crops: for beans, the optimal concentration is 1% more, while for corn, it is 10%. A positive result was obtained from the use of fish hydrolysate to stimulate germination and growth of beans and corn at low growing temperatures (frost). However, the germination rate, even after treatment with fish hydrolysates under cold conditions, is lower than that under optimal growing conditions. Therefore, the use of these hydrolysates will allow for less yield loss in case of unforeseeable sharp temperature drops after seed sowing, but not enough to recommend sowing treated seeds earlier.
AUTHORS’ CONTRIBUTION
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
ABBREVIATION
| IPCC | = Intergovernmental Panel on Climate Change |
AVAILABILITY OF DATA AND MATERIAL
The authors confirm that the data supporting the findings of this study are available within the article.
FUNDING
This study was conducted as part of the ECOTWINS (Research Capacity Building and Upskilling and Upgrading the Research Team in NUBiP (Ukraine) on Agroecological Intensification for Crop Production) project. ECOTWINS has received funding from the Horizon Europe Framework Programme (HORIZON) under grant agreement No. 101079308.


