All published articles of this journal are available on ScienceDirect.
Impact of Alien Genes on Disease Resistance, Drought Tolerance, and Agronomic Traits in Winter Wheat Commercial Varieties
Abstract
Background:
Global climate change facilitates the spread of diseases of the winter bread wheat (Triticum aestivum L.) and increases the yield losses caused by a combination of these diseases and drought. Prevention of the yield losses depends on the identification of the resistance genes and the introduction of these genes into commercial cultivars.
Objective:
The objective of the study was to evaluate resistance to widespread diseases, drought and other agricultural traits amongst the members of diversity panel consisting of introgression wheat lines derived from complex interspecies crosses.
Materials and Methods:
The field trials were conducted during 2018-2019 and 2019-2020 seasons. The winter wheat diversity panel consists of seventy-eight introgression lines and two check cultivars developed for the arid climate zone of southern Ukraine. The data on nine agronomic (quantitative scores) and five-pathogen response (point scores) traits were collected and subjected to both variance and correlation analysis to determine the contribution of the individual genetic backgrounds towards plant resiliency.
Results:
Most lines were found resistant to rust species. The distribution of responses in the analyzed lines was bimodal in response to rusts and unimodal in response to powdery mildew or septoria. The resistance traits showed no correlation with heading date, plant height, and protein content. The yield traits did not correlate with the resistance to diseases except for stem rust (Rsp = 0.34**) and Septoria blight (Rsp = −0.23*). A negative correlation was observed between quality traits and grain yield, but quality traits correlated positively with each other.
Conclusion:
High diseases resistance trait correlates with low yield, reduced protein content or small grain. The frequency of lines combining pathogen resistance as measured by total yield with grain quality is low. The introgression lines provide a source of genes for improving disease and drought resistance of winter wheat.
1. INTRODUCTION
In recent years, global climate change has resulted in the desertification of the southern regions of Ukraine and the expansion of dry climatic zone to the north. The average rainfall in April and May in the Odesa region has been recorded below the norm in 10 out of the last 20 years [1]. A reduction in the rainfall promotes the spread of the main diseases of winter bread wheat (Triticum aestivum L.) and increases the yield losses caused by both these diseases and drought [2-4]. Furthermore, an increase in temperature, especially in winter, exacerbates the spread and diversification of many pathogens. The racial composition of several wheat diseases changes, and new more aggressive and virulent biotypes (races, isolates) that overcome the resistance of existing cultivars emerge more frequently [2, 5-7]. Prevention of the devastating impact of diseases on yield depends on the development of new donors carrying the resistance genes for improving current cultivars [8]. Moreover, breeding of disease-resistant cultivars decreases pollution of the biosphere with pesticides and their decomposition products.
Among others, the introgression lines developed from interspecies crosses between commercial highly productive cultivars and different primary sources of alien origin offer rich genetic diversity for the identification of genes for disease resistance [9-11] and drought tolerance [12]. Here, we analyze the phenotypic diversity of new introgression wheat lines derived from complex interspecies hybrids and their backcrosses for resistance to widespread diseases and drought, yield and other important agricultural traits.
2. MATERIALS AND METHODS
2.1. Wheat Cultivars and Lines
The winter bread wheat diversity panel consists of seventy-eight introgression lines corresponding to different generations, degrees of saturation and origin, as well as two check cultivars for the arid climate zone (Antonivka and Kuyalnik). The lines were derived from wide hybridization of several winter bread wheat cultivars (Odesskaya 267, Albatros, Selyanka, Kuyalnik, Gurt, etc.) with a collection sample (Н74/90-245), three original introgression strains (E200/97-2, 592PH16 and Е214/09-1), as well as five amphiploid lines (AD Zhirov, ES4, ES17, ES20 and ES25) derived using Aegilops tauschii Coss. In addition, several advanced lines were obtained with the participation of the cultivar Vigen, developed from hybridization with octoploid partial wheat-Elymus amphiploid Elytricum fertile [13] or after final crossing of backcrossed hybrids with the MA1 line, which has a modified translocation of 1BL.1RSm in the genetic background of the spring variety Pavon 76 [14] (Table 1).
Line H74/90-245 was created at the Dobrudzha Agricultural Institute-General Toshevo (former Institute of Wheat and Sunflower, Bulgaria) from the cross Tom Pouce Blanc/AD (T. timopheevii Zhuk./Ae. tauschii ssp. strangulata)//Aurora/3/Rusalka [15, 16]. This line was registered under the number IU029995 by the National Center for Plant Genetic Resources of Ukraine (NCPGRU) of the V.Ya. Yuryev Plant Production Institute (Kharkiv, Ukraine). Line AD Zhirov (genome formula AuGD) was created by Dr. E.G. Zhirov in the Krasnodar Research Institute of Agriculture (Russia) by crossing T. militinae Zhuk. et Migusch./Ae. tauschii like T. miguschovae Zhir [17]. Both lines are identical in morphological traits and parameters of disease resistance. However, T. miguschovae headed 4-5 days later than AD Zhirov, had lower height and larger grains. They have NCPGRU catalog numbers UA0500015 and UA0500016, respectively [18]. Hybrids from Odesskaya 267/T. miguschovae cross and the hybrids from inverse crosses died after several backcrosses from cytoplasmic male sterility. The elite synthetic (ES) lines (T. durum Desf. cv. Altar 84/Ae. tauschii samples, ABD) were created at CIMMYT (Mexico) [19, 20] and provided by Dr. O.I. Rybalka. According to the published data and our preliminary work, all strains, lines and amphiploids, have high resistance to powdery mildew, leaf, yellow and stem rusts [13-22].
Experimental material was obtained by the Pedigree method as a result of numerous permanent individual selections, starting from the first segregating generation. Hybrid populations were screened for the studied diseases. Elite plants were selected for the presence of the disease resistance and alien morphological traits both in the backcrosses and after each self-pollination. One of the selection criteria was the consistency of both individual characteristics, including the alien traits and their complex. Consequently, some alien characteristics persisted amongst the progenies (Table 1).
| Pedigree1) | Traits 2) | Number of Lines | Type3) | Source4) |
|---|---|---|---|---|
| Od.267/AD Zhirov//Od.267*10 | Hg | 1 | NIL | [17, 18] |
| Od.267/Н74/90-245//Od.267*12/3/MA1 | Pm Lr Sr | 2 | AIL | [14, 15] |
| Kuyalnik/4/Od.267/Н74/90-245//Od.267*4/3/Selyanka | Pm Lr Yr Sr | 16 | AIL | [15, 16] |
| Kuyalnik/4/Od.267/Н74/90-245//Od.267*4/3/Selyanka/5/Zmina (or Vatazhok, or MA1) | Lr Sr | 9 | AIL | [14, 16] |
| Od.267/E200/97-2//Od.267*10/3/Kuyalnik | Lr Yr Sr | 4 | AIL | [21] |
| Selyanka/ES4 F2//Od.267 | Pm Lr Yr Iw | 1 | PIL | [19, 20] |
| Selyanka/ES17 F2//Od.267 (or Selyanka)/3/Borviy (or Gurt, or Zmina, or Vatazhok, or Selyanka) | Lr Sr Pc | 15 | PIL | [19, 20] |
| Selyanka/ES20 F2//Od.267 (or Selyanka) | Pm Lr Yr Hg Bg Iw Pc | 4 | PIL | [19, 20] |
| Selyanka/ES20 F2//Od.267 (or Selyanka)/3/Vatazhok (or Zmina, or Vigen, or Podyaka) | Pm Lr Yr Sr Bg Iw Pc | 8 | PIL | [19, 20] |
| Selyanka/ES25 F2//Albatros (or Selyanka) | Pm Lr Yr Sr Hg Pc | 3 | PIL | [19, 20] |
| Selyanka/ES25 F2//Albatros/3/Zmina | Lr | 3 | PIL | [19, 20] |
| 592PH16/Meloiya//Mudrist | Lr Hllow | 1 | AIL | |
| Е214/09-1/Borviy (or Gurt)//Gurt (or Zhaivir) | Pm Lr Yr Sr | 4 | AIL | [22] |
| Е214/09-1/Gurt*2//Vatazhok (or Vigen) | Lr | 4 | AIL | [22] |
| Vigen/Od.267//Selyanka | Lr Yr Sr | 2 | BL | [13] |
| Vigen/Mudrist//Shchedrist/3/Nasnaga | Lr Yr Sr | 1 | BL | [13] |
2)Hg – hairy glume; Bg– brown glume; Pc – purple calm; Iw – waxy absence; Hllow – pubescence of the lower surface of the leaves; Pm, Lr, Yr, Sr – different levels of resistance, respectively, to powdery mildew, leaf, yellow and stem rusts.
3)NIL – near isogenic line, PIL – primitive introgression line, AIL – advanced introgression line, BL – breeding line.
4) Study reporting the status, pedigree or cross combination of the alien sources.
2.2. Field Experiments
The field experiments were performed in the experimental fields of PBGI–NCSCI. Yield, morphological traits, and resistance to biotic and abiotic stresses were evaluated in the control nursery at the institute experimental base “Dachna” during 2018-2019 and 2019-2020 seasons. Resistance to common diseases was studied under natural infection environments in the nursery in 2016-2020. In parallel, the leaf and stem rust resistance was assessed against provocative backgrounds in an infectious field nursery of the Department of Phytopathology and Entomology. In the control nursery, sowing was carried out by a breeding tractor seeder SSFK-7 without replication; the estimated plot area was 10 m2 at the rate of 5 million germinating grains/ha. Standard cultivars were sown in two replicates every 20 plots. Fertilizers were applied as follows: 1) 150 kg/ha of nitroammophoska was applied during pre-sowing cultivation; 2) early spring root fertilization was carried out with ammonium nitrate at a dose of 150 kg/ha; 3) foliar feeding was carried out by spraying a tank mixture using urea at the rate of 7.7 kg/ha of the nitrogen-active substance. The harvesting was performed with “Sampo-130” combine. In the infectious nursery, the material was sown in 1-row plots with row length 1.15 m and feeding area of an individual plant of 30 × 5 cm2.
2.3. Plant Disease Resistance
All the genotypes were screened for widespread diseases both in control and in infectious nurseries. Adult plant disease susceptibility was examined using powdery mildew (Blumeria graminis (DC) Speer f. sp. tritici March.), leaf (Puccinia triticina Erikss. & Henn.), stem (Puccinia graminis sp. tritici Erikss. & Henn.) and stripe (Puccinia striiformis West.) rusts, as well as wheat septoria leaf blotch (Septoria tritici Rob. ex Desm.). However, the resistance against artificial provocative pressures was assessed only for leaf and stem rusts; the reaction to other diseases was evaluated on natural backgrounds. Plant inoculation was performed in an infectious field nursery at the growth stage (GS) of ear formation (GS49 according to the Zadoks scale [23]). Racial composition of rust species, terms and methods of inoculation has been described earlier [6, 24].
The impact of pathogens on plants was evaluated using the 9-point unified integrated COMECON scoring scale [6, 24, 25], where 1=very susceptible and 9=immune. For heterogeneous infections, the score for each line was converted to the individual scale of moderate susceptibility or moderate resistance (4-6 points) depending on the severity of the race virulence. This scale is based on modified Saari and Prescott (0-9) single-digit scoring scale [26] but in reverse order. It allows estimating adult plant response to mildew and septoria on the basis of the vertical progression of the pathogen [27] in reverse point order), and to rusts on the basis of the top leaves damage intensity (disease severity) [28]. The scale is adopted in Ukraine [6, 11, 28] from the former scales used in the USSR [10], COMECON [24], and some other countries [29]. This scale allows calculating the pathogen response in points using common statistical methods [24, 30]. Plant pathological appraising under both natural and artificial infection conditions was assessed at the phase of maximal disease development. The Zadoks developmental growth stage [23] of lines evaluated under infection pressure ranged between GS55-57 (i.e., ear half emergence to ear three quarters emergence stage) for the mildew, GS73-75 (early milk stage - milk ripe) for the septoria, leaf and stripe rusts, and GS77-83 (end of milk maturity - soft dough stage) for the stem rust, respectively (Table 2).
2.4. Drought Resistance and Laboratory Methods
Although the 2019 growth season was characterized as drought [1], the total rainfall was 172 mm. The maximum amount of precipitation was recorded as 55 mm in January 2019. This generated sufficient soil moisture for the growth and development of plants. The total precipitation during winter 2020 was 26 mm. Furthermore, almost no precipitation occurred in the spring of 2020. Thus, the drought resistance index was calculated as the fraction of yield in acute drought conditions in 2020 growth season relative to the yield in relatively good rainfall season of 2019.
The protein content was determined by the Kjeldahl method [31]. The weight of a thousand kernels (WTK) was measured using the standard method (DSTU 4138-2002) [32] with two portions in 500 kernels. The test (hectolitre) weight was determined on the same 1000 kernel samples. Flour density was detected using the flour samples that were prepared for the protein content determination. To select lines with high protein content, additional protein criteria were calculated: “Yield of protein per unit area” [t/ha] = (“Yield of dry grain”, t/ha × “Protein content in grain”, %) / 100% and “Absolute protein content per 1000 grains” [g] = (“WTK”, g × “Protein content”,%) / 100% as reported earlier [33, 34]. Their use will eliminate the variance of the protein content due to variations in the anatomical structure of the grain or plant productivity under the influence of environmental factors. The designations of diseases and morphological traits in the tables and text are given in accordance with the International Catalog of Gene Symbols for Wheat [35].
2.5. Statistical Analyses
Data were subjected to analysis of variance and correlation analysis using the STATISTICA program package. Before statistical analysis, the weight of 500 kernels was converted into values for 1000 kernels to get two WTK replications for ANOVA. Fisher's F criterion, LSD0.05 test and standard deviation (SD) were performed to compare the means. The mean values (M), standard deviation (SD), and limits of variation (LV) were used to analyze the distributions of values. The linear correlation coefficients (Pearson’s r) for quantitative scores and non-parametric coefficients (Spearman’s Rsp) for point scores were calculated using overall values per lines. To simplify the material presentation, the unified common notations of the degree of significance of our defined or taken from the literature indicators, criteria and coefficients have been reported in the tables and text: *, ** and *** – significant at the 0.05, 0.01 and 0.001 probability levels, respectively.
3. RESULTS
Most lines were resistant to rust species with average resistance scores of 5.0-5.9 points according to the prescribed 1 to 9 scale (Table 2). This resistance was due to the successful introgression of alien Lr, Yr and Sr genes from all sources involved in hybridization. A higher long-term resistance to stem rust was primarily observed among the derivatives of line H74/90-245 from Bulgaria. The derivatives of sample H74/90-245 include introgression lines developed from crosses with strains E200/97-2, 592 PH16 and E214/09-1 (Table 1). In the primary introgression strains E200/97-2, H242/97-1 and E125/03, which were the parents for those crosses, the wheat-rye translocation 1BL.1RS of type Caucas/Aurora from sample H74/90-245 was determined using electrophoresis of storage protein [21]. The translocation is likely inherited by their progeny – advanced lines. The 1RS arm of this translocation is known to carry the Lr26/Sr31/Yr9/Pm8 gene complex [35]. Although the effectiveness of the resistance genes in this locus is partly lost due to the emergence of new races of pathogens, some positive effect of 1BL.1RS on the economically valuable and adaptive traits depends on the genetic background of the line and on climate [36]. In particular, the Sr31 gene was reported effective for pathogen resistance in Ukraine [9].
Among the derivatives of amphiploids generated using Ae. tauschii, the disease tolerant lines were rare. The majority of stem rust-resistant lines scored as resistant (7-8 points) at the milk phase GS75-77 gradually lost the resistance during grain maturation stage with the score 2-4 points up to GS90-91. Only several lines showed resistance to powdery mildew, but this trait depended on the weather conditions. None of the analyzed lines showed tolerance to septoria; the best lines showed moderate susceptibility (4-5 points) relative to the standards. This outcome contradicts the reported high resistance to septoria leaf blotch among introgression lines in the Forest-Steppe of Ukraine [11]. The bimodal distribution of the response to all types of rust suggests that resistance to the rust species is controlled by several major gene loci. On the contrary, the unimodal distribution of resistance to powdery mildew and septoria with a high proportion of intermediate points and low values of standard deviation (Table 2) suggests polygenic mechanisms determined by a positive transgression and originates from multiple minor sources of resistance. Unlike resistance to rusts, crossing cannot easily transfer such traits.
Yield analysis demonstrated that high diseases resistance traits correlated with low yield, reduced protein content, or small grain. Thus, there is a non-linear relationship between disease resistance and the agronomical traits. Several lines with a high WTK or protein content retained a set of target traits of the group resistance and exceeded the standards in individual traits or their complexes. However, the frequency of lines with the group resistance, high yield and grain quality was low (3.8%).
| Disease2) | GS3) | % Lines with Reaction (Points) | Statistical Parameters4) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Susceptible | Resistance | |||||||||
| 1-2 | 3-4 | 5 | 6-7 | 8-9 | M | SD | LV | RSp (F) | ||
| Pm | 55-57 | 2.4 (6.70)5) |
46.3 (6.53) |
26.8 (6.61) |
23.2 (7.18) |
1.2 (4.20) |
4.6 (6.70) |
1.33 (0.86) |
2-8 (4.20-8.19) |
0.08 (0.95) |
| Lr | 73-75 | 2.4 (6.16) |
36.6 (7.17) |
12,2 (6.44) |
62.2 (6.70) |
7.3 (6.55) |
5.5 | 1.60 | 2-8 | -0.05 (0.53) |
| Yr | 73-75 | 2.4 (6.40) |
50.0 (6.74) |
8.5 (6.64) |
39.0 (6.71) |
- | 5.0 | 1.52 | 2-7 | -0.01 (0.14) |
| Sr | 77-83 | 3.7 (5.36) |
25.6 (6.63) |
6.1 (6.49) |
37.8 (7.44) |
26.8 (7.60) |
5.9 | 1.88 | 2-8 | 0.34** (1.87) |
| Stb | 73-75 | 1.2 (6.13) |
93.9 (6.75) |
4.9 (5.36) |
- | - | 3.6 | 0.60 | 2-5 | -0.23* (2.65) |
The resistance traits did not correlate with heading date, plant height (except for stem rust, Rsp=-0.33*), and protein content. The yield traits also did not correlate with the resistance to powdery mildew or leaf and stripe rust (Table 2). A weak positive correlation (Rsp=0.34**) between yield and stem rust resistance could be due to the positive effect of the 1BL.1RS translocation on both traits. This fact suggests that the genetic background of some introgression lines was favorable for generating the positive effect of the 1BL.1RS translocation on both yield and adaptive traits in Southern Ukraine. It is also likely that 1BL.1RS translocation worked cooperatively with other resistance loci. The correlation between yield and resistance to Septoria blight was somewhat negative (Rsp=- 0.23*). The WTK showed low but significant correlation with the resistance to powdery mildew (Rsp=0.31**), stem rust (Rsp=-0.26*) and Septoria blight (Rsp=0.31**). Also, resistance to stem rust correlated with the protein yield (Rsp=0.37***), test weight (Rsp=0.55***) and flour density (Rsp=-0.46***). The latter could be the consequence of the 1BL.1RS influence.
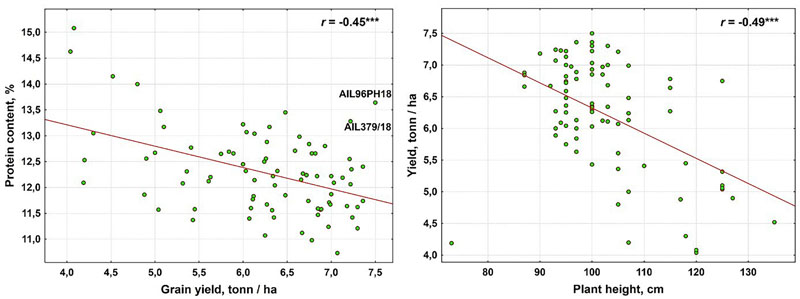
Although the protein content correlated negatively with grain yield (r=-0.45***), lines AIL379/18 and AIL96PH18 (Kuyalnik/4/Od.267/Н74/90-245//Od.267*4/3/Selyanka/5/Zmina) scored high values of both traits (Fig. 1a). A negative linear dependence was also observed between other quality traits and grain yield, but the grain quality traits correlated positively with each other except for the test weight with the volume of 1000 kernels and the test weight with flour density. The plant height exhibited the highest contribution to grain yield (r=-0.49***), followed by the volume of 1000 kernels (r=-0.29**) and WTK (r=-0.23*), respectively. However, the relationship between plant height and yield was not linear (Fig. 1b). The maximum yield was in line with plant height ca.100 cm, whereas greater or lower plant height was accompanied by lower yield. No correlation was detected between the date of heading and other traits (Table 3) despite the rather vast variation of this trait (14 to 19 May).
According to the 2018/19 data, fifteen introgression lines (AIL341/18, AIL345/18, AIL347/18, AIL353/18, etc.) with a different level of resistance to powdery mildew or rusts were isolated and sown for the next season. The lines combined a high breeding performance with productivity at or above the nearest standard level in a more favorable environment of 2019 (Table 4), and none showed the alien morphological traits. Ten lines were the advanced lines (six or more crosses to modern cultivars) derived from the line H74/90-245. Based on the apparent resistance to stem rust, these lines are likely to carry the 1BL.1RS translocation from cv. Aurora.
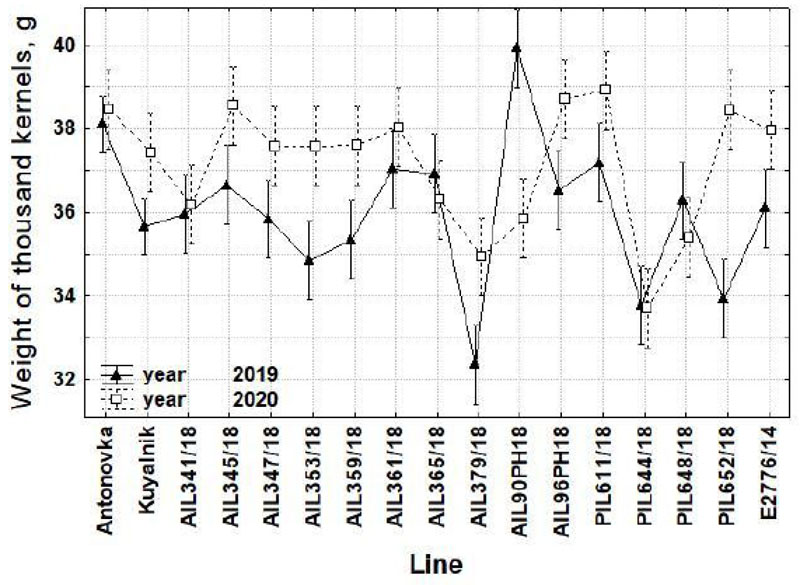
An analysis of variance indicated the effect of the year to be significant on grain yield (F(1;18)=416.7***), WTK (F(1;16)=19.7***) and protein yield (F(1;18)=214.2***). The grain yield was not significantly affected by the factor ‘Line’, and most traits were affected by the environmental conditions rather than by the genotype. Interaction of ‘Line’ × ‘Year’ was significant for WTK (F(16;38)=4.5***) with a change of ranks in some lines. Although the differences between the line values obtained in different years were insignificant, WTK was higher in the drought season of 2020 in the majority of lines (Fig. 2), which led to significant differences between the years (35.7 g in 2019 and 37.2 g in 2020). Protein yield was much higher in 2019 (8.50 t/ha vs. 5.12 t/ha in 2020) because of the grain yield, which was also much higher in the more favorable climate of 2019 (Table 4 and 5).
The protein content in dry 2020 was seemingly lower (12.0% in 2019 versus 11.7% in 2020), but the differences between the values in each year were not statistically significant. The only outlier was line E2776/14, for the yield (5.21 t/ha) and protein content (13.2%) were the highest amongst all lines in 2020. The same tendency was observed for values of absolute protein content per 1000 kernels (data not shown).
| Traits | Date of Heading | Plant Height | Yield | WTK1) | VTK1) | Test Weight | Protein Content |
|---|---|---|---|---|---|---|---|
| Plant height | -0.19 | ||||||
| Yield | 0.14 | -0.49*** | |||||
| WTK | -0.10 | 0.25* | -0.23* | ||||
| VTK | -0.11 | 0.29** | -0.29** | 0.85*** | |||
| Test weight | 0.03 | -0.14 | -0.18 | 0.07 | -0.47*** | ||
| Protein content | -0.06 | 0.35** | -0.45*** | 0.21 | 0.02 | 0.32** | |
| Flour density | -0.21 | 0.26* | -0.23* | 0.28* | 0.38*** | -0.28* | -0.10 |
| Line | Resistance to Diseases (Points)1): |
Common Performance Points |
Yield t/ha |
Test (Hectolitre) Weight kg/hl |
Flour Density kg/m3 |
||||
|---|---|---|---|---|---|---|---|---|---|
| Pm | Lr | Yr | Sr | Stb | |||||
| Antonivka (St) | 3 | 3 | 3 | 1-2 | 4 | 4+ | 6.93 | 71.2 | 568 |
| Kuyalnik (St) | 3 | 4 | 4 | 2-3 | 3 | 4 | 6.89 | 72.5 | 608 |
| AIL341/18 | 4 | 6-7 2) | 7 | 6-8 | 4 | 5 | 7.21 | 78.2 | 549 |
| AIL345/18 | 6 | 7 | 4 | 7-8 | 3 | 4+ | 7.30 | 76.4 | 582 |
| AIL347/18 | 6 | 7 | 4-7 | 7-8 | 3 | 4+ | 7.22 | 79.6 | 553 |
| AIL353/18 | 6 | 6-7 | 4 | 7-8 | 3 | 4- | 7.23 | 77.5 | 603 |
| AIL359/18 | 5 | 6-7 | 5 | 7-8 | 3 | 4- | 7.13 | 78.5 | 563 |
| AIL361/18 | 6 | 6-7 | 5 | 7-8 | 3 | 5+ | 7.24 | 77.2 | 573 |
| AIL365/18 | 6 | 6-7 | 5 | 6-8 | 3 | 4- | 7.03 | 78.6 | 569 |
| AIL379/18 | 6-7 | 4-6 | 7 | 6-7 | 3 | 5- | 7.22 | 77.0 | 592 |
| AIL90PH18 | 6-7 | 5-7 | 7 | 7-8 | 5 | 5 | 7.07 | 75.3 | 611 |
| AIL96PH18 | 4-7 | 4-6 | 7 | 7-8 | 4 | 4 | 7.50 | 77.7 | 552 |
| PIL611/18 | 4 | 6-8 | 3 | 5-6 | 3 | 5- | 6.93 | 75.9 | 553 |
| PIL644/183) | 4 | 4 | 3 | 7-8 | 4 | 4+ | 6.88 | 76.8 | 530 |
| PIL648/18 | 4 | 6-8 | 3 | 6-8 | 3 | 5- | 7.00 | 75.9 | 616 |
| PIL652/18 | 4 | 5-7 | 4 | 7-8 | 3 | 5+ | 6.84 | 75.4 | 537 |
| E2776/14 | 5 | 4-6 | 5-6 | 3-6 | 4 | 4+ | 6.89 | 75.2 | 572 |
| SD4) | - | - | - | - | - | - | 0.88 | 3.0 | 44 |
Test weight correlated with the grain size and shape (r=-0.47*** between test weight and 1000 kernel volume) and protein content (r=0.32**), and did not depend on WTK (r=0.07). This outcome is consistent with the fact that small round grains with high protein content could be packed better. Quite notable differences between the genotypes were observed in flour density (LV=509 to 718 kg/m3). Noteworthy, despite the weak negative correlation between the yield and flour density (r=-0.23*), selection for this trait would likely increase WTK and the line yields, regardless of the grain size.
The lack of rain in spring 2020 shortened the heading time by an average of 4.4 days (p<0.001), reduced the plant height by 10 cm, and caused a 3.6 t/ha decrease in yield relatively to 2019. Average grain yield reduction (the drought resistance index) in 2020 was 24.4% to 50.7% lower than in 2019 for the experimental lines, and 25.3% (Kuyalnik) or 33.4% (Antonivka) lower for the standards (Table 5). As the drought resistance index is inversely proportional to the yield values, the lines with lower yield in 2019 had the smallest yield reduction, and thus, were classified as drought resistant. As a result, three promising drought-tolerant lines Е2776/14 (Vigen/Od.267//Selyanka), PIL644/18 and PIL652/18 (Selyanka/ES17 F2//Od.267 F4/3/Gurt) were selected. Although these lines did not exceed the yield standards in 2019, Е2776/14 showed the highest protein content in 2020 (13.2%), and PIL644/18 and PIL652/18 were resistant to stem rust (Table 4).
4. DISCUSSION
In the history of wheat breeding, disease and drought-resistant germplasm, such as wheat-rye 1BL.1RS translocation, have made outstanding contributions [10, 37]. Our work also shows the value of using derivatives of the line H74/90-245 for generating advanced introgression lines combining genes resistant to fungal pathogens localized in wheat-rye translocation 1BL.1RS with complexes of effective resistance genes from other sources. In addition, productivity and yield traits in lines with the translocation 1BL.1RS are greater relative to other introgression lines.
The positive effect of the 1BL.1RS translocation on yield, adaptive traits, and the effect of other resistance genes have been reported in several genetic backgrounds. For example, the effective disease resistance genes Yr9 and Sr31were introduced into PBGI cultivars by combining the intact 1BL.1RS or modified 1BL.1RSm (carrying two interstitial segments from wheat chromosome arm 1BS into the 1RS arm) translocations with the high-quality allele Glu-B1al [38, 39]. In addition, a recombination of resistance genes from the 1RS chromosome of different rye cultivars was reported [40]. However, the resistant lines derivative from the strain MA1 and probably having the 1BL.1RSm translocation showed reduced grain yield in the field, relative to the introgression lines with the complete 1RS arm. In agreement with this view, a short segment of wheat 1BS chromosome in the distal region of the 1RS arm was shown to be associated with reduced grain yield, biomass, root length and canopy water status [41, 42], caused by triplicated gene dosage of 1BS insertion collinear with the 1RS duplication [43]. Thus far, only a few disease-resistant genes from the 1BL.1RS translocation have been utilized in wheat breeding. At the same time, wheat alien species provide rich sources of genetic variability. The germplasm with varying amounts of the alien chromosome material offers new opportunities for improving disease resistance.
| Line |
Date of Heading May |
Plant Height cm |
Grain Yield t/ha |
Drought Tolerance Index % |
Weight of Thousand Kernels g |
Protein Content % |
Protein Yield t/ha |
Protein Content Per 1000 kernels g |
|---|---|---|---|---|---|---|---|---|
| Antonivka (St) | 15 | 96 | 5.77 | 33.4 | 38.3 | 11.3 | 6.57 | 4.28 |
| Kuyalnik (St) | 14 | 100 | 6.01 | 25.3 | 36.6 | 11.4 | 6.84 | 4.22 |
| AIL341/18 | 16 | 101 | 5.74 | 40.6 | 36.1 | 11.7 | 6.84 | 4.31 |
| AIL345/18 | 14 | 95 | 5.79 | 41.5 | 37.6 | 11.4 | 6.61 | 4.27 |
| AIL347/18 | 14 | 92 | 5.39 | 50.7 | 36.7 | 11.8 | 6.40 | 4.32 |
| AIL353/18 | 15 | 90 | 5.49 | 48.3 | 36.2 | 11.9 | 6.59 | 4.29 |
| AIL359/18 | 15 | 93 | 5.69 | 40.4 | 36.5 | 12.4 | 7.02 | 4.53 |
| AIL361/18 | 15 | 90 | 5.65 | 43.9 | 37.5 | 11.9 | 6.65 | 4.48 |
| AIL365/18 | 15 | 95 | 5.81 | 34.7 | 36.6 | 11.7 | 6.83 | 4.27 |
| AIL379/18 | 14 | 94 | 5.78 | 40.0 | 33.6 | 12.9 | 7.51 | 4.34 |
| AIL90PH18 | 14 | 84 | 5.54 | 43.4 | 34.3 | 10.8 | 5.97 | 4.10 |
| AIL96PH18 | 15 | 96 | 5.99 | 40.3 | 37.6 | 12.6 | 7.72 | 4.74 |
| PIL611/18 | 14 | 92 | 5.67 | 36.5 | 38.1 | 12.7 | 7.21 | 4.83 |
| PIL644/181) | 14 | 90 | 5.89 | 28.8 | 34.0 | 11.1 | 6.60 | 3.89 |
| PIL648/18 | 16 | 93 | 5.63 | 39.1 | 35.8 | 12.0 | 6.79 | 4.30 |
| PIL652/18 | 15 | 82 | 5.71 | 33.2 | 36.2 | 11.8 | 6.76 | 4.25 |
| E2776/14 | 15 | 91 | 6.05 | 24.4 | 37.0 | 12.4 | 7.43 | 4.32 |
| SD2) | 1.8 | 12 | 1.39 | - | 1.7 | 0.7 | 1.83 | 0.27 |
| LSD0.05 | 1.8 | 10 | 0.83 | - | 1.0 | 1.3 | 1.47 | 0.48 |
We found a negative correlative between the grain yield and plant height, volume and weight of 1000 kernels (Table 3). It means semi-dwarf and small-grained genotypes were more productive (Table 5). Several disease tolerant introgression lines approached or exceeded the standards for grain yield and protein content. Sometimes, using our cultivars in the hybridization programs generated lines in which greater material productivity was accompanied by grain size reduction. This contradicts the previous report where an increase in yield was achieved, partly by grain enlargement (WTK up to 50 g or more) [44]. Overall, introgression lines have been widely studied with regards to different valuable traits in Ukraine [11, 30] and in the world [12, 45]. These lines were used: (i) to study theoretical aspects of genome evolution, mapping genes, alien trait inheritance or stability in wheat background [9, 10], [40, 46]; and (ii) to diversify the genetic variability of wheat and increase the efficiency of breeding [13, 37, 47, 48]. Hybridization with distant relatives contributed to the transfer of genes for disease resistance [7, 22, 48, 50, 51], pests tolerance [52], and adaptation to abiotic stress factors [11, 12, 53] of bread wheat. To date, the officially designated genes originating from wheat alien species include 17 Yr genes, 35 Lr gens, 30 Sr genes, 41 Pm genes and one Septoria tritici blotch resistance gene [45]. The number of results with the successful use of the obtained hybrids indicates the potential of this approach for improving winter wheat in the southern regions of Ukraine [47].
Introgression lines exceeding the standards were isolated in several breeding programs, and since then, they have become commercial cultivars [6, 8, 37, 51]. In this work, 15 of the 78 lines with disease resistance genes exhibited satisfactory yield traits in the favorable environment of 2019, but only 1 line exceeded the nearest standards in drought season of 2020. 3 lines of the 15 could be classified as drought-resistant, 2 were also resistant to diseases, and 1 was a high-grain protein content line. The lines are devoid of many negative traits that are inherent to the wild species, and as such, they are a promising source of resistance genes to diseases or drought for the ongoing breeding programs.
CONCLUSION
As a result of crosses and backcrosses of different primary sources of alien traits with modern wheat cultivars, breeding introgression lines with alien genetic complexes for resistance to diseases, high protein content and morphological traits were obtained. A high long-term resistance to stem rust was observed mainly among the derivatives of line H74/90-245. Among the derivatives of Ae.tauschii, the majority of stem rust-resistant lines gradually lost the resistance during the grain maturation stage. Frequently, the high diseases resistance correlated with low yield, reduced protein content or small grains. The 1BL.1RS translocation has a positive effect on both productive and adaptive traits in several lines carrying other resistance genes.
The resistance traits lacked correlation with the heading date, plant height and protein content. Only stem rust resistance correlated negatively with the plant height. The yield traits did not correlate with the resistance to powdery mildew and leaf or stripe rusts. The WTK correlated with the resistance to powdery mildew, stem rust and Septoria blight. Furthermore, resistance to stem rust correlated with the grain and protein yield, test weight and flour density. Protein content correlated negatively with grain yield, but the yield quality traits correlated positively with each other except for the test weight-volume of 1000 kernels and test weight-flour density. The plant height had the highest contribution to grain yield, followed by a volume of 1000 kernels and WTK, respectively.
Fifteen advanced introgression lines (AIL341/18, AIL345/18, AIL347/18, AIL353/18, etc.) combined resistance to powdery mildew or rusts with productivity at or above the standard level in a favorable environment. Three promising lines Е2776/14, PIL644/18 and PIL652/18 were drought-resistant relatively to the standards.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This work was supported by CRDF Global Grant FSA3-19-65504-0.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Dr. O.A. Vasilyev for helping in the creation of artificial infection pressure of rust diseases.


