All published articles of this journal are available on ScienceDirect.
Purple Nonsulfur Bacteria Rhodopseudomonas palustris Improve Soil Phosphorus Availability and Yield of Lemon Balm (Melissa officinalis L.) in Alluvial Soils via Plant and Ratoon Seasons
Abstract
Background
Poorly nutritious soils limit the growth of crops. Moreover, lemon balm (Melissa officinalis L.) has never been applied with purple nonsulfur bacteria (PNSB). Therefore, this study was performed to (i) evaluate the phosphorus (P) providing capacity of PNSB and (ii) determine the effectiveness of the PNSB in ameliorating P uptake, growth, and yield of lemon balm in alluvial soils.
Materials and methods
The experiment was designed in completely randomized blocks with nine treatments and three replications. The treatments from (1)-(4) were fertilizations of 100% P, 75% P, 50% P, and 25% according to the recommended fertilizer rate for lemon balm (RFRLB). The treatments (5-7) corresponded to the treatments (2-4), but they were combined with PNSB; the treatment (8) was applied with only PNSB, and the treatment (9) was applied without either chemical fertilization or PNSB.
Results
The results showed that P fertilization combined with PNSB increased Pavailable by 14.1-24.2% as compared with the treatments with only chemical fertilization. Supplying both 75% P and PNSB improved lemon balm growth, such as the number of leaves per plant by 8.63%, the number of secondary branches by 7.69%, and essential oil content by 43.8% in season 1, and increased P uptake by 15.0-29.6% in both seasons.
Conclusion
A reduction of 25% P combined with PNSB maintained the yield of lemon balm like the 100% P fertilization in the two consecutive seasons because the PNSB solubilized unavailable P nutrient in the soil, leading to another P source rather than the chemical fertilizer.
1. INTRODUCTION
Lemon balm (Melissa officinalis L.) is known as a medicinal plant due to its possession of beneficial components for health, such as flavonoids, terpene, and phenolics [1,2]. The plant is suitable for growing in soils with pH 5.0-7.0 [3]. However, in the South of Vietnam, the pH is fairly low, roughly 5.0 [4], which could make the yield and medicinal values of the plants poor because the pH can limit the soil nutrient availability [5]. For example, at pH 5.5, the availability of P, Mo, Ca, and Mg is poor, while that of Fe, Al, and B is greater. On the other hand, at neutral pH, the availability of N, S, and P is great [5]. In addition, using too much chemical fertilizer reduces the use efficiency and causes nutrient imbalance, subse quently leading to soil contamination, degradation, envi ronmental pollution, and disturbances in ecological biodiversity [6-9]. In soil, the total P content is roughly 100-3,000 mg kg-1, but plants can absorb only a small portion of available P from the soil solution under the forms of HPO42- and H2PO4- [10]. Soil minerals immobilize soluble P provided by fertilizer under both high and low pH conditions [11-13].
Moreover, the state of P deficiency can alter root structure, suppress photosynthesis and carbohydrate metabolism, activate or repress some enzymes, and increase organic acid production [14-16]. To make use of the immobilized P in farming soils, some biological approaches have been presented based on mechanisms that produce dissolving substances, such as organic acids [17]. Soil microbes, especially bacteria, can solubilize P in soils [18, 19]. In particular, some bacteria belonging to the Bacillus, Pseudomonas, Rhizobium, and Enterobacter genera can solubilize P the most efficiently [20]. On the other hand, to ameliorate the content of available P under poorly nutritious conditions, PNSB is a potential group of bacteria that can provide P for upland crops, such as sesame and pineapple [21-23].
Furthermore, Rhodopseudomonas palustris can produce plant growth-promoting substances, such as IAA, siderophores, ALA, and EPS, which can facilitate plant growth under conditions that lack nutrients [24, 25]. The R. palustris strains have been used in mixtures to make use of the different strengths of each strain. The TLS06 and VNW64 can strongly solubilize Al-P and Fe-P and produce siderophores, while VNS89 can strongly produce ALA, and VNW02 can remove Fe2+ toxicity [24, 26]. For instance, a mixture of R. palustris VNW02, TLS06, VNW64, and VNS89 was applied along with rice straw from mushroom farming to improve sesame yield in alluvial soil [27]. Moreover, this mixture was also used on canary melon in alluvial soil and showed positive results [28]. However, the mixture has never been used on herbs, particularly lemon balm. Therefore, this study was proposed to evaluate the efficiency of phosphorus-solubilizing PNSB in improving soil phosphorous availability and lemon balm in alluvial soils. It was hypothesized that the application of phosphorus-solubilizing PNSB into lemon balm seeds and alluvial soil should improve soil P availability and, thereby, the P uptake, growth, and yield of lemon balm. This should be one of the first studies applying PNSB strains to enhance the yield of an herb in alluvial soil.
2. MATERIALS AND METHODS
2.1. Materials
The experiment was performed through 2 continual seasons, including plant and first ratoon from February 2022 to May 2022, in the wet season for the first crop and wet season for the first ratoon crop, in the greenhouse of the Agricultural Research and Practice Camp, College of Agriculture, Can Tho University, Can Tho City, Vietnam (10.029783,105.767414).
The chemical features of alluvial soil are described in Table 1 .
|
Depth (cm) |
pHH2O | pHKCl | Ptotal (%) | Available P | Al-P (mg kg-1) | Fe-P (mg kg-1) | Ca-P (mg kg-1) |
Kexchangeable (meq 100 g-1) |
Soil texture (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg kg-1) | Clay | Silt | Sand | ||||||||
| 0-20 | 5.97 | 4.63 | 0.163 | 36.9 | 160.4 | 488.3 | 298.4 | 0.233 | 57.6 | 40.9 | 1.50 |
The fertilizers used were Phu My urea (46% N), superphosphate Long Thanh (16% P2O5 and 15% CaO), and potassium chloride (60% K2O).
2.1.1. Bacteria
The liquid biofertilizer of P-PNSB, Rhodopseudomonas palustris VNW64, VNS89, TLS06, and VNS02 used was developed by Khuong et al. [25]. The mixture was used based on its ability to produce synergic effects. The optimal condition for multiplying their density was as per the findings of Khuong et al. [24]. The biofertilizer was contained in 250.0 mL Duran bottles of 200.0 mL of liquid BIM containing 1.0 g L-1 (NH4)2SO4, 0.5 g L-1 K2HPO4, 0.2 g L-1 MgSO4, 2.0 g L-1 NaCl, 5.0 g L-1 NaHCO3, 1.5 g L-1 yeast extract, 1.5 g L-1 glycerol, and 0.03 g L-1 L-cysteine in SDW and adjusted for 1 x 108 CFU mL-1 density.
2.2. Methods
2.2.1. Experiment Design
Soils were dried, filtered from plant residues, and mixed well before use. A total of 8 kg of dry soil was put into each plastic pot, in which three seeds were sown. The experiment had 9 treatments. Therein, the first four treatments (1-4) were correspondingly 100% P, 75% P, 50% P, and 25% P, respectively, and the treatments (5-7) included the treatments 2-4 but they were added with P-PNSB, the treatment (8) was applied with only P-PNSB, and the treatment (9) was applied with neither chemical fertilizer nor P-PNSB. Each treatment was conducted in three pots corresponding to three replications. Each pot has 3 plants.
2.2.2. The Recommended Fertilizer Rate for Lemon Balm
The fertilizer formula in the current study was 300N-125P2O5-300K2O (kg ha-1) [29], which was 0.872 N-1.104 P2O5-0.667 K2O (g pot-1). The fertilization was divided into four portions. Therein, the first portion was 100% P used 1 day before plantation; the second one was 30% N, and 50% at 10 DOFs; the third one was 40% N at 40 DOFs, and the last one was 30% N and 50% K at 70 DOFs.
2.2.3. Seed Preparation
The surface of the seeds was sterilized with 70% ethanol for 1 min and sodium hypochlorite 1% for 10 min and rinsed three times by SDW for 5 min each time. Seeds were incubated on moisturized filter paper in Petri dishes until there were buds and roots. Then, seeds were divided into two equal portions in two 250.0 mL beakers, each of which contained SDW, and the other contained 100 mL of the P-PNSB mixture (Rhodopseudomonas palustris VNW64, VNS89, TLS06, and VNS02), and soaked for 1 h before plantation. Each pot was sown with 3 seeds corresponding to each designated treatment. A total of three plants were maintained until harvest. For each treatment with the P-PNSB, 5.0 mL of bacteria mixture was supplied at 30 and 60 DOFs.
2.2.4. Agronomic Traits
The different growth traits were determined according to Khalid and Cai [30] on plant height (cm), leaf length (cm), the quantity of primary branches (branches), the quantity of secondary branches (branches), and the SPAD indices. For the plant yield (or fresh biomass, g pot-1), the weight of the peak and stem cut from 7 cm above the ground was measured. For dry biomass (g pot-1), plant samples were dried at 70 °C for 72 h, and weighed. The measurement was conducted on 3 three plants of each replication per treatment at harvest.
2.2.5. Analysis of Plant and Soil
The soil analysis was conducted according to Sparks et al. [31] on pHH2O, pHKCl, EC, NTotal, NH4+, PTotal, PAvailable, Al-P, Fe-P, Ca-P, and exchangeable K+. The N, P, and K contents and uptake in stem and leaves were determined according to Houba et al. [32].
2.2.6. Essential Oil Determination
Essential oil determination followed the steam distillation method [33]. Samples were cut into small species and placed into the distillation flask containing 400.0 mL SDW, which was positioned on the electric stove to extract essential oil. After distillation, the essential oil was purified from water and determined for weight.
3. RESULTS
3.1. Effects of P-PNSB Rhodopseudomonas Palustris on Alluvial Soil for Farming Lemon Balm
The results in Fig. (1) illustrate that the treatments with both P fertilizer and P-PNSB had a greater soluble P concentration (100.5-133.7 mg kg-1) than the treatments with only P fertilizer (89.0-92.1 mg kg-1). Furthermore, when no P fertilizer was used, the treatments with P-PNSB resulted in a greater soil soluble P concentration than the treatments without bacteria, with 86.9 compared with 84.0 mg kg-1, respectively. Additionally, fertilizations at three different P fertilizer rates combined with P-PNSB improved soil pH and K+ content with 6.23-6.63 compared with 5.60-5.91 mg kg-1 and 0.198-0.235 compared with 0.174-0.188 mg kg-1. However, only at 75% P, according to the RFRLB, P-PNSB supplementation improved the soluble P content. The Al-P, Fe-P, and Ca-P contents in soil were approximately 110.3-130.1 mg kg-1, 328.9-430.8 mg kg-1, and 159.9-182,2.mg kg-1 in the treatments with P-PNSB, while when only P fertilizer was applied, the results were 123.0-152.4 mg kg-1, 403.9-433.7 mg kg-1, and 181.7-248.7 mg kg-1, respectively. However, only the Al-P and Ca-P contents were reduced in the treatments with P-PNSB in the soils that were not supplied with P fertilizer. Moreover, the P-PNSB supplementation reduced the EC. However, both P fertilizer and P-PNSB did not significantly affect the values of pHKCl, Ntotal, and Ptotal, whose means were 4.56, 0.452, and 0.100%, respectively (Table 2). The treatments with P-PNSB among different P fertilizer rates resulted in bacterial density of 6.15-6.32 g-1 DSW, which was greater than those of the treatments with only chemical fertilizer (1.87-2.01 g-1 DSW) (Fig. 2).
| Treatment | pHH2O | pHKCl | EC | Ntotal | Ptotal | NH4+ | Al-P | Fe-P | Ca-P | K+ |
|---|---|---|---|---|---|---|---|---|---|---|
| - | - | mS cm-1 | % | mg kg-1 | ||||||
| 100% P | 6.02c | 4.55 | 0.345a | 0.467 | 0.102 | 2.10b | 154.7a | 472.5a | 252.2a | 0.192bcd |
| 75% P | 5.91c | 4.50 | 0.317b | 0.369 | 0.105 | 1.82c | 152.4a | 433.7b | 248.7a | 0.188cd |
| 50% P | 5.91c | 4.47 | 0.310b | 0.481 | 0.096 | 1.77c | 138.1b | 426.9b | 201.1b | 0.182cd |
| 25% P | 5.60d | 4.54 | 0.308b | 0.457 | 0.101 | 1.75c | 123.0d | 403.9c | 181.7bc | 0.174de |
| 75% P + P-PNSB | 6.63a | 6.60 | 0.297b | 0.467 | 0.092 | 2.82a | 130.1c | 430.8b | 182.2bc | 0.235a |
| 50% P + P-PNSB | 6.26b | 4.56 | 0.265c | 0.462 | 0.098 | 1.90bc | 112.3e | 387.8d | 164.9c | 0.214ab |
| 25% P + P-PNSB | 6.23b | 4.52 | 0.255cd | 0.471 | 0.101 | 1.77c | 110.3e | 328.9d | 159.9c | 0.198bc |
| 0% P + P-PNSB | 5.37e | 4.58 | 0.254cd | 0.434 | 0.102 | 1.72c | 102.5f | 309.0f | 117.3f | 0.154e |
| 0% P | 5.28e | 4.64 | 0.235d | 0.462 | 0.104 | 1.70c | 111.0e | 322.5ef | 130.2e | 0.125f |
| Level of significance | * | ns | * | ns | ns | * | * | * | * | * |
| CV (%) | 1.71 | 1.25 | 4.57 | 21.6 | 6.32 | 7.04 | 1.69 | 2.20 | 3.20 | 6.75 |
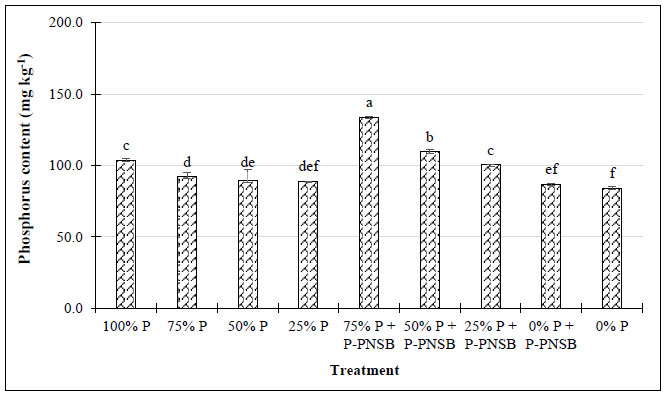
Note: Lower-case letters show significant differences between treatments at p-value 0.05; P-PNSB: the mixture of the four phosphorus-solubilizing purple nonsulfur bacteria Rhodopseudomonas palustris TLS06, VNW02, VNW64, and VNS89.
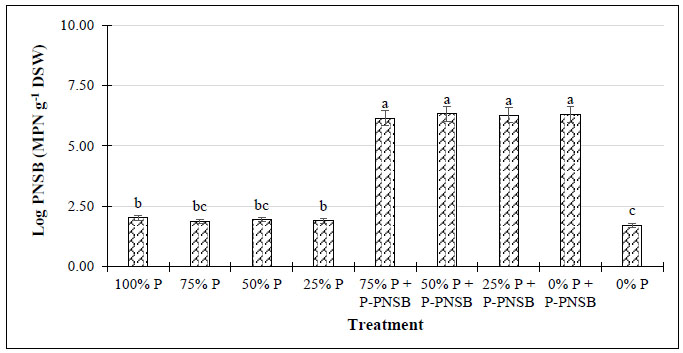
Note: Lower-case letters show significant differences between treatments at p-value 0.05; P-PNSB: the mixture of the four phosphorus-solubilizing purple nonsulfur bacteria Rhodopseudomonas palustris TLS06, VNW02, VNW64, and VNS89.
3.2. Effects of P-PNSB Rhodopseudomonas palustris on P Uptake of Lemon Balm in Alluvial Soil
Fig. (3a) indicates that the combination of both P fertilization and P-PNSB increased P content via two farming seasons. When no P fertilizer was used, the treatments with P-PNSB had greater P contents (0.046% in season 1, and 0.146% in season 2) than the treatments without bacteria (0.042 and 0.126%, respectively). Simultaneously, at three P fertilizer rates, the treatments with P-PNSB (0.252 and 0.546%) resulted in greater results than the treatments without P-PNSB (0.136 and 0.313%).
P uptake values in the treatments with P-PNSB in both seasons were 49.2-53.2, and 102.7-152.2 mg kg-1, which were greater than the treatments without P-PNSB (23.4-34.4 and 60.7-92.7 mg kg-1, respectively), when P fertilizer was used at different rates. However, P uptake in the treatments with or without P-PNSB was equivalent at 0%, according to RFRLB (Fig. 3b). Dry biomass in the treatments with both P fertilization and P-PNSB supplementation was greater than that of the treatments with only P fertilization, with 46.6-47.7% compared with 35.1-42.3% in season 1, and 53.7-40.6% compared with 35.2-49.4% in season 2, respectively. However, only at 50% P, according to RFRLB, supplying with or without P-PNSB did not significantly change P uptake in season 2 (Fig. 3c).
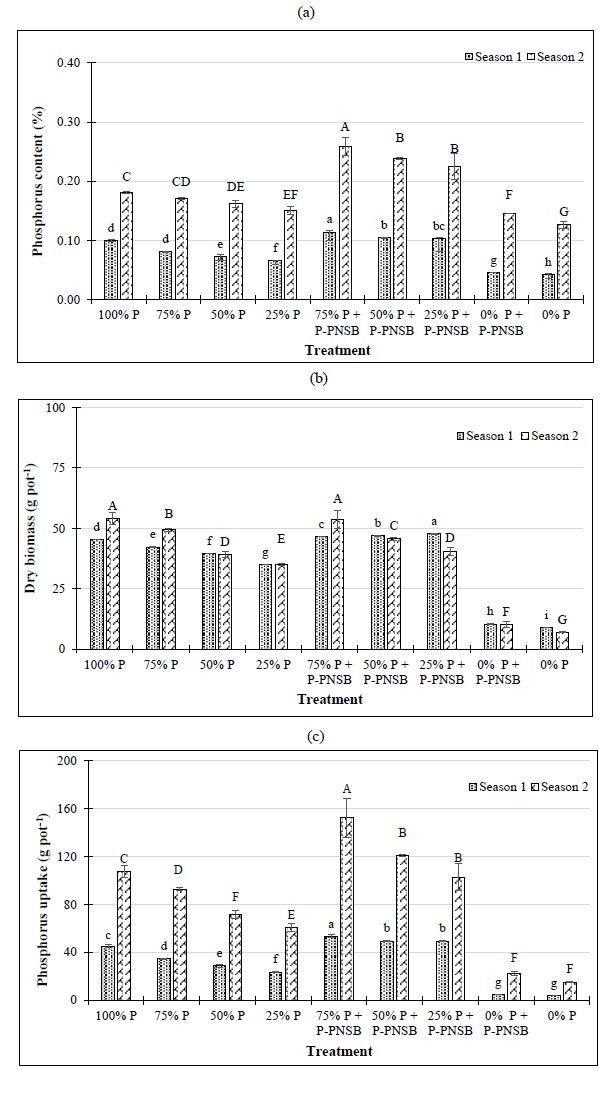
Note: Lower-case and upper-case letters show significant differences between treatments at p-value 0.05 in seasons 1 and 2, respectively; P-PNSB: the mixture of the four phosphorus-solubilizing purple nonsulfur bacteria Rhodopseudomonas palustris TLS06, VNW02, VNW64, and VNS89.
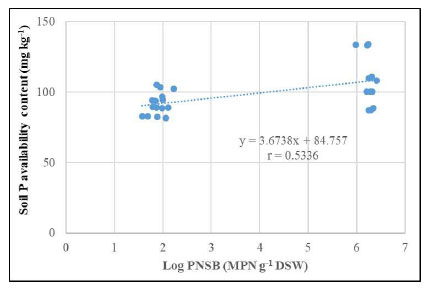
The density of PNSB found in the soil increased, leading to the greater available P content in the soil. This proportional correlation with a coefficient of 0.5335 is shown in (Fig. 4).
The N and K contents and uptakes are also recorded in Table 3. In particular, two factors significantly affected the content and uptake of N and K. However, during two consecutive seasons, only the treatment with 100% P showed consistent dominant results, as compared with other treatments with lower P percentages, whose differences were inconstantly significant. On the other hand, when applying both the P-PNSB and P fertilizer, the N content and uptake only in season 2 showed significant differences proportionally according to the P fertilizer level, while in season 1, the differences were insignificant. However, for the K content and uptake, season 1 and season 2 followed the same trend, which showed the greatest result in the treatment with P-PNSB and 75% P and significant decreases in the results following the decline in P fertilizer level. Significantly, in the result of K content, the treatment with only P-PNSB outweighed the treatment without P-PNSB and chemical P fertilizer.
| Treatment | Content (%) | Uptake (mg kg-1 dry plant weight) | ||||||
|---|---|---|---|---|---|---|---|---|
| N | K | N | K | |||||
| Season 1 | Season 2 | Season 1 | Season 2 | Season 1 | Season 2 | Season 1 | Season 2 | |
| 100% P | 0.218bc | 0.441c | 1.40c | 2.63d | 99.0b | 260.4b | 633.4d | 1555.5c |
| 75% P | 0.196cd | 0.378de | 1.28d | 1.96e | 82.8c | 205.4c | 541.6e | 1063.5e |
| 50% P | 0.175de | 0.364de | 1.25d | 1.83e | 69.0cd | 160.5e | 494.4f | 808.6f |
| 25% P | 0.167def | 0.343ef | 1.21de | 1.78e | 48.8d | 137.8f | 426.0g | 713.9f |
| 75% P + P-PNSB | 0.252a | 0.546a | 1.87a | 4.24a | 117.4a | 320.2a | 868.8a | 2493.5a |
| 50% P + P-PNSB | 0.231ab | 0.497b | 1.73b | 3.93b | 108.6ab | 251.5b | 812.1b | 1987.2b |
| 25% P + P-PNSB | 0.224abc | 0.399d | 1.46c | 2.89c | 106.8ab | 182.3d | 693.4c | 1320.9d |
| 0% P + P-PNSB | 0.147ef | 0.341ef | 1.14e | 1.35f | 15.5e | 51.8g | 117.3h | 205.4g |
| 0% P | 0.136e | 0.313f | 0.980f | 0.977g | 12.2e | 37.4g | 87.9h | 117.2g |
| Level of significance | * | * | * | * | * | * | * | * |
| CV (%) | 9.08 | 5.62 | 3.95 | 6.16 | 10.8 | 6.23 | 4.68 | 9.21 |
3.3. Effects of P-PNSB Rhodopseudomonas Palustris on Growth and Yield of Lemon Balm in Alluvial Soil
In soil not fertilized with P, the treatment with P-PNSB resulted in greater growth parameters, such as plant height, leaf length, quantity of primary branches, leaf width, and quantity of secondary branches, than the treatment without P-PNSB (Table 4). However, the number of leaves per plant did not statistically change via two continual seasons. In the case of P fertilization, combining the three fertilizer rates and P-PNSB resulted in greater leaf length (3.52-4.01 cm) than the treatment with P-PNSB (2.88-3.67 cm). Simultaneously, the number of secondary branches shared the same trend, with 24.0-29.2 cm compared with 7.50-23.7 cm, respectively, except for the 25% P according to RFRLB, where the treatments with and without P-PNSB showed statistically the same results. For the leaf quantity per plant, only at 75 and 25% P according to RFRLB, the treatments with P-PNSB outperformed the treatments without ones in both seasons. Furthermore, the treatments without P fertilization but with P-PNSB had greater SPAD indices (20.5 and 27.8) than the treatments without P-PNSB (18.1 and 27.3) in the two seasons, respectively (Fig. S1).
| Treatment | Plant height | Leaf length | Leaf width | Leaf number per plant | Primary branches quantity | Secondary branches quantity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | (leaves) | (branches) | ||||||||||
| Season 1 | Season 2 | Season 1 | Season 2 | Season 1 | Season 2 | Season 1 | Season 2 | Season 1 | Season 2 | Season 1 | Season 2 | |
| 100% P | 57.3a | 68.3a | 5.75ab | 3.67b | 4.33a | 3.13a | 540.0ab | 1870.0a | 21.0a | 28.5a | 6.00ab | 23.7b |
| 75% P | 56.6a | 67.0ab | 5.65ab | 3.60bc | 4.18a | 2.90a | 513.0ab | 1638.0b | 20.8a | 26.2b | 5.00bc | 23.3b |
| 50% P | 52.5bc | 64.5ab | 5.55ab | 3.21e | 4.34a | 2.88a | 453.0b | 1562.8b | 16.3c | 26.8b | 3.67c | 18.8c |
| 25% P | 50.1c | 63.6b | 5.48ab | 3.26de | 4.43a | 2.81a | 539.7ab | 1409.3c | 16.4c | 20.0c | 3.75c | 17.4c |
| 75% P + P-PNSB | 57.4a | 68.3a | 5.68ab | 4.01a | 4.53a | 2.93a | 591.0a | 1812.3a | 20.5a | 28.5a | 6.50a | 29.2a |
| 50% P + P-PNSB | 53.5b | 65.5ab | 5.85a | 3.52bcd | 4.41a | 2.93a | 550.5ab | 1620.3b | 20.5a | 26.9ab | 6.08ab | 28.6a |
| 25% P + P-PNSB | 52.8bc | 59.3c | 5.35b | 3.68b | 4.15a | 3.03a | 572.3a | 1589.3b | 17.8b | 25.8b | 4.50c | 24.0b |
| 0% P + P-PNSB | 41.6d | 31.8d | 3.93c | 3.35cde | 3.18b | 2.93a | 168.0c | 110.0d | 6.00d | 5.83d | 2.00d | 13.7d |
| 0% P | 37.1e | 27.8e | 3.30d | 2.88f | 3.16b | 2.40b | 142.5c | 98.0d | 4.66e | 3.27e | 1.00d | 7.50e |
| Level of significance | * | * | * | * | * | * | * | * | * | * | * | * |
| CV (%) | 3.24 | 3.86 | 4.06 | 4.13 | 5.56 | 4.91 | 12.8 | 4.19 | 4.01 | 4.63 | 18.6 | 4.06 |
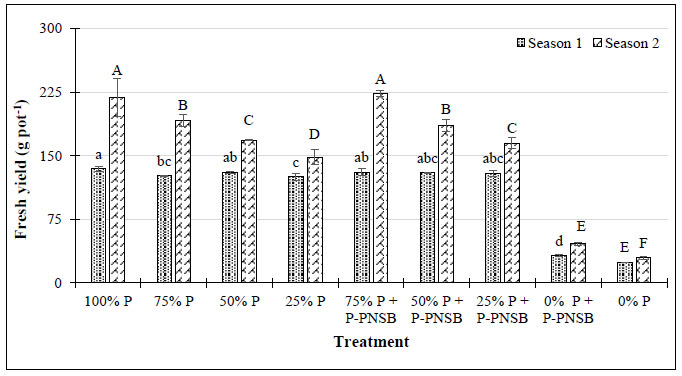
Note: Lower-case and upper-case letters show significant differences between treatments at p-value 0.05 in seasons 1 and 2, respectively; P-PNSB: the mixture of the four phosphorus-solubilizing purple nonsulfur bacteria Rhodopseudomonas palustris TLS06, VNW02, VNW64, and VNS89.
In Fig. (5), 75% P fertilization combined with P-PNSB improved the yield of lemon balm. Moreover, the essential oil content after extraction in the treatment with both 75% P and P-PNSB was 0.272 g 100 g-1, and greater than 0.153 g 100 g-1 of the treatment with only 75% P according to RFRLB (Tables S1 and S2).
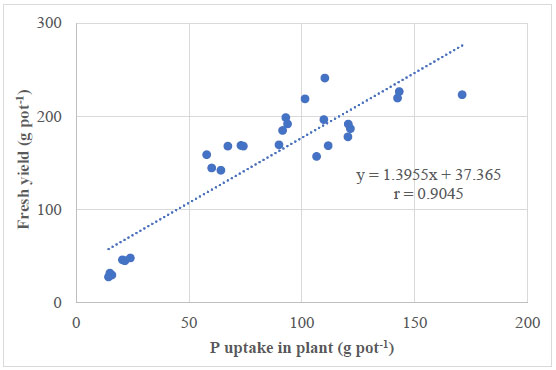
There was a proportional correlation between the P uptake in plants and the whole plant yield of lemon palm in two consecutive seasons, with a correlation coefficient of 0.9045. In other words, the greater the P uptake was, the greater the fresh yield was obtained (Fig. 6).
4. DISCUSSION
As reported in Table 2, the treatment with both 75% P and P-PNSB improved pHH2O (6.63) as compared with the one with only 75% P, according to RFRLB (5.91). This showed that the P-PNSB Rhodopseudomonas palustris VNW64, VNS89, TLS06, and VNS02 strains produced EPS and ALA to enhance soil pH [26, 34, 35]. EPS contains many functional groups, such as carboxyl, sulfhydryl, hydroxyl, and phosphoryl, that bear negative charges when binding with H+ [36]. This explains why supplying PNSB can increase P availability in soil, i.e., PNSB solubilizes the insoluble forms to produce the soluble P compound for plants and also increases soil pH while increasing soil pH ameliorates the P availability [34, 37]. Furthermore, PNSB can solubilize P by producing compounds that can dissolve insoluble P forms, such as organic acids and siderophores, by producing extracellular enzymes or by releasing P during organic degradation [38]. P contents increased by 41.2-41.7% in treatments with P fertilization and by 8.70-13.7% in treatments with P-PNSB when no P fertilization was used (Fig. 1). Previous studies indicate that supplying P- PNSB, such as Luteovulum spp., increases soluble P content in saline soils for rice and acid sulfate soils for pineapple [21, 23]. The increasing availability of P was accompanied by the decreasing content of insoluble P compounds (Table 2). In other words, P-PNSB solubilized insoluble P to provide soluble P for plants. Additionally, some biological approaches have been presented based on mechanisms to create mineral solubilizers, such as organic acids, H+, and OH-, to make use of the immobilized P content in soils [17]. PNSB has been used to solubilize insoluble P in soil without releasing heavy metals [39]. In acid sulfate soils, PNSB biofertilizers can not only solubilize P and reduce the used amount of chemical P fertilizer by 25-50% while maintaining the optimal yield but also remediate soils by reducing Al3+ and Fe2+ toxicity [25, 39]. Furthermore, supplying P-PNSB can also increase concentrations of NH4+ (2.82 mg kg-1) and exchangeable K+ (0.235 mg kg-1) as compared with the treatment without P-PNSB. Moreover, a biofertilizer can increase nutrient contents in soils [40]. Moreover, PNSB reduces P fertilizer by 25-50% of the recommended rate but still ensures the rice grain yield [24]. As shown in Fig. (3b), P uptake in the treatments with both 25-75% P and P-PNSB increased as compared with the treatment with only chemical fertilization. However, when chemical fertilization was not used, the treatments with and without P-PNSB varied insignificantly in two seasons. Nevertheless, there were proportional correlations between the PNSB density and the soil P available content (Fig. 4). Higher available soil P resulted in higher P uptake (Fig. 5) and between the P uptake and the lemon balm (Fig. 6). In other words, the application of PNSB increased the yield of lemon balm by promoting available P content. This is consistent with the study by Khuong et al. [21], where rice yield was improved by P-solubilizing PNSB strains.
In Table 1, supplying P-PNSB R. palustris VNW64, VNS89, TLS06, and VNS02 promoted plant growth via plant height, leaf length, and quantity of primary branches in two seasons in treatments with no chemical P fertilizer. This is in accordance with the studies by Huu et al. [22] and Khuong et al. [25], where using P-PNSB R. palustris and L. sphaeroides increased the height of rice and pineapple plants. Furthermore, supplying PNSB also increased root length by 25%, root dry weight by 57%, number of branches by 26%, number of seeds by 38%, grain yield by 33%, 1,000-seed weight by 1.6%, and harvesting indices by 41% when compared with no bacteria application [41]. Moreover, as reported by Liu et al. [42], PNSB strains can provide plant growth-promoting substances, such as IAA and ALA under disadvantaged and ameliorate plant growth. In season 1, in the treatment with only P-PNSB, fresh biomass increased as compared with the one without P-PNSB. However, in season 2, either fertilizing chemical P or not, supplying P-PNSB increased fresh biomass. This showed that P-PNSB adapted to the alluvial soil and actively solubilized P for plants, which resulted in increasing soluble P in soil and P uptake in plants. This is the reason why the application of P-PNSB can reduce 25% P due to the response of low lemon balm requirement for P. Thus, the yield of lemon balm increased in the treatments with P-PNSB in the two seasons.
CONCLUSION
Combining P fertilization and P-PNSB increased pHH2O by 9.20-10.1% and Pavailable by 11.4-22.4% compared to the treatments with only chemical fertilization. Supplying P-PNSB, R. palustris VNW64, VNS89, TLS06, and VNS02 reduced 25% P according to RFRLB but still improved soil fertility, fresh biomass yield (by 31.4 g pot-1), and P uptake (by 8.00 mg P kg-1 in season 1 and by 45.1 mg P kg-1 in season 2). Furthermore, fertilizing 75% P and supplying P-PNSB altogether had equivalent plant height to the treatment with 100% P, according to RFRLB in both seasons. Further, reducing 25% P and supplying P-PNSB yielded greater essential oil content than the treatment with only 75% P. Moreover, the P-PNSB should be evaluated on lemon balm in different series of soils and an optimal fertilizer formula combined with P-PNSB should be designed for lemon balm.
AUTHORS' CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| ALA | = 5-aminolevulinic Acid |
| BIM | = Basic Isolation Medium |
| DOFs | = Days of Farming |
| DSW | = Dry Soil Weight |
| EPS | = Exopolymeric Substances |
| IAA | = Indole Acetic Acid |
| P-PNSB | = P-solubilizing Purple Nonsulfur Bacteria |
| PNSB | = Purple Nonsulfur Bacteria |
| RFRLB | = Recommended Fertilizer Rate for a Lemon Balm |
| SPAD | = Soil Plant Analysis Development |
| SDW | = Sterilized Distilled Water |
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author, [N.Q.K]., on special request.


