All published articles of this journal are available on ScienceDirect.
Study on the Biological Drug Enterocol’s Effect on the Nile Tilapia Breeding
Abstract
Background
Nile tilapia (Oreochromis niloticus) is a promising fish species for fish farming development in the Republic of Kazakhstan. It exemplifies a balanced and fast-growing source of flesh for future food production. In this context, optimising breeding methods is crucial for a high flesh yield and a safe product. The study delves into the potential of the Enterocol drug in enhancing Nile tilapia breeding, focusing on assessing muscle state and blood parameters.
Objects
Two groups of fish were utilized, with 20 specimens in each group. One set was the control group, and the other was the experimental group. The single difference between the fish groups was the Enterocol consumption by the experimental specimens, which were given an E. coli 64G strain concentration of 108 CFU in mL and a 5% daily dose of fish body weight three times daily for two months.
Methods
Blood parameters were analysed to assess the health of the fish groups. Subsequently, classical pathological methods were employed, such as macroscopic and microscopic assessments of each group's fish material. A histological analysis was also performed to assess the condition of the muscles.
Results
Examining the experimental group's muscles revealed a significant average increase in fish mass and 6.74% total body weight growth. Moreover, the total thickness of the muscle layer decreased as villi height increased, indicating a clear fibre structure. Haematological analysis unveiled elevated levels of haemoglobin, erythrocytes, leucocytes, and total protein in the experimental fish group, indicating improved health. These findings underscore the potential of the Enterocol drug in enhancing fish health and productivity.
Conclusion
Based on the results, it can be concluded that using Enterocol in fish breeding plants can effectively enhance flesh quality and safety, providing a secure and reliable solution for the aquaculture industry.
1. INTRODUCTION
Fish and seafood are nutritious and valuable meal options that could form a significant share of the population's diet. It is important to note that aquaculture is rapidly growing and can substantially contribute to the production industries across various countries [1, 2]. One of the perspective breeds for this purpose is Nile tilapia (Oreochromis niloticus), which belongs to the Cichlid family. This fish type was known in Ancient Egypt more than five thousand years ago. Most tilapia species are commonly used in artificial aquaculture production and can be introduced for manufacturing in Southeast Asia, particularly for fish manufacturing and breeding [3, 4].
Sarsembayeva et al. [5] stated fish farming in the Republic of Kazakhstan as one of the fastest-growing economic sectors. The fish breeding potential in the Republic of Kazakhstan is only partially utilized. Currently, only 3% of the potential is used for rivers, 29% for lakes, 6% for ponds, and the remaining significant portion (73%) is used for artificial reservoirs. Significantly, artificial breeding must consider the animals' well-being, including ensuring they are free from hunger and thirst, discomfort, pain, injury, disease, fear, and distress, and allowing them to express normal behaviour [6]. One of the fish farming problems in artificial reservoirs is providing fish wellness [7], considering the factors of overfishing or fish stocking density, water pollution (water quality), microclimate parameters, and feeding methodologies with nutrient load checking [8]. Cortés-Sánchez et al. [9] reported that environmental pollution and diseases (both viral and bacterial) cause massive fish mortality, especially in artificial conditions. This fact is valid for all regions with developed fish industries. Hence, most farmers use antibiotics to prevent and overcome such problems. Nevertheless, the use of antibiotics in aquaculture can change the microbiota and lead to the emergence of resistant bacteria that can be harmful to aquatic organisms [10, 11]. Therefore, both practitioners and scientists are looking to resolve this problem. One potential approach is to consider the introduction of probiotic feed supplements as a replacement for anti- biotics currently utilized in aquaculture. Singh et al. [12] cited the importance of a lipid and fatty acid supplementary diet for fish's successful reproduction and survival in artificial conditions.
Nandi et al. [13] reported that protein and polyunsaturated fatty acid supplements (as prebiotics) positively affect breeding performance, gonadal matu- ration, and spawn recovery in female broodstock. Later, Xu et al. [14] confirmed Nandi et al.'s data and stated that the positive outcome of fish breeding is better fish wellness and fertility parameters. This can be achieved by improving the body's immune response due to the action of the ß-glucans that activate defense mechanisms after cell damage, including antigen-presenting cells and increased immune response cells [15, 16]. Additionally, limited data are available on the impact of probiotic supplements attributed to the action of ß-glucans. Nonetheless, it has to be proven [15]. Another reason for introducing probiotics in aquaculture is the demand for environmentally friendly trends. Consequently, probiotics have become an environmentally friendly strategy for sustainable aquaculture [17], using fewer drugs, like antibiotics, chemotherapeutic medicines, and sterilization agents [16]. Soltan et al. [18] proved that probiotics can be given as viable microbial food additives to benefit fish growth, immune response, intestinal microbial balance, and digestive enzyme activity. Amin et al. [19] indicated the safety of various feeding probiotic strains. They demonstrated that probiotic strains usually disappear from the gastrointestinal tract within a few weeks after food intake ceases. Hence, these microbes are in transit and do not become allochthon strains.
Microbiologist Nissle initially researched the potential probiotic effects of non-pathogenic strains of Escherichia coli in 1917. Various animal studies have been conducted using these strains [20]. The feed additive Enterocol, a biological product, has been extensively utilized in animal husbandry and poultry [21, 22]. Nevertheless, more information on its use in fish farming must be provided. In this regard, this study aimed to evaluate the effect of the Enterocol drug on fish muscle state and haematology parameters.
2. MATERIALS AND METHODS
The study was conducted in artificial reservoirs from March to May 2021, with three repeated trials involving two commercial hybrid Nile tilapia (Oreochromis niloticus) sets. Each set consisted of 20 fish, one serving as the control group and the other as the experimental group. LLP TengryFish provided the experimental facility.
The laboratory tests were conducted at the Antiparasitic Biotechnology facility within the Kazakh National Agrarian Research University Department of Biosafety.
The Enterocol study was initiated efficiently, considering the Nile tilapia's haematological parameters and the histological muscle structures of the control and experimental groups.
2.1. Study Design
The experiment involved two sets of fish, each consisting of three replicates. The control and experimental groups consisted of 20 specimens each, with an average weight of each fish of 3.65±0.05 g. The specimens were selected randomly based on their well-being. The experiment lasted for 61 days (two months), during which the control group was fed the standard diet, while the experimental group received the Enterocol supplement in addition to their regular feed. The fish were monitored daily to ensure their health and well-being. In the event of mortality, proper recording and immediate removal of deceased fish were carried out as a precautionary measure.
In each replication, two reservoirs were used for each group, with a size of 2×4 meters for each. The water level in each reservoir was one meter, with a total water volume of 5 m3. The temperature was 24±2°C, and the water pH was 7.2-7.4. The reservoirs were aerated at 70%, and the light rate was 10%. The reservoir was maintained by cleaning once a week. Each reservoir's water condition was checked twice during the experiment by fixing toxic chemical elements (Pb, Cd, Cu, and Zn) and total microbial content by fixing the Enterobacteriaceae bacteria family content.
Starved fish were periodically removed from the reservoirs every two weeks to be measured and examined for external abnormalities. The data were used to adjust the diet provided in different experimental stages. Half the settled fish waste and water were removed daily and replaced with fresh tap water from the storage tank.
Measurements were conducted on all the fish in the control and experimental groups on the 62nd day of the experiment. For future studies, an average specimen was selected from each group. At the end of the test, the fish were deprived of food for 24 hours. The final weight of all fish in separate reservoirs was determined by measuring their number and weight. Therefore, nine fish with an average total length of 26.4±2.19 cm and a wet body weight of 345.7±4.35 g were selected from the experimental reservoir. The specimens from the control group were almost identical in length, measuring 26.2±1.7 cm, and the average weight was 322.4±3.96 g. Subsequently, classical pathological methods were used to conduct macroscopic and microscopic assessments of the material from each fish group.
2.2. Fish Feeding
The fish were fed a complete ration with an energy value of 364.96 kcal per 100 g and a nutritional value of 40.53 g of protein, 8.03 g of fat, 2.75 g of cellulose, 28.4 g of biologically active substances, 10.4 g of minerals (ash), 3.8 g of starch, and 1.61 g of sugars per 100 g of the ration (Table 1).
| Feed Ingredients | Percentage, % |
|---|---|
| Feed (hydrolytic) yeast | 26.4 |
| Fish flour | 18 |
| Meat and bone meal | 12 |
| Soy flour | 17 |
| Wheat bran | 5 |
| Wheat | 5 |
| Blood meal | 2 |
| Wheat germ | 5 |
| Corn gluten | 6 |
| Soybean meal | 2.5 |
| Premix (PM-2) | 1 |
| Antioxidant | 0.05 |
| Detergent | 0.05 |
The experimental group's ration was supplemented with Enterocol, containing a concentration of the E. coli 64G strain of 108 CFU/mL. The supplement dosage was calculated based on the fish's weight, using 5% of the specimen's body weight. The fish were fed three times a day.
2.3. Production of the Biological Preparation Enterocol
The E. coli 64G strain (No. 25918; deposited at the National Centre of Biotechnology of the Republic of Kazakhstan, Scientific Research Agricultural Institute, number 4595) was used, taking into account the data reported on the strain [23].
The E. coli 64G strain was inoculated to obtain a pure culture of 1 mL in two vials with Hottinger's broth (medium volume 100 mL). The culture was grown in a thermostat for 16-18 h at +37-38°C (primary generation culture).
Culture purity was assessed by smear microscopy and Gram staining. After that, it was inoculated to 50 mL in a bottle containing 10 L of Hottinger's broth and cultured for 18-20 h at 37°C (generation culture). Afterward, the second culture was checked for growing microorganisms. The nutrient medium was 100-110 L at pH 7.6-7.8, and the amine nitrogen content was 200-250 μg%.
The matrix culture was pumped in a volume of 8-10 L and distributed among the cuvettes by rotating the apparatus. The material was exposed in cuvettes for 15-20 min. The excess materials were poured into a bottle. The culture was left to grow in the apparatus out for 16-18 h under the control of automatic devices at a temperature of +37-38°C with 15-minute aeration. A sterile flushed liquid (drying medium) in a volume of 5-6 L was introduced into the apparatus and washed off by rotating the apparatus after the indicated period of bacterial culture cultivation.
Drying medium (pH 7.8-8.0) comprised 1.5-2% gelatin and 10% sucrose. After checking the obtained microbial mass for purity and typical growth, it was brought to a concentration of 1010 CFU in mL according to the optical turbidity standard. The drug was tested for sterility and packaged in sterile 4 mL vials with continuous stirring. The vials were sealed with caps and subjected to freezing and drying (70-76 h). The drug preparation date was the lyophilisation completion day. According to Biyashev et al. [24], Enterocol’s expiration date is a year from the manufacturing date if stored in a dry, dark place at +4-15°C. The experimental group's probiotic drug was prepared by gently spraying the required bacterial suspension on the feed experimental version and mixing it in portions to obtain the final probiotic concentration (108 CFU/mL).
2.4. Haematology Testss
Nine fish from each group were anaesthetised and had their blood sampled. The muscles were subsequently extracted and subjected to histological testing. The blood was collected from starved fish after 10 minutes in well-aerated water. The blood for the experiment was collected by removing the scales with a scalpel, and the mucus was wiped off. The skin was disinfected using 70% ethanol. Then, the blood samples were obtained using a syringe from the tail vein and placed into a clean tube containing 10% EDTA solution for the determination of Haemoglobin (Hb), Red Blood Cells (RBCs), and White Blood Cells (WBCs). After centrifugation at 3000 revolutions per minute for ten minutes, serum samples were separated.
The Hb content was determined by employing Sahli's method. The number of RBCs was determined using the test tube method in Goryaev's chamber. The number of WBCs was defined by directly counting them in 80 large squares of Goryaev's chamber and calculated using formula (1):
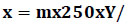 |
(1) |
where,
x – the number of leukocytes in 1 μL;
m – the total cell number in counted squares;
У – the degree of blood dilution, and n – the number of viewed squares.
2.5. Histology Examination
Histological examinations were performed on organs and tissues to identify microscopic changes and analyse the internal organ ratio when utilising a biological product. They were conducted by employing GOST R 19496-93 “Meat. Method of histology examination”. The material for the histological study was fixed in 10% neutral formalin. After that, serial paraffin sections with 5-6 μm thickness were prepared.
Ultrathin sections were prepared with a semiautomatic microtome HEOTION ERM 3100 and a microtome МС-2. Celloidin, paraffin, and frozen sections were utilised and stained using traditional and specific histological techniques with hematoxylin-eosin on a Leica processor for staining sections No. S4040/No. 000000358 (BioLine LLC, Russia, St. Petersburg). Histological slides were studied using a binocular microscope MBI-6 under various magnifications. A total of 40 blocks were created from the tilapia muscle samples, and 30 were subjected to histological examination.
2.6. Statistical Analysis
The experimental study results were evaluated by employing modern measurement methods and statistical reliability results using Microsoft Office Excel and STATISTICA 6.0 software packages, with calculated Student’s t-distribution. The data on haematology and blood biochemistry were analysed using the ANOVA method. A reliability of 0.05 was used to account for the statistical difference between the means.
3. RESULTS
3.1. Fish Wellness Study
To evaluate the effects of Enterocol, a supplement based on a non-pathogenic E. coli strain, on fish health, we analysed the blood parameters of both the control and experimental groups (Table 2).
The blood index analysis revealed that introducing a non-pathogenic E. coli strain did not affect the fish's health. The experimental group exhibited good physical health and positive activity measures, consumed all proposed feeds, and showed body mass growth. The blood parameters in both the control and experimental groups supported the hypothesis. For example, we could compare the Hb level, which indicates the specimen's well-being and is often influenced by feeding [25].
| Index | Groups | |||
|---|---|---|---|---|
| Control | Experimental | |||
| Hemoglobin, g/L | 91.5±0.11 | 92.1±0.21 | ||
| Red blood cells, х1012/L | 0.93±0.01 | 0.86±0.32 | ||
| Leukocytes, х109/L | 11.3±0.03 | 23.6±0.01 | ||
| Total protein in blood serum, g/L | 6.8±0.14 | 7.2±0.05 | ||
The experimental group exhibited a higher Hb level than the control group, 92.1±0.21 g/L versus 91.5±0.11 g/L (p≤0.05). The average values in each group could be observed within the reference range of 80-120 g/L. The experimental group showed better RBC filling, particularly regarding RBC content. Although the number of RBCs in the experimental group was slightly lower than that in the control group (7.5%), both groups had normal RBC content levels (0.8-3.5x1012/L). Table 2 displays that the experimental group had a fixed level of 0.86±0.32x1012/L, while the control group had a 0.93±0.01x1012/L level.
The white blood cell count in the blood indicates the body's immune reactivity. The physiological WBC count in fish can change significantly throughout the year due to rapid hematopoiesis. Upon analysis of the data, it was noteworthy that both groups of studied fish specimens had a higher white blood cell count than the reference values. Specifically, the experimental group had a significantly higher count than the control group, with 23.6x109/L and 11.3±0.03х109/L, respectively. The enhanced immune reactivity of fish in the experimental group could be observed.
After a meticulous analysis of the two groups' body weights, a significant difference was found in the average body weight. The control group had a precise combined weight of 2901.92 grams, while the experimental group weighed 3111.6 grams. Hence, we could conclude an approximately 6.74% increase in body weight prevalence in the experimental group.
3.2. Fish Muscle Histology Study
For the next experimental phase, both groups of fish were subjected to a two-stage histological study (macroscopic and microscopic).
3.2.1. Macroscopic Examination of Fish Muscle
The quality of the flesh depends on the diameter of the muscle fibre and the amount of fat present in the muscles. The ratio of muscle compared to connective tissue is what defines this. This balance can be used to analyse the presence of full-fledged muscle proteins instead of defective proteins found in the connective tissue layer [26]. It should also be noted that physiological characteristics and environmental factors can alter this balance [27]. To analyse the state of the tilapia, muscle samples were taken from the dorsal side above the lateral line of its body.
The macroscopic study revealed the fish's muscles in the control group to be whitish in colour and soft in consistency, with a fibrous standard structure. Additionally, the pattern of the inner surface of the muscle tissue was preserved.
The condition of the fish muscles improved in the group that received the Enterocol drug as part of the experiment. The muscle fibres had a better appearance and overall structure, displaying characteristic features, such as a whitish colour, dense consistency, and clear internal pattern. A fixed enormous flesh mass was obtained from the experimental group (Fig. 1).
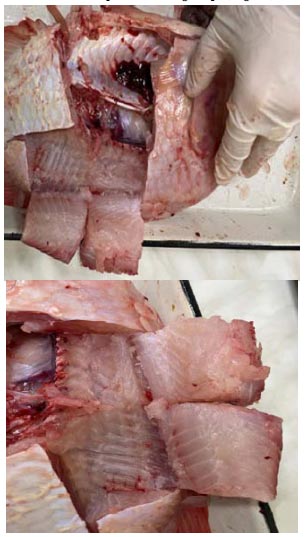
The fish-flesh macroscopic study of the control (A) and experimental (B) groups.
3.2.2. Microscopic Examination of Fish Muscle
Histological analysis provides insights into muscle structure and variations in specific body areas. Additionally, by analysing tissue and cellular structures, it is possible to identify the structure of the product and determine its quantity. Therefore, a study examined the histology of tilapia's white muscles. The samples showed a moderate dissociation of muscle fibres, but the integrity of muscle fibre myofibrils was preserved in all the samples analyzed. The muscles exhibited apparent crossed striations.
In the control group, some improvements were observed in the fibre structure. The fibre bundles were slightly separated, with some thinner in their extended sections. Some muscle fibres were damaged in the fish flesh samples of the control group (Fig. 2). It can be hypothesised that muscle tears may have been caused by the stress factor when catching and fixing the fish. The muscle tissue pathology of the examined tilapia was analysed using Altufiev's criteria for evaluating muscle pathology. According to the criteria, the muscle fibres in the samples from the control group displayed structural pathologies scoring between 0 and 1.
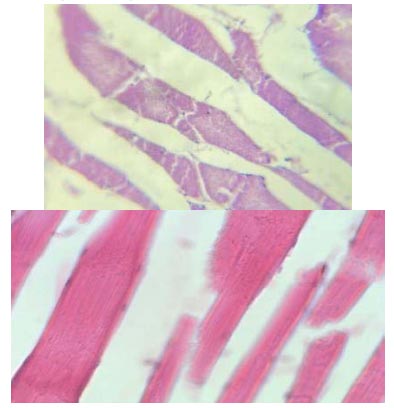
Histological section of the control group tilapia muscle: muscle fibre ruptures. Inc. x 10 (A) and x 40 (B). Haemotoxylin and eosin staining.
The quality of fish products is widely recognised to be contingent upon the quality and structure of the muscle fibres. The skeletal muscle of the control group exhibited indicators of dystrophy alongside dissociation, cleavage, and minor rupture of muscle fibres. Nonetheless, striations in the muscle fibres were evident in all samples analysed. Distended myocyte nuclei were detected in some muscular regions (Fig. 3).
After implementing Enterocol into the feeding schedule, the subsequent step was to examine samples from the experimental group. The structure of the muscle fibres was maintained, and the sarcolemma remained intact. The myocyte nuclei were located on the periphery of the fibres, and the fixed areas demonstrated the vacuolation of the intramuscular connective tissue (Fig. 4).

Histological section of the control group tilapia muscle: dystrophy and dissociation of muscle fibres. Inc. x 10 (A) and x 40 (B). Haemotoxylin and eosin staining.
According to the histology and morphology evaluation, the biological product positively affected the villi length and the thickness of the fish muscle layer. The examination has revealed the length of the Nile tilapia's villi in the experimental group to be notably longer, on average, in the muscle compared to the samples from the control group.
4. DISCUSSION
Feeding is essential in animal breeding because it provides vital nutrition for growth and development [28]. Applying bio-friendly feed additives (probiotics, prebiotics, and synbiotics) is becoming popular. Such additives can improve fish's growth potential in artificial conditions, immune resistance, and overall well-being [16].
The chemical composition of fish flesh can differ in different species as well as in fish body parts. Also, it significantly depends on the nutrition and physiological state [29, 30]. Haematology is a vital parameter that demonstrates animals' general state and feeding's effects on them [31]. Khan et al. [32] discovered that unfavourable environmental factors can cause pathological changes in blood indexes, which can be detected. Our research study has tested haematological parameters in fish specimens from both the control and experimental groups (which received a biological supplement based on the non-pathogenic E. coli strain).
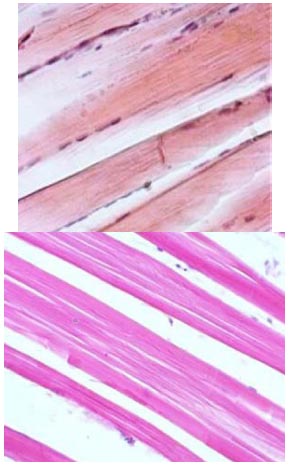
Histological section of the experimental group tilapia muscle. Inc. x 40 (A) and x 60 (B). Haemotoxylin and eosin staining.
Earlier research studies have provided data on the use of probiotic drugs in fish breeding. Liu et al. [33] reported an increase in the thickness of the fish muscle layer associated with introducing probiotics into the fish diet. The authors suggested that this effect could be significant for the fishing industry. Furthermore, it has been discovered that providing tilapia with a probiotic element, like non-pathogenic E. coli, can enhance muscle fibre growth. A difference was observed in the average wet weight of the fish, with 345.7±4.35 g for the experimental group and 322.4±3.96 g for the control group. Silva et al. [34] obtained positive results in tilapia breeding, with an increase in feed conversion rate of 13%–37% for the group that received additional feeding with the probiotic component. Nevertheless, the authors of a study used a combination of Bacillus subtilis, Bifidobacterium bifidum, Enterococcus faecium, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus lactis, Lactobacillus plantarum, and Pediococcus acidilactici (at a dosage of 1.0 × 109 CFU/g). In contrast, we have developed our supplement using an E. coli strain isolated from the lamprey digestive tract [23]. The supplement significantly increased the overall mass of the fish throughout the experiment as a result of the feeding trial. The experimental group showed a 6.74% increase in total weight mass, with a final weight of 3111.6 g compared to 2901.92 g. We also conducted macroscopic and microscopic analyses of the muscle state to understand this effect.
Fish somatic muscles are divided into white and red muscles in different body parts. White muscles, the main component of skeletal muscles responsible for locomotor function, mainly comprise a protein highly dependent on the fish's diet [27, 35]. Nemova et al. [36] provided data indicating that teleost fish muscle proteins consist of approximately 20-30% sarcoplasmic protein, 60-70% myofibril protein, and about 2% stromal protein. The diameter of muscle fibres varies depending on the fish species, age, size, and feeding conditions. This study has compared the state of tilapia muscle in a control group with the group supplemented with a biological drug based on the non-pathogenic E. coli strain.
The study results have indicated the biological product to benefit the development of Nile tilapia muscle growth and its histological and morphological composition. It is important to note that the product has demonstrated the potential to support Nile tilapia growth, leading to improved health and well-being. The obtained results can be used to study the product’s microbiological impact (as a probiotic component) in animal farming and animal resistance possibilities (immunity and stress defense) in the education of future veterinary specialists. As Muca et al. [37] stated, introducing students to the practical experience positively affects the students’ skills and interest in education.
Enterocol aids in the efficient absorption of nutrients by enhancing digestive reactions, muscle layer thickness, and enzyme activity. Our findings have supported those of Abarike et al. [38], who found enhanced digestive enzyme activity in Nile tilapia upon the supplementation of Bacillus subtilis, resulting in higher quality scores for fish meat. Significantly, the use of probiotics in fish farming is becoming increasingly common. Empirical evidence provided by Allameh et al. [39], Jang et al. [40], and Deng et al. [41] shows that various strains of yeast, fungi, bacteria, and bacilli, including Lactobacillus spp., are utilized in the aquaculture farming process. Supple- menting fish rations with probiotics can enhance their growth and immunity and improve gastrointestinal tract functionality. Recent research in this field has revealed heightened immune reactivity among fish in the experimental group. An increase in total protein content was observed in the blood serum of these animals (7.2 vs. 6.8 g/L), which can be attributed to their increased metabolism [42].
Reda et al. [43], Istiqomah et al. [44], and Won et al. [45] have presented the impact of probiotic drugs on fish. They noted that probiotic microbial strains can regulate intestinal homeostasis and support physiological functioning. Their results have demonstrated the positive impact of probiotics on fish breeding. Regardless, it is crucial to consider certain factors when introducing probiotics to any living organism. Pradhan et al. [46] have demonstrated the safety of the biological supplement drug for living organisms. Yu et al. [47] indicated that B. coagulants can improve fish blood values by stimulating the immune response, as evidenced by the increased activity of immune components, such as WBCs. The study has identified the optimal dose for achieving the desired effect. The fish's wellness was tested by comparing the blood parameters of the control and experimental groups. The results suggested that the biological drug Enterocol stimulated increased levels of Hb and RBCs and elevated concentrations of WBCs (Table 2). The safety of the proposed probiotic supplement added to the fish ration can be discussed, particularly when considering the behavioural characteristics of the observed fish groups. It is worth noting that the fish in the control and experimental groups exhibited similar activity and appetite levels.
Biyashev et al. [23] demonstrated the safety of Enterocol through an analysis of haematology results in lambs. Bulegenova et al. [22] also investigated the effects of Enterocol on newborn calves, specifically on total protein levels and the number and specific content of immunoglobulins in blood serum. According to Bulegenova et al. [22] and Biyashev et al. [23], newborn calves given the probiotic experienced an increase in total protein concentration and all categories of antibodies in their blood. The growth degree in blood parameters depended on the amount of the supplement.
Zhakupova et al. [21] studied the biological properties of the E. coli 64G strain and demonstrated its non-pathogenic and non-toxic nature. The authors suggested the E. coli 64G strain could colonise the gastrointestinal tract as an active allochthonous resident. Further research is required to investigate this possibility. Zhakupova et al. [21] observed that the 64G strain enhanced animal growth and disease resistance. Therefore, the authors suggested using this bacterial strain in animal breeding as it is stable and viable under industrial conditions. It is important to note that positive outcomes were consistently observed in the breeding of fish when Enterocol was added to their diet. This was evidenced by the experimental group's higher total mass than the control group.
Attention should be paid to the dosage of probiotics for feeding. An experimental recommendation is a dosage of 108 CFU/mL. Yet, it is necessary to test the optimal concentration of the biological component in each proposed drug. Al-Hisnawi et al. [48] reported that the probiotics dosage in aquaculture is usually 106 CFU/g of feed, while Allameh et al. [39], Jang et al. [40], and Deng et al. [41] noted the optimal dosage of 107-108 CFU/mL. Therefore, this parameter can be specific to each preparation.
CONCLUSION
Both morphometric and histological tests on two groups of fish performed in this study have indicated the biological feeding supplement, Enterocol, containing the non-pathogenic E. coli 64G strain (dosage being 5% of the animal’s weight with concentration of 108 CFU/mL), to facilitate positive growth of Nile tilapia muscle. Upon examining the muscles at a macroscopic level, the experimental group's fish has been found to exhibit an improved overall structural condition, with a thicker muscle layer. Nevertheless, the muscle’s colour was whitish with dense consistency and an explicit internal pattern that showed good muscle development. Results obtained from the microscopic analysis have also indicated improved muscle condition. The experimental samples have shown greater thickness in their longer sections than control fish samples with partially damaged fibres. Hence, increased muscle productivity (by 6.74% of the wet weight) and improved fish flesh quality in the specimens with Enterocol consumption have been observed. Nonetheless, animal wellness is an essential parameter in the breeding process. In conclusion, the fish in the experimental group displayed good wellness, as evidenced by the blood parameters tested in both groups of fish. The results have revealed raised levels of Hb (92.1±0.21 g/L vs. 91.5±0.11 g/L; p≤0.05) with RBC content in the normal range in both the groups (0.86±0.32x1012/L and 0.93±0.01x1012/L) and WBC being higher in the experimental group (23.6x109/L and 11.3±0.03х109/L). Considering blood parameters, Enterocol input can be concluded as safe for fish feeding. Additionally, based on the macroscopic and microscopic results of the muscle, its positive impact on fish growth is notable. Thus, Enterocol could serve as a viable feed supplement in fish breeding, contributing to enhanced growth.
LIMITATION
Our research was conducted in artificial reservoirs. An in-depth study in natural conditions and on research groups with more than 20 animal specimens in the reservoir needs to be performed.
AUTHORS’ CONTRIBUTION
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to itssubmission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| Hb | = Haemoglobin |
| RBCs | = Red Blood Cells |
| WBCs | = White Blood Cells |
ETHICAL STATEMENT
This study received approval from the Asfendiyarov Kazakh National Medical University’s ethics review committee (report no. 4; 24th February, 2021). The experimental fish underwent manipulation in accordance with the Code of Professional Ethics for Veterinarians and the ethical principles for animal research outlined by the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes.


