All published articles of this journal are available on ScienceDirect.
The Effect of Boiled Oil (Olive Oil Produced using Boiled Olive Fruits) on Gut Microbiota in the Rat
Abstract
Aims
To investigate the common belief amongst locals that boiled oil (BO) is more beneficial than virgin olive oil (VOO).
Background
The effect of olive oil on gut microbiota varies according to the species of the microbe. It was reported to promote the growth of some beneficial bacteria and inhibit the growth of others, in addition to its inhibitory effect on pathogenic bacteria. In certain villages in Northern Jordan, a portion of the harvest of olive fruits is boiled before oil extraction. This product is known locally as “boiled oil,” and locals believe that it is more beneficial than VOO.
Objective
This study aims to investigate the effect of oil extracted from boiled olive fruits on gut microbiota in comparison with virgin olive oil.
Methods
Forty-seven Wistar Albino rats were divided into six groups of 7 rats per group in addition to the reference group. The rats were fed 10 ml/Kg body weight/ day of corn oil, VOO, or boiled oil for the duration of the experiment. The reference group was slaughtered before ingesting any oil. Then, after 3 and 6 weeks, a group from each treatment was slaughtered, and faeces samples were collected from the cecum and the adjacent part of the colon. The collected faeces samples were diluted for bacterial enumeration
Results
After 3 weeks, the groups that were fed boiled oil showed a significant increase in Bifidobacteria in comparison with the control and the VOO group by a mean growth of 8.54 log10 CFU/g, 7.75 log10 CFU/g, and 6.85 log10 CFU/g, respectively. Boiled oil showed a significant increase in Lactobacilli count in comparison with the control and the olive oil group with a mean growth of 9.57 log10 CFU/g, 9.21 log10 CFU/g, and 9.38 log10 CFU/g, respectively. Moreover, for the Escherichia coli count, boiled oil showed a significant increase at 5.84 log10 CFU/g in comparison with the VOO group at 5.24 log10 CFU/g, but boiled oil showed a non-significant increase in comparison with the control group. Moreover, boiled oil showed a significant decrease of total aerobic bacteria at a mean growth of 8.50 log10 CFU/g, whereas the VOO group counted 8.89 log10 CFU/g but showed a non-significant decrease with the control group. After 6 weeks, there was a non-significant increase in Bifidobacteria and lactobacilli for boiled oil in comparison with the control group. In addition, boiled oil showed a significant increase in Bifidobacteria and lactobacilli in comparison with the olive oil group at a mean growth of 6.68 log10 CFU/g and 8.79 log10 CFU/g, respectively. For Escherichia coli, it shows a significant increase at 5.97 log10 CFU/g for boiled oil in comparison with 5.36 log10 CFU/g for the control group and a significant increase compared with the VOO group with a mean growth of 4.94 log10 CFU/g. Moreover, the boiled oil group caused a significant increase in total aerobic bacteria at 8.75 log10 CFU/g, in comparison with the VOO group at 8.37 log10 CFU/g. In addition, boiled oil caused a non-significant increase in total aerobic bacteria in comparison with a control group. Boiled oil did not have a significant effect on total anaerobic bacteria.
Conclusion
Boiled oil exhibited less antimicrobial activity in comparison with virgin olive oil, probably because of the loss of total phenolic compounds.
1. INTRODUCTION
Gut microbiota is a complex bacterial system that resides in the gastrointestinal tract [1]. It plays an essential role in maintaining gastrointestinal health and in protecting against pathogenic bacteria, and any alteration in its composition can lead to numerous disorders [2-4]. Diet is a prominent factor that plays a pivotal role in the gut microbiota, and one such factor is olive oil [5]. Olive oil has a beneficial effect on gut health due to the presence of polyphenolic compounds, unsaturated fatty acids, antioxidants, anti-inflammatory and prebiotic effects [6, 7]. Polyphenolic compounds affect the gut microbiota homeostasis, including the ratio of Firmicutes/ Bacteroidetes, and stimulate Bifidobacterium growth. In some villages in Northern Jordan, farmers boil olive fruits and then sun-dry them for two weeks before pressing them using traditional millstone to obtain boiled oil [8-10]. Boiled oil is distinguished by its deep green colour and strong taste compared with the known olive oil [11]. Villagers claim that this process increases the quantity and enhances a preferred acquired flavour, and it has healing activity [11, 12]. Villagers boiled olive fruits in iron half barrels on a wood fire until fruits matured to increase the amount of oil produced during the pressing process [10, 11]. The boiled olive fruits are then sun-dried for 2 weeks to remove excess water before the extraction process [12]. The boiled olive paste is then mixed in a stone basin and spread by hand on pressing mats. Furthermore, the piston applies mechanical hydrostatic force to squeeze and separate boiled oil droplets from the olive paste [12]. The boiled oil is then collected in metallic 20 L containers [11]. The effect of olive oil produced by boiling olive fruits was not investigated earlier thus in the current study, the effect of boiled oil on the gut microbiota in the rat was assessed using Bifidobacteria, Lactobacilli, Escherichia coli, total aerobic and total anaerobic bacterial counts.
2. MATERIALS AND METHODS
2.1. Test Animals
Forty-seven male Wistar albino rats (170-240 g; about 6 weeks of age) were purchased from the Animal House at Jordan University of Science and Technology. The rats were divided randomly into seven groups in separated metallic cages in an air-conditioned room at 20+3 °C with an alternating 12h light-dark cycle. They were accli- matized for 14 days, and then the feeding began for the six groups. An additional group of 5 rats was anaesthetized with diethyl ether and slaughtered at zero time (day zero) to be considered as a reference group. The rats were fed a standard chow and tap water. The rats were fed corn oil or VOO or BO via gavage for 3 or 6 weeks. The rats were weighed two times per week, and the dose was calculated according to their body weight. All the experiments were approved by the Animal Care and Use Committee (ACUC) at Jordan University of Science and Technology.
2.2. Experimental Design
Forty-seven rats were divided randomly into seven groups, each group containing seven rats except the day zero group containing 5 rats as follows:
2.2.1. Group 1
The day zero group (Reference group) contained 5 rats that were slaughtered immediately after groups were assigned and before the acclimatization period.
2.2.3. Group 3
The Olive oil group received 10ml/kg body weight/day of olive oil via gavage for 3 weeks.
2.2.4. Group 4
The boiled oil group received 10ml/kg body weight/day of boiled oil via gavage for 3 weeks.
2.2.5. Group 5
The control group received 10ml/kg body weight/day of corn oil via gavage for 6 weeks.
2.2.6. Group 6
The Olive oil group received 10ml/kg body weight/day of olive oil via gavage for 6 weeks.
2.2.7. Group 7
The boiled oil group received 10ml/kg body weight/day of boiled oil via gavage for 6 weeks.
After 3 or 6 weeks, the rats were anaesthetized using diethyl ether. The rib cage was opened by a midline incision using a surgical scissor. The cecum and the proximal part of the colon were excised using a surgical scissor that was sterilized using 70% ethanol. Then, samples were directly transformed into labelled sterile cups and kept in an insulated icebox
2.3. Test Sample Preparation
A one-gram of faeces samples were collected using a sterilized surgical blade, a sterile spatula, and sterile forceps. The collected samples were transferred to a sterile capped tube that contained 9 ml peptone water, and the content of the capped tubes was homogenized using a vortex mixer for 30 seconds. One ml of the first diluent was transported into a second tube to achieve a 10-2 dilution. This procedure was performed repeatedly until 10-8 dilution was achieved.
2.4. Bacterial Examinations
The procedure of preparing media and dilutions was done according to the British standard methods procedure [13].
2.5. Sample Inoculation
Pour plate method: From each tube, 0.1 ml was inoculated into a Petri dish. Then Rogosa SL agar [14] and Plate count agar (PCA) [15] were poured into Petri dishes, moved clockwise and counterclockwise to mix well, and left until solidifying at room temperature.
Spread plate method: From each tube, 0.1 ml was placed into a petri dish containing anaerobic basal agar (ABA) [16], Bifidus selective medium (BSM) agar [17], and Tryptone bile x-glucuronide (TBX) medium [18], respectively. Then, the inoculate was spread on the Petri dish's surface using a sterile L-shaped rod, and then the dish was left for 5 minutes until the agar absorbed the inoculate.
2.6. Sample Incubation
The inoculated Petri dishes of BSM agar, ABA, and Rogosa SL had flipped, were placed in anaerobic jars, and anaerobic sachets were added. BSM agar was incubated for 72 hours at 37 °C, and ABA and Rogosa SL were incubated for 48 hours at 37 °C. In addition, the inoculated Petri dishes of PCA and TBX medium were flipped and incubated in aerobic conditions for 24 hours at 37 °C.
2.7. Colony Counting
After the incubation period ended, the colonies that appeared were counted depending on their respective media-expected colony colour and morphology.
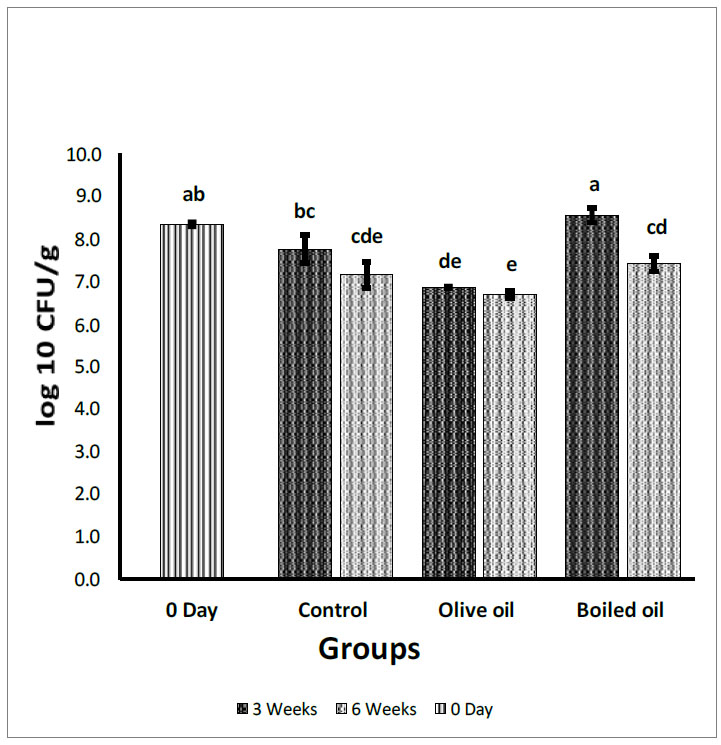
Fecal Bifidobacteria count expressed in log10 (CFU/g). Collected from the cecum and adjacent part of the colon of rats fed corn oil (control), VOO (olive oil), and boiled oil via oral gavage after 0, 3, and 6 weeks of treatment. The p-values were calculated using the Student T-test. Means with different superscripts a, b, c… differ significantly (P<0.05).
2.8. Statistical Analysis
The statistical analysis of data was performed by the statistical package for social science (SPSS, version 25.0, Chicago, 110 IL). The one-way analysis of variance (ANOVA) test was performed to test the difference between the treatments, followed by mean separation using LSD Analysis. The p-values were calculated using the student t-test. Means with different superscripts a, b, c… differ significantly at (P<0.05).
3. RESULTS
3.1. Effect on Bifidobacteria
Fig. (1) depicts the effect of boiled oil on Bifidobacteria counts in Wistar Albino rats compared with VOO and corn oil (control) groups. After 3 weeks of via gavage ingestion of the different oils, boiled oil showed a significant increase (P<0.05) in Bifidobacteria with a mean growth of 8.54 log10 CFU/g, in comparison with control (corn oil) and VOO groups with a mean growth of 7.75 log10 CFU/g, and 6.85 log10 CFU/g, respectively. After 6 weeks, there was a non-significant increase (P>0.05) in Bifidobacteria with a mean growth of 7.40 log10 CFU/g for boiled oil compared with 7.14 log10 CFU/g for the control group was found. Moreover, boiled oil showed a significant increase (P<0.05) in comparison with the VOO group, with a mean growth of 6.68 log10 CFU/g. When the means of 3- weeks groups were compared with the zero time group, boiled oil showed a non-significant increase (P>0.05) in Bifidobacteria with a mean growth of 8.54 log10 CFU/g compared with a mean growth of 8.33 log10 CFU/g at the day zero group. After 6 weeks, the boiled oil group showed a significant decrease (P<0.05) in Bifidobacteria mean growth at 7.40 log10 CFU/g compared with the day zero group and a significant decrease (P<0.05) compared with 3 weeks group at a mean growth of 8.54 log10 CFU/g.
3.2. Effect on Lactobacilli
Fig. (2) illustrates the effect of boiled oil on Lactobacilli counts in Wistar Albino rats compared with VOO and corn oil groups. After 3 weeks of treatments, boiled oil showed a significant increase (P<0.05) in Lactobacilli with a mean growth of 9.57 log10 CFU/g, compared with a mean growth of 9.21 log10 CFU/g for the control group. In contrast, boiled oil showed a significant increase (P<0.05) in Lactobacilli at 9.57 log10 CFU/g compared with the VOO group at 9.38 log10 CFU/g. After 6 weeks, there was a non-significant increase (P>0.05) in Lactobacilli with a mean growth of 9.16 log10 CFU/g for boiled oil compared with 9.08 log10 CFU/g for the control group. In addition, the boiled oil group showed a significant increase (P<0.05) compared with the VOO group, with a mean growth of 8.79 log10 CFU/g. After 3 weeks, boiled oil showed a significant increase (P<0.05) in Lactobacilli with a mean growth of 9.57 log10 CFU/g compared with a mean growth of 9.28 log10 CFU/g on day zero. On the 6th week, boiled oil showed a non-significant decrease (P>0.05) in Lactobacilli mean growth at 9.16 log10 CFU/g compared with day 0 and a significant decrease in Lactobacilli at a mean growth of 9.16 log10 CFU/g compared with 3 weeks at a mean growth of 9.57 log10 CFU/g.
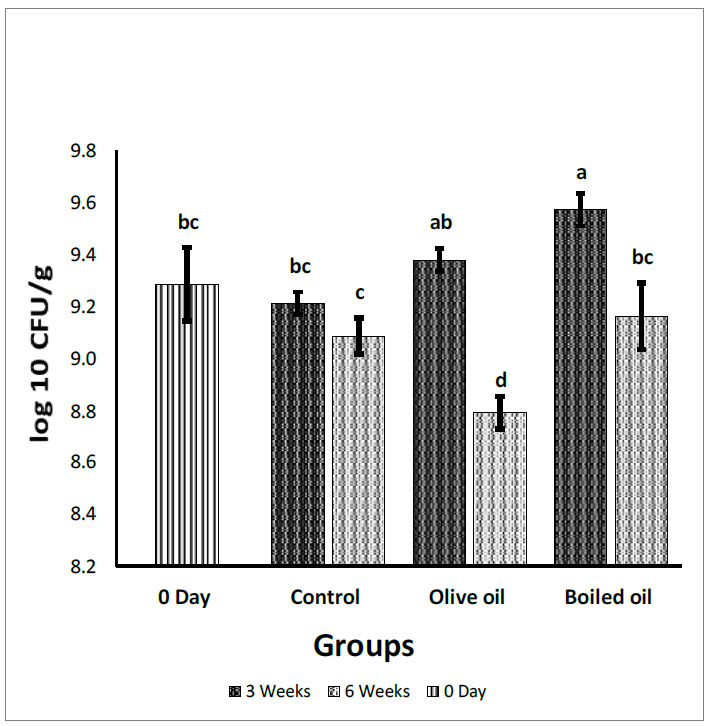
Fecal Lactobacilli count expressed in log10 (CFU/g). Collected from the cecum and adjacent part of the colon of rats fed corn oil (control), VOO (olive oil), and boiled oil via oral gavage after 0, 3, and 6 weeks of treatment. The p-values were calculated using the Student T-test. Means with different superscripts a, b, c… differ significantly (P<0.05).
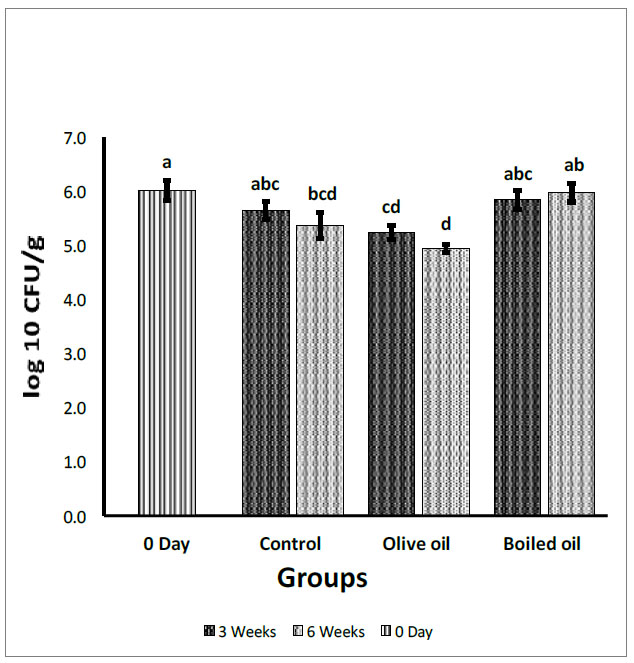
Fecal Escherichia coli count expressed in log10 (CFU/g). Collected from the cecum and adjacent part of the colon of rats fed corn oil (control), VOO (olive oil), and boiled oil via oral gavage after 0, 3, and 6 weeks of treatment. The p-values were calculated using the Student T-test. Means with different superscripts a, b, c… differ significantly (P<0.05).
3.3. Effect on Escherichia coli
Fig. (3) depicts the effect of boiled oil on Escherichia coli counts in Wistar Albino rats in comparison with olive oil and control groups. After 3 weeks of treatments, boiled oil showed a non-significant increase (P>0.05) in Escherichia coli with a mean growth of 5.84 log10 CFU/g, compared with a mean growth of 5.64 log10 CFU/g for the control group. In contrast, boiled oil showed a significant increase (P<0.05) in Escherichia coli at 5.84 log10 CFU/g compared with the VOO group at 5.24 log10 CFU/g. After 6 weeks, there was a significant increase (P<0.05) in Escherichia coli with a mean growth of 5.97 log10 CFU/g for boiled oil compared with 5.36 log10 CFU/g for the control group. In addition, boiled oil showed a significant increase (P<0.05) compared with the olive oil group with a mean growth of 4.94 log10 CFU/g. When the 3 and 6-week means were compared with day zero and with each other, boiled oil showed a non-significant decrease (P>0.05) in Escherichia coli compared with day zero. On the 6th week, boiled oil showed a non-significant increase (P>0.05) in Escherichia coli mean growth at 5.97 log10 CFU/g compared with 3 weeks at mean growth at 5.84 log10 CFU/g.
3.4. Effect on Total Aerobic Bacteria
Fig. (4) shows the effect of boiled oil on total aerobic count compared with olive oil and the control group. After 3 weeks, the total aerobic count of the boiled oil group was 8.50 log10 CFU/g, which decreased significantly (P<0.05) in the VOO group count of 8.89 log10 CFU/g, with a reduction of 0.39 log10 CFU/g for boiled oil. In contrast, the boiled oil showed a non-significant decrease (P>0.05) in total aerobic bacteria with a mean growth of 8.50 log10 CFU/g, compared with the control group at 8.76 log10 CFU/g. After 6 weeks, the results showed a significant increase (P<0.05) of boiled oil at 8.75 log10 CFU/g, compared with the VOO group (8.37 log10 CFU/g). In addition, there was a non-significant increase (P>0.05) of boiled oil at 8.75 log10 CFU/g, compared with a control group with a mean growth of 8.61 log10 CFU/g. When the 3- and 6-week means were compared with day zero and with each other, boiled oil showed a non-significant decrease (P>0.05) in total aerobic bacteria after 3 and 6 weeks compared with day zero. On the 6th week, boiled oil showed a non-significant increase (P>0.05) in total aerobic bacteria mean growth at 8.76 log10 CFU/g compared with 3 weeks at mean growth 8.50 log10 CFU/g.
3.5. Effect on Total Anaerobic Bacteria
Fig. (5) illustrates the effect of boiled oil on total anaerobic counts in Wistar Albino rats compared with VOO and control groups. The total anaerobic count showed a non-significant difference after 3 and 6 weeks of the treatment except for the boiled oil at 6 weeks, which revealed a significant increase (P<0.05) in anaerobic bacteria at a mean growth of 9.10 log10 CFU/g, compared with the control group. After 3 weeks, boiled oil showed a non-significant (P>0.05) decrease in total anaerobic bacteria mean growth at 8.86 log10 CFU/g, compared with control and olive oil groups with a mean growth of 9.03 log10 CFU/g and 8.92 log10 CFU/g, respectively. After 6 weeks, boiled oil showed a non-significant (P>0.05) increase in total anaerobic bacteria at 9.10 log10 CFU/g, compared with VOO at a mean growth of 8.94 log10 CFU/g. At 3 and 6 weeks, boiled oil and VOO showed a non-significant increase (P>0.05) in comparison with day zero. In addition, there was a non-significant increase (P>0.05) between the 3rd and 6th week.
4. DISCUSSION
The results of this study showed that VOO caused a significant decrease in Bifidobacteria, Lactobacilli, Escherichia coli, and total aerobic bacterial counts in comparison with BO. This might be explained by the presence of higher phenolic compounds in VOO than in BO, which act as bioactive compounds and antimicrobials to pathogenic bacteria and some beneficial bacteria such as Bifidobacteria [9]. Previous studies have shown that olive oil can also show a strong antibacterial effect on beneficial bacteria such as Bifidobacterium bifidum [8]. Moreover, the type and extraction method of olive oil was also reported to affect its chemical composition, causing some changes to its phenolic compounds, which have a strong antibacterial effect [8]. This effect on Bifidobacteria can be explained by the effect of heat on polyphonic compounds during boiling, which decreased by 55%, leading to a decline of the antioxidant activity in BO in comparison with VOO. This decrease in polyphonic compounds might have led to decreased antimicrobial activity [9].
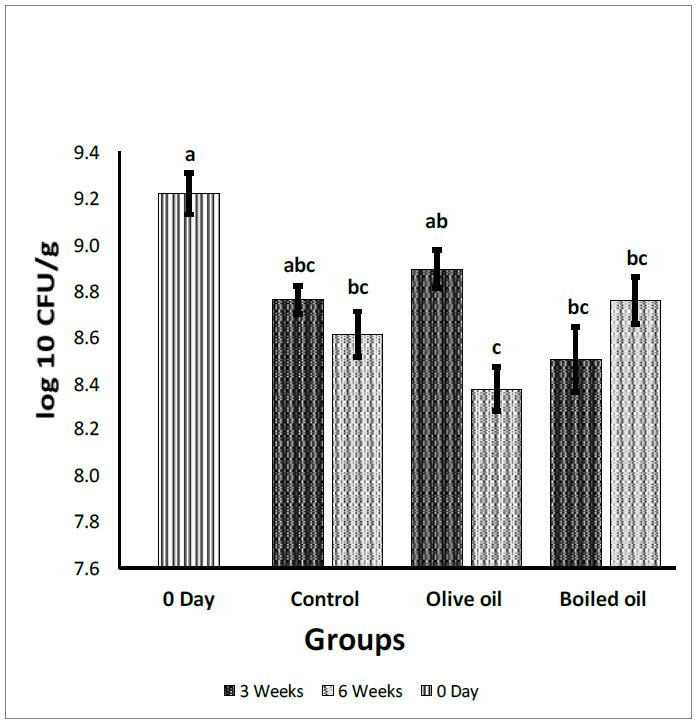
Fecal total aerobic bacteria count expressed in log10 (CFU/g). Collected from the cecum and adjacent part of the colon of rats fed corn oil (control), VOO (olive oil), and boiled oil via oral gavage after 0, 3, and 6 weeks of treatment. The p-values were calculated using the Student T-test. Means with different superscripts a, b, c… differ significantly (P<0.05).
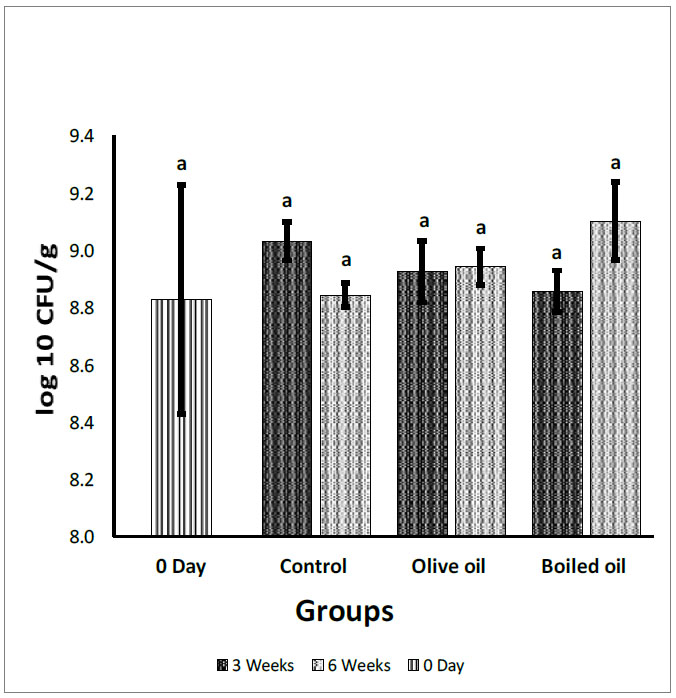
Fecal total anaerobic bacteria count expressed in log10 (CFU/g) collected from the cecum and adjacent part of the colon of rats fed corn oil (control), VOO (olive oil), and boiled oil via oral gavage after 0, 3, and 6 weeks of treatment. The p-values were calculated using the Student T-test. Means with different superscripts a, b, c… differ significantly (P<0.05).
Other constituents of olive oil, such as oleuropein, act as a prebiotic, and Lactobacilli use it as a carbon source for proliferation and convert it to hydroxytyrosol which increases the growth of Lactobacilli, especially L. johnsonii [9]. Antimicrobial activity is higher in VOO than in ordinary olive oil and pomace oil, and some antioxidants, such as ligstroside in extra virgin olive oil, have a strong antibacterial effect on Lactobacilli [19].
Extra virgin olive oil can reduce certain lactic acid bacteria species, especially Lactobacillus animalis, Lactococcus, Lactobacillus taiwanensis, and Lactobacilli acidophilus [9, 19, 20]. Therefore, the significant decrease in Lactobacilli growth in the VOO-receiving group after 6 weeks can be explained by the presence of antibacterial activity in VOO due to the presence of antioxidants and polyphenolic compounds that may reduce the Lactobacilli growth compared to the day zero group which was used as a reference. The high temperature that was used during boiling olive fruits before the pressing might have caused a decrease in polyphenolic compounds and antioxidant activity, hence the change in its antimicrobial effect. Moreover, a significant loss of polyphenolic compounds and antioxidant activity was already established using the same oil samples that were used in this study in an earlier study. Thus, this may have led to an increase in the number of Lactobacilli in boiled oil [9].
A significant decrease in Escherichia coli growth using VOO was expected. Once again, the presence of polyphenolic compounds with antimicrobial activity in the VOO inhibited the growth of Escherichia coli. The corn oil, which was used as a control, did not contain any phenolic compounds, therefore, there was no antibacterial activity [21, 22].
Factors affecting the loss of the total polyphenolic compounds include temperature, malaxation time, and contact duration of olive fruits with water [23]. Loss of polyphenolic compounds during malaxation arises due to enzymatic oxidation, causing a decrease in phenolic compound concentration in olive oil [23]. The Boiling of olive fruits before the pressing process showed a reduction in antibacterial activity efficacy towards Escherichia coli bacteria, which was caused by the reduction of the contents of the polyphenolic compound due to high temperature.
Total aerobic bacteria counts have higher quantities in the gut epithelium and mucosa in some diseases, including Crohn's and inflammatory bowel disease [24]. The increase in the number of total aerobic bacteria is associated with the incidence of inflammatory bowel disease [24]. Boiled oil increased the number of total aerobic bacteria after 6 weeks compared with VOO.
CONCLUSION
These results show the beneficial impact of VOO, which significantly reduced the number of total aerobic bacteria after 6 weeks compared to BO. The most predominant anaerobic bacteria are Bacteroides, Prevotella, Clostridium, and Peptostreptococcus species [25]. Boiled oil increased the number of total anaerobic bacteria after 6 weeks compared to corn oil, but there was no significant effect compared with VOO after 3 or 6 weeks. Anaerobic bacteria are the predominant bacteria in the colon, and an increase in the number and overgrowth of total anaerobic bacteria may cause irritable bowel syndrome [25]. The change in the colonization of anaerobic species on gut mucosa is strongly related to diseases, including inflammatory bowel disease [24, 26-28]. Overall, the effect of boiled oil is considered detrimental to gut health.
AUTHOR’S CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
ABBREVIATIONS
| BO | = Boiled oil |
| VOO | = Virgin olive oil |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All the experiments were approved by the Animal Care and Use Committee (ACUC) at Jordan University of Science and Technology.
HUMAN AND ANIMAL RIGHTS
This study adheres to internationally accepted standards for animal research, following the 3Rs principle. The ARRIVE guidelines were employed for reporting experiments involving live animals, promoting ethical research practices.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available from the corresponding author [S.J] upon reasonable request.


