RESEARCH ARTICLE
Screening of Soybean Advanced Breeding Lines for Resistance Against the Southern Green Stink Bug Nezara viridula L (Hemiptera: Pentatomidae) under Free-Choice and No-Choice Tests
Nuryati Nuryati1, Rudy Soehendi1, Catur Hermanto2, Ruly Krisdiana3, Saptowo Jumali Pardal1, Jumakir Jumakir1, Mochammad Muchlish Adie1, Ayda Krisnawati1, *, Yuliantoro Baliadi1, Basri Abubakar1
Article Information
Identifiers and Pagination:
Year: 2023Volume: 17
E-location ID: e18743315268755
Publisher ID: e18743315268755
DOI: 10.2174/0118743315268755231031104416
Article History:
Received Date: 23/06/2023Revision Received Date: 23/08/2023
Acceptance Date: 30/08/2023
Electronic publication date: 13/11/2023
Collection year: 2023

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
The southern green stink bug (SGSB), Nezara viridula, is a potentially harmful pod sucker insect found in soybean.
Objective:
The objective of this study was to evaluate the resistance level of several soybean advanced breeding lines against the southern green stink bug N. viridula under the free-choice and no-choice tests.
Methods:
The research materials were 14 advanced breeding lines and four check cultivars. The experiment was conducted in Malang, East Java, Indonesia, during the dry season I and II 2022. The resistance evaluation of the soybean genotypes against the N. viridula was under the Free-Choice test (FC) and the No-Choice test (NC). The newly emerged adults N. viridula were infested when plants reached the R5 stage. The data were observed for damage intensity, yield and yield components.
Results:
The intensity of pod damage in the FC ranged from 20.09 to 46.40%, meanwhile, in the NC was 25.63 to 67.63%. This shows that the NC condition provides more selection pressure than the FC. Each genotype exhibited different resistance reactions in the FC and NC. The pod damage intensity had a significant negative correlation with seed yield both in the FC and NC.
Conclusion:
The No-Choice test (NC) provided a higher selection pressure for resistance to SGSB than the Free-Choice test (NC). The use of SGSB-resistant cultivars will prevent soybean seed yield losses. A new finding in this study, two soybean genotypes Anj/Rjbs-305 and Anj/Rjbs-306 were resistant to SGSB. Both genotypes exhibit good agronomic performance (high yield and large seed size), thus can be proposed as new soybean varieties to be developed in the country.
1. INTRODUCTION
The pod-sucking pest complex is one of the causes of the decline in soybean yields in various production centers around the world. Significant yield losses were reported in terms of both quality and quantity [1, 2]. Potentially harmful hemipteran pod-sucking pests include Mirperus jaculus Thumberg (Alydidae) as well as Riptortus dentipes F. (Alydidae), Clavigralla tomentosicolis Stal. (Coreidae); Anoplocnemis curvipes (Coreidae), Nezara viridula (Pentatomidae), and Aspavia armigera F. (Pentatomidae) [1].
In South America, the main species that reduce soybean yields are N. viridula, Piezodorus guildinii, and Euschistus heros [3]. In India, the major insect pests observed were whitefly (Aleurodicus dispersus), leaf webber (Anarsia ephippias), flea beetle (Systena sp.), and stink bug (N. viridula) [4]. In Indonesia, the most common soybean pod-sucking pests are N. viridula and R. linearis [5-7]. N. viridula was reported to cause significant losses from negligible to significant loss [6, 8]. The level of pod damage varies depending on the variety, planting time, climatic conditions, population level, and plant development stage [9, 10].
Using pest-resistant varieties is one method of preventing yield loss due to the southern green stink bug (SGSB). The soybean mechanism of resistance to these pests varies according to research findings. Ayu and Suharto [11] found that pod trichome density, pod wall hardness, and thickness all significantly influenced the damage intensity caused by N. viridula, therefore it was included in the antixenosis resistance mechanism. The high level of seed damage caused by pentatomids was related to their feeding behaviour, the morphology of their mouth parts, and their saliva, though information on the specific composition of the oral secretion is limited [12]. Previous research suggests that non-preference, antibiosis, and temporal separation were potential mechanisms underlying the high stink bug resistance that has been seen for PIl71444 in the field [13]. However, pod trichome density has been suggested as one of the most effective morphological defenses against pod-sucking bugs [7].
The development of resistant varieties must consider the availability of donor gene resistance, as well as the factors that influence resistance and the mechanisms underlying resistance. Initially, the study of soybean pest resistance was based on three genotypes: PI 171451, PI 227687, and PI 229358. The three soybean genotypes were tested for SGSB resistance, and PI229358 was found to be the most consistently resistant to SGSB ([14]. During the following period, the Brazilian soybean genotype IAC 100 was widely used as a donor gene for pest resistance. According to Campos et al. [15], IAC-100, PI 558040, and V00-0870 may provide a source of genetic traits for resistance by minimizing seed weight loss caused by southern green stink bug feeding. IAC 100 was also found to be resistant to the brown stink bug, E. heroes [16]. Victor et al. [17] reported that IAC-100 exhibited high resistance when infected in the R5 and R7 phenological phases, and this resistance was associated with higher peroxidase activity.
The development of SGSB-resistant soybean cultivars needs to consider plant agronomic characteristics. N. viridula has been reported to prefer soybean genotypes with short reproductive phases and small seed sizes as food sources [18]. Another study, however, discovered that small seed size was not a major determinant of soybean resistance to pod suckers [19]. Krisnawati and Adie [20] found soybean genotypes with consistent resistance to pod-sucking bugs, as well as early maturity (78 days) and large seed size (15.57 g/100 seed). In Egypt, the successful identification of soybean resistance to SGSB revealed that Giza 111 and Crawford were susceptible, while Giza 35, Giza 21, and H30 appeared to be low resistant [21]. The use of choice test revealed that PI 085665 and PI 097139 had the lowest rates of damage from the brown marmorated stink bug and seed weight loss caused by BMSB [22]. Soybean and pea were identified as the most suitable hosts for pod sucker insects due to their shorter developmental duration, higher survivability, longevity, and fecundity, and higher population trend index on these hosts [23].
This study aimed to evaluate the resistance level of several soybean advanced breeding lines against the southern green stink bug N. viridula under the free-choice and no-choice tests.
2. MATERIALS AND METHODS
2.1. Plant Materials and Study Area
Eighteen soybean genotypes which consist of 14 advanced breeding lines and four check cultivars were selected for this study. The genotypes were derived from several hybridizations obtained from the Indonesian soybean breeding program. The check cultivars were Detap 1 (large seed size and early maturity), Dega 1 (large seed size and early maturity), Anjasmoro (susceptible to the pod-sucking pest), and G100H (resistant to the pod-sucking pest). The experiment was conducted at the Indonesian Legumes and Tuber Crops Research Institute, which is located at Malang, East Java, Indonesia, during the dry season I and II 2022 (May to December 2022). While the climatic conditions in the experimental station were somewhat similar; we can include the data from one growing season.
2.2. Insect Rearing and Soybean Planting
The insect of N. viridula imago was collected from a soybean field at Jambegede Research Station (Malang, Indonesia) during the dry season I (May to August 2022), which is located at 8°10'30” South Latitude and 112°33'32.4” East Longitude. The type of soil was Inceptisol, elevation of 335 m above sea level, and C3 of Oldeman climate type. Adults of N. viridula were collected from mature soybean plants using a 15-inch-diameter sweep net. After collection, the insects were reared in the laboratory. Insects were reared in a mesh cage (length= 120 cm, width= 120 cm, and height= 100 cm) with a food source of fresh green beans Phaseolus vulgaris L. The first generation (F1) from the rearing of N. viridula imago was used in this study.
Soybean genotypes were grown in the screen house. Each genotype was planted in a plastic pot (Φ =18 cm) containing a 4:1 mixture of soil and manure, with two plants per pot. Planting is done following the days to maturity of each genotype to ensure that flowering occurs simultaneously. The pests and diseases were optimally controlled until 30 days after planting.
2.3. Insect Resistance Evaluation
The resistance test of the soybean genotype against the N. viridula was conducted under the Free-Choice test (FC) dan No-Choice test (NC). The experiment of FC, as well as NC, was arranged as a randomized completely block design with three replicates. In the FC, 18 genotypes were enclosed in a large nylon mesh cage (length= 120 cm, width= 120 cm, and height= 100 cm). The infestation of N. viridula was done when plants reached the R5 stage (n=2 per plant or 36 adults N. viridula per cage). In the NC, each genotype was placed in a nylon mesh cage (50 cm in height and 26 cm in diameter) and arranged randomly according to replication. Each cage was then infested with a pair of newly emerged adult N. viridula when plants reached the R5 stage.
2.4. Observation
The data were collected on the agronomic traits in the NC as well as FC, consisting of plant height, number of branches/plant, number of nodes/plant, number of pods/plant, 100 seed weight, and seed yield/plant. The fully matured soybean pods (R8 stage) were used for the pod observation. The number of attacked pods was recorded. The pod damage intensity by N.viridula is calculated using the formula:
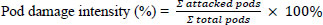 |
The level of resistance of the soybean genotypes to the pest N.viridula was classified using the Standard Deviation (SD) according to a method by Chiang and Talekar [24], as follows:
HR (Highly Resistant):
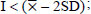 ;
;
R (Resistant):
 ;
;
MR (Moderately Resistant):
 ;
;
S (Susceptible):
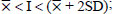 ;
;
HS (Highly Susceptible):
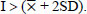
Where:
I = Pod damage intensity of each genotype
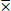 = Mean of pod damage intensity
= Mean of pod damage intensity
SD = Standard deviation
2.5. Data Analysis
The damage intensity and agronomic data were subjected to the analysis of variance, and the mean differences were compared by LSD test (α = 5%). The relationship between pod damage intensity with the agronomic traits in the FC as well as NC was investigated using Pearson’s correlation and then visualized using the Corrplot and Hmisc packages of the RStudio program version 1.3.959 [25].
3. RESULTS AND DISCUSSION
3.1. The Analysis of Variance
The resistance evaluation for the 18 soybean genotypes against the Southern Green Stink Bug (SGSB), which was performed under the Free-Choice test (FC) and No-Choice test (NC) showed a significant difference in the pod damage intensity and agronomic traits among genotypes (Table 1). The pod damage intensity of SGSB on FC and NC was significantly different, and each soybean genotype showed a range of damage intensity. The agronomic performance of each genotype in the FC and NC conditions was also significantly different, except for the number of branches. Agronomic performance among soybean genotypes differed significantly as well. This variation emerged as a result of the genetic backgrounds of the studied types differing, including the targeted agroecological variations [18].
3.2. The Classification of Resistance to N. Viridula
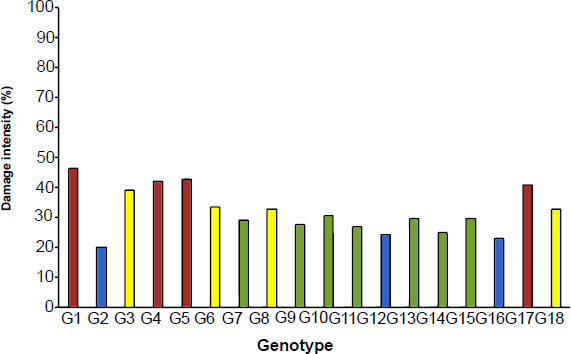
The pod damage intensity caused by N. viridula in 18 soybean genotypes under the Free-Choice test (FC) and No-Choice test (NC) were presented in Figs. (1 and 2), respectively. The intensity of pod damage caused by SGSB ranged from 20.09 to 46.40% under FC, with an average of 31.96%, and from 25.63 to 67.63% under NC. This shows that the NC condition generates 38.42% more selection pressure than the FC condition. A previous study also obtained a higher damage intensity in the NC than in the FC [2]. This was reasonable because individual insect pests have the opportunity to choose their host plant in the FC, meanwhile, the NC forced the insect pest to feed the host plant, without other choices of hosts.
| Character | Symbol | - | Mean Ssquare | CV (%) | ||
|---|---|---|---|---|---|---|
| - | Test (T) | Genotype (G) | T×G | |||
| Pod damage intensity (%) | DI | - | 21454.6453** | 777.0614** | 783.5585** | 39.35 |
| Plant height (cm) | PHG | - | 100.0416ns | 445.4632** | 249.7965** | 14.54 |
| Number of branches/plant | NOB | - | 3.6296 ns | 2.8616** | 0.8943 ns | 33.81 |
| Number of nodes/plant | NON | - | 3.6296 ns | 33.3093** | 30.5315** | 25.68 |
| Number of pods/plant | NOP | - | 4.4490 ns | 269.1843** | 187.4000** | 27.50 |
| 100 seed weight (g) | SSZ | - | 118.5777** | 40.3700** | 5.6667* | 12.29 |
| Seed yield/plant (g) | SYP | - | 3290.1977** | 73.0863** | 78.7045** | 25.70 |
Based on the pod damage intensity, the resistance level of 18 soybean genotypes to SGSB under FC (Fig. 1) was classified into four categories: three resistant genotypes, seven moderately resistant genotypes, four susceptible genotypes, and four highly susceptible genotypes. In the NC (Fig. 2), three soybean genotypes were classified as resistant, six genotypes were moderately resistant, six genotypes were susceptible, and three genotypes were highly susceptible. The check cultivar of G100H was susceptible to SGSB under NC but moderately resistant in the FC. A study reported that G100H was resistant to the pod-sucking bug R.linearis [7], and has moderate resistance to the pod pest of armyworm [26]. The G100H is the progeny from the IAC 100 and Himeshirazu cross [26]. Meanwhile, the IAC-100 has previously been found to be resistant to a variety of insects, including the neotropical stink bug complex (E. heros, N. viridula, and P. guildinii) [22].
In this study, different resistance reactions were exhibited by each soybean genotype in the NC as well as FC. Other studies also showed differences in resistance response to the stink bug among soybean cultivars [18, 27]. These differences in resistance levels between genotypes could be due to oxidative stress response and isoflavonoid production following an attack by stink bugs [3, 28], the digestive activity and organic compounds in watery saliva [12], different content of soluble leaf phenolics [29], cultivar differences [30], and the developmental stage of the plant [31] as well as the stinkbug [32]. According to a study, the pentatomid feeding habit, mouthpart morphology, and saliva all contribute to the high level of seed damage [12].
The evaluation for SGSB resistance in 18 soybean genotypes under the NC (Fig. 1) revealed a higher intensity of pod damage than the FC (Fig. 2). Three soybean genotypes (G2, G12, G16) were resistant to SGSB under the FC, meanwhile three other genotypes (G5, G10, G11) were resistant to SGSB under the NC. A study found that the resistance genotype with higher pod penetration resistance had a lower peroxide content after stink bug attack, and higher guaiacol peroxidase (GPOX), catalase (CAT), and superoxide dismutase (SOD) activities in seeds [3, 17]. Those three soybean genotypes that were classified as resistant (G2, G12, G16) in the FC were changed to highly susceptible, moderately resistant, and moderately resistant, respectively. Meanwhile, three soybean genotypes were identified as resistant in the NC, two of which changed to moderately resistant and one genotype to highly susceptible in the FC. Accordingly, the NC provides a higher resistance selection pressure than the FC, thus the soybean genotypes G10 (Anj/Rjbs-305) and G11 (Anj/Rjbs-306) were suggested as resistant genotypes to SGSB. Another study found genotypes IAC-100, V00-0742, V00-0842, and V99-1685 are less preferred by the southern green stink bug, and genotypes IAC-100, PI 558040, and V00-0870 were suggested as a source of genetic traits for resistance by reducing seed weight loss caused by southern green stink bug feeding [15]. The use of insect resistance genotypes could be an effective method to reduce the application of chemical pesticides and promote eco-friendly pest control and environmental protection.
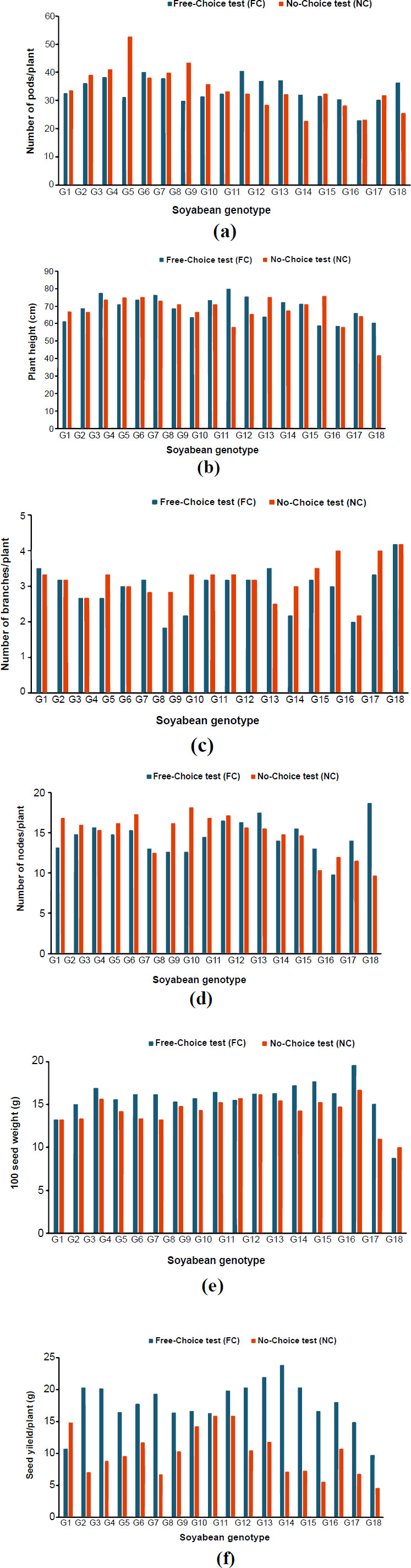
3.3. Agronomic Performance
Agronomic character performance differed between genotypes and resistance evaluation methods (FC and NC) (Fig. 3). In the FC, the average plant height was 33.80 cm, the number of branches/plant was 2.94, the number of nodes/plant was 14.56, the number of pods/plant was 33.80, 100 seed weight was 15.76 g, and seed yield/plant was 17.75 g. On the other hand, in the NC, the average plant height was 34.08 cm, the number of branches/plant was 3.20, the number of nodes/plant was 14.81, the number of pods/plant was 34.08, 100 seed weight was 14.28 g, and seed yield/plant was 9.95 g.
Yield is considered one of the most important traits of soybeans. A stink bug attack can lead to a major reduction in yield quantity and quality [12]. In this study, the susceptible genotypes had a low yield. Other studies reported that stink bugs result in fewer pods, fewer seeds per pod, smaller seeds, altered fatty acid compositions, and poorer quality soybean seeds [33, 34]. The soybean crop may also exhibit irregular growth, delayed maturity, and foliar retention as a result of a severe stink bug infestation [34]. Using their piercing-sucking stylets, stink bugs feed on soybeans by puncturing the seed pods and releasing poisonous digestive enzymes that induce tissue damage or even seed abortion [12].
A lower ratio of pod damage and seed yield are two major indicators of a plant's pest resistance [35]. The soybean genotypes G10 (Anj/Rjbs-305) and G11 (Anj/Rjbs-306) have low pod damage intensity, and a good agronomic trait, particularly for 100 seed weight and seed yield. Both of these genotypes produced large seeds, with only a minor difference in yield between FC and NC. The novelty of this research lies in the fact that in addition to being resistant to SBSB, the soybean genotypes G10 (Anj/Rjbs-305) and G11 (Anj/Rjbs-306) have the potential for high seed yield and are supported by another important characteristic, namely the relatively large seed size. Since the NC gave a higher selection pressure than the FC, the genotypes that have been shown to be resistant to NC may also develop high resistance in the field. The superior and resistant genotypes in this study will be continued to the next step of breeding. However, the evaluation of these improved breeding lines for insect resistance in field conditions for two years needs to be considered in further research.
3.4. Interrelationship Among Traits
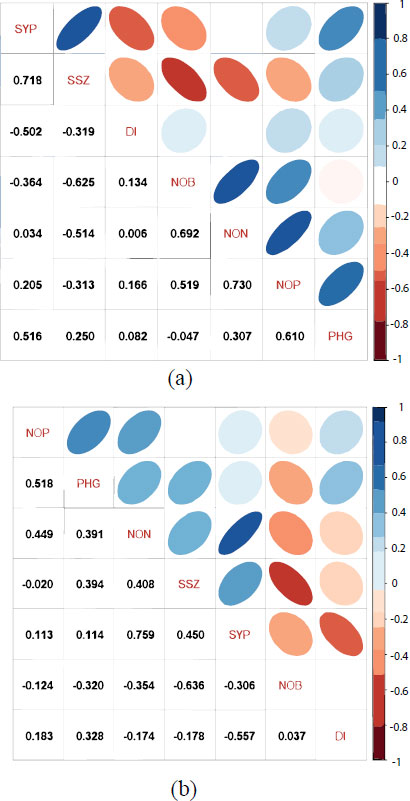
The quantity and quality of soybean seeds determine the economic value of the soybean. The stink bug attack results in a reduction in seed quality and quantity. In this study, the pod damage intensity had a significant negative correlation with seed yield in the FC (r = -0.502*) as well as in the NC (r = -0.557*) (Figs. 4). Accordingly, using SGSB-resistant cultivars will prevent soybean seed yield losses.
In the FC, seed yield was positively correlated with PHG and SSZ. The PHG significantly supported the NOP, and NOP was supported by NOB and NON. In this case, it is demonstrated that the PHG plays an indirect role in seed yield. In the NC, seed yield was only significantly determined by the NON. However, the NON was positively correlated with NOP, and the NOP has a significantly strong correlation with the PHG. A study by Kuswantoro et al. [18] found that resistance to insect pest N. viridula was significantly correlated with all agronomic characteristics, except the number of branches per plant. Resistance to N. viridula was found to have a negative correlation with days to maturity, duration of the reproductive phase, number of unfilled pods, and weight of 100 seeds. Further study in pod-sucking bug R. linearis by Adie et al. [2] found a significant negative correlation between pod damage intensity and the number of pods/plant in the NC, but there was no significant correlation in the FC. In pecky rice, there was no significant difference in the sucking frequency of N. viridula on mature husks of CRR-99-95 W and the check genotypes. These findings indicate that CRR-99-95 W has no antixenosis effect against rice stink bugs [36].
CONCLUSION
The No-Choice test (NC) provided a higher selection pressure for resistance to SGSB than the Free-Choice test (NC). The pod damage intensity had a significant negative correlation with seed yield both in the FC and NC. Soybean genotypes that are resistant under NC may develop resistance in the field. New findings of this research are two SGSB-resistant soybean genotypes Anj/Rjbs-305 and Anj/Rjbs-306, which had desirable agronomic performance (large seed size and high yield) and can be proposed as new soybean varieties developed in various soybean production centers in Indonesia.
LIST OF ABBREVIATIONS
| SGSB | = Southern Green Stink Bug |
| FC | = Free-Choice Test |
| NC | = No-Choice Test |
RESEARCH INVOLVING PLANTS
The referred plant species used in the study was not endangered.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data of current study are available from corresponding author [A.K], on a reasonable request.
FUNDING
This research was funded by the Research Organization for Agriculture and Food, National Research and Innovation Agency, Republic of Indonesia (ORPP – BRIN) under the Research Programme of Superior Crop Varieties and Livestock Breeds year 2022 with contract number 29/III.1.1/HK/2022.
CONFLICT OF INTEREST
The authors declare that there are no conficts of interest.
ACKNOWLEDGEMENTS
The authors gratefully acknowledged the Research Organization for Agriculture and Food, National Research and Innovation Agency, Republic of Indonesia (ORPP – BRIN) for the financial support.