All published articles of this journal are available on ScienceDirect.
Evaluation of Heat Distribution for the Diagnosis of the Hoof with Abscess by Infrared Thermography in Horses
Abstract
Background:
The study of normal temperature distribution for the diagnosis of hoof disease in horses using Infrared Thermography (IRT) is rare, therefore, the normal temperature distribution is to be investigated. In this study, we investigated the possible use of IRT in the diagnosis of hoof disease in horses.
Methods:
A total of 66 horses (56 healthy horses and 10 horses with hoof abscess) were used in this study. Veterinarians and farriers performed physical examinations, such as lameness examination, palpation, hoof test, and radiography, on all horses. IRT was performed in standard conditions. The plantar of the hoof was lifted upward by the owner, and the distance of the thermal camera between the plantar of the hoof was 0.3 - 0.5 m. For evaluation, the heat pattern of the plantar of the hoof was divided into seven regions of interest (ROIs), and statistical analysis was performed.
Results:
On performing IRT, the temperature of healthy hooves and hooves with hoof abscess was found to be 25.77 °C ± 3.87 °C (mean ± SD) and 31.94 °C ± 2.60 °C, respectively (p < 0.0001). The mean temperature of ROIs in hooves with abscesses was 6.17 °C higher than that of healthy hooves. Sole regions were found to be especially prominent. Additionally, all ROIs of hooves with hoof abscess classified IRT with high sensitivity (74.3%) and specificity (87.5%).
Conclusion:
The present study demonstrated the usefulness of IRT in diagnosing hoof with abscess. Moreover, this study suggests that IRT may be useful as a new temperature measurement analysis system in terms of determining differences in the heat distribution of the hoof.
1. INTRODUCTION
The hoof complex comprises the hoof capsule, sole, frog, digital cushion, ungual cartilages, and the deep digital flexor tendon [1, 2]. These biological structures are susceptible to trauma and various disease processes, including infections (hoof abscesses, puncture wounds, and keratomas), white line disease, and canker. These diseases generally originate in the foot’s ground surface, penetrate the hoof capsule, and extend toward/into the foot’s dermal structure [1]. Many factors may play a role in the development of hoof diseases or abscesses, including the environment, hoof quality, trauma, and shoeing conditions [1].
Hoof abscesses are probably the most common cause of acute severe lameness in horses encountered by veterinarians and farriers [1]. In particular, the soles of horses that exercise a lot on stony ground are often wounded by sharp pebbles. Such wounds may suddenly result in severe (acute) lameness. It is difficult to diagnose because the degree of lameness varies from being subtle in the early stages to non-weight bearing [1]. Hoof abscess, defined as the focal accumulation of purulent exudate between the keratinized and germinal layers of the epithelium, is often associated with a defect in the white line [1]. Bacteria or foreign material that penetrate the stratum corneum induce an inflammatory response that causes the abscess [1]. Surgical treatment and medication are combined in the treatment of hoof abscess, which often includes paring out the abscess to establish drainage and the use of poultice to promote draining [1, 3].
Shin et al. recently reported the prevalence of hoof disorder in racing and riding horses in South Korea, including thrush 4.2% (122/2,891), superficial hoof wall cracks 1.2% (35/2,891), white line lesions 1.0% (29/2,891), hoof wall separation 0.6% (17/2,891), hoof wall defect 0.5% (14/2,891), laminitis 0.3% (8/2,891), and wounds 0.2% (6/2,891) [4].
Infrared Thermography (IRT) has the advantage of enabling a quicker and more accurate diagnosis of physiological changes in the region of interest (ROI) compared with conventional imaging techniques [5]. IRT has been used as a diagnostic tool in the veterinary field since the mid-1960s. This technique has been used mainly for the diagnosis of fractures and ruptures in the orthopedic field [6-8]. IRT is a non-contact, non-invasive diagnostic technique, which measures the physiological function by recording the exothermal status of the emitted infrared rays [9-11]. The skin temperature depends on the underlying tissue metabolism and local blood circulation. Therefore, the changes in physiological activity result in abnormal thermal patterns. Alterations in cutaneous blood flow and sweat patterns occur in the case of overload and inflammation [12]. Despite the various benefits of IRT, few studies have been reported that were used to diagnose hoof-related diseases with IRT.
The study of normal temperature distribution for the diagnosis of hoof disease of horses using IRT is rare therefore, we aimed to investigate whether IRT could aid in the diagnosis of hoof disease in horses; we also aimed to propose a new temperature measurement analysis system in terms of detecting differences in the heat distribution of the hoof.
2. MATERIALS AND METHODS
2.1. Horses
A total of 66 horses [56 (40 thoroughbreds and 16 ponies, 34 geldings and 22 mares, 2 – 16 years old) healthy horses and 10 thoroughbred horses (8 geldings and 2 mares, 2 – 4 years old) with hoof abscess] were used in this study. The diagnosis of hoof disease was performed by veterinarians and farriers using radiography and hoof tester, etc., at the KRA equine hospital. All horses were kept in stables and fed high-quality feed during the experiment.
2.2. IRT and Radiography
Radiography was carried out according to the general standards, and thermography was performed according to the methods described by Soroko and Howell [13], and Yang et al. [14]. First, the imaged area was brushed, and dirt and mud were removed, if present, 1 h before the examination. For minimizing the risk of disrupting factors, the examination was taken in an enclosed area 15 min after brushing, which ensured that the transient heat generated by brushing had subsided [13]. For calming the horse, a 0.8 mL dose of Detomidin (Provet Veterinary Products LTD., Turkey) was administered. The camera was calibrated to the ambient temperature; the temperature measurement was adjusted to degree Celsius, and the distance was adjusted to meters. At this time, the emissivity was 0.97, the ambient temperature ranged between 26.4°C and 33.8°C, and the examination area was blocked from outside wind and direct sunlight. The plantar of the hoof was lifted upward by the owner, and the distance of the thermal camera (T420, FLIR systems Inc., Oregon, USA) was kept from the plantar surface of the hoof in the range of 0.3 – 0.5 m.
2.3. Data Analysis
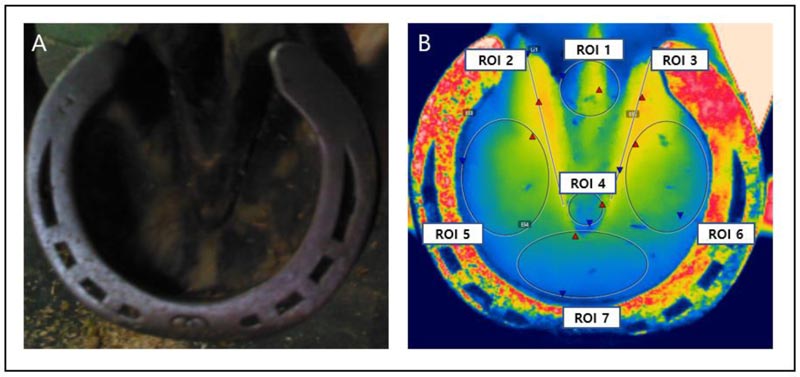
The heat pattern of the hoof area was evaluated using images taken of those hooves that were healthy and those diagnosed with hoof abscess and divided into seven regions of interest (ROI 1 – 7) (Fig. 1). The first ROI represented the location of the central cleft. ROI 2 and ROI 3 reflected the lateral clefts on the medial and the lateral, respectively. ROI 4 reflected the frog, and ROI 5, ROI 6, and ROI 7 reflected areas of the sole (medial, lateral, and middle between the medial and lateral regions, respectively). Each horse had different hoof sizes, but the ROIs covered as much of the area as possible. The average temperature for each area was calculated using specialized software (FLIR Tools Professional, FLIR Systems Brazil, Brazil). The thermal images were analyzed by a rainbow color scheme, and the temperature assigned to each color was uniform for all images.
The estimation of the main trends of basic ROI temperatures for all 66 plantar was performed using an unpaired t-test with Welch’s correction or the Mann–Whitney test. ROI temperatures were also compared for grouped data using The Kruskal–Wallis test with Dunn’s multiple comparisons test to be able to discuss the data obtained in the context of relevant scientific articles. Sensitivity and specificity were measured using receiver operating characteristics (ROC) curves. The sensitivity (%) reflected the ability to detect a hoof abscess given that an abscess was present, while specificity (%) estimated the ability to avoid classifying a healthy hoof as a hoof with abscess. All statistical analyses were performed using SPSS software (version 25, IBM SPSS Statistics, USA), where probability (p) values < 0.05 were considered to indicate statistical significance.
3. RESULTS
3.1. Hoof Temperature
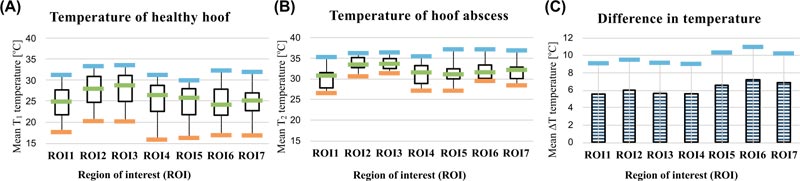
ROI temperature data gathered from all 66 plantar along with differences in temperature data with respect to each part of the hoof are compared in Table 1. The overall temperature of healthy hooves (n = 56) and hooves with hoof abscess (n = 10) as measured by IRT was 25.77 °C ± 3.87 °C (mean ± SD) and 31.94 °C ± 2.60 °C, respectively (p < 0.0001; Table 1).
The temperature of ROIs 2 and 3 was higher in both types of hooves because the lateral cleft was closer to the depths in the hoof. The mean temperature of ROIs in hooves with abscess was 6.17 °C higher than that of healthy hooves, a finding that was especially prominent for ROIs 5-7. Meanwhile, the mean ROI temperature was significantly higher (p < 0.0001) in hooves diagnosed with hoof abscess than healthy hooves (Table 1).
A comparison of ROIs according to hoof temperature of healthy hooves and hooves with abscess is displayed in Figs. (2a and 2b). Temperature differences (ΔT) between the ROIs of healthy hooves and hooves with hoof abscess are displayed in Table 1 and Fig. (2c). ΔT values were significantly higher in ROI 5 – 7 compared with those of other ROIs; the highest ΔT was observed for ROI 6.
3.2. Feasibility of IRT in the Detection of a Hoof Abscess
The ability of IRT to predict a hoof abscess according to ROI classification is summarized in Table 2. In general, the cut-off value was higher in the affected area (lateral cleft and sole regions). IRT classified hooves with hoof abscess with high sensitivity (mean: 74.3%, range: 60% – 100%) and specificity (mean: 87.5%, range: 62.5% – 100%) across all ROIs, and analysis of the area under the curve (AUC) showed excellent discrimination (mean: 0.934, range: 0.881 – 0.964, Fig. (3) and Table 2).
| ROI | Hoof area | T1 ± SD | T2 ± SD | p | ΔT |
|---|---|---|---|---|---|
| 1 | Central cleft | 24.80 ± 3.56 | 30.32 ± 2.79 | < 0.0001 | 5.52 ± 3.45 |
| 2 | Lateral cleft (left side) | 27.67 ± 3.61 | 33.66 ± 1.65 | < 0.0001 | 5.99 ± 3.39 |
| 3 | Lateral cleft (right side) | 27.97 ± 3.56 | 33.63 ± 1.55 | < 0.0001 | 5.66 ± 3.33 |
| 4 | Frog | 25.52 ± 3.55 | 31.05 ± 2.55 | < 0.0001 | 5.53 ± 3.42 |
| 5 | Sole (left side) | 24.78 ± 3.83 | 31.30 ± 2.56 | < 0.0001 | 6.52 ± 3.67 |
| 6 | Sole (right side) | 24.66 ± 3.91 | 31.85 ± 2.25 | < 0.0001 | 7.19 ± 3.71 |
| 7 | Sole (middle side) | 24.98 ± 3.44 | 31.79 ± 2.43 | < 0.0001 | 6.81 ± 3.31 |
| 1 – 7 | Entire hoof body | 25.77 ± 3.87 | 31.94 ± 2.60 | < 0.0001 | 6.17 ± 3.71 |
| ROI | Cut-off value (°C) | Sensitivity (%) | Specificity (%) | AUC | SE | Significance |
|---|---|---|---|---|---|---|
| 1 | 27.75 | 80 | 75.0 | 0.881 | 0.050 | < .0001 |
| 2 | 32.35 | 90 | 91.1 | 0.963 | 0.030 | < .0001 |
| 3 | 32.05 | 80 | 85.7 | 0.951 | 0.030 | < .0001 |
| 4 | 28.75 | 70 | 76.8 | 0.884 | 0.055 | < .0001 |
| 5 | 29.05 | 90 | 87.5 | 0.945 | 0.041 | < .0001 |
| 6 | 29.40 | 90 | 87.5 | 0.964 | 0.021 | < .0001 |
| 7 | 28.80 | 80 | 83.9 | 0.950 | 0.027 | < .0001 |

4. DISCUSSION
Puncture wounds to the sole of the foot can introduce bacteria and debris to the solar surface of the distal phalanx, which frequently results in fracture or septic pedal osteitis. Septic pedal osteitis involves bone lysis of the distal phalanx and is accompanied by the presence of purulent exudate (which differentiates this condition from non-septic pedal osteitis) [15]. Radiography is sometimes useful to identify a gas or fluid pocket; however, it is difficult to locate a suitable drainage location by radiography. Moreover, drainage is possible only by experienced veterinarians or farriers. This study did not investigate whether the location of the inflammation matches the radiographic pockets of the abscessed hoof and the thermal pattern of thermography. Because, in the study, the thermal pattern showed the affected range as temperature distribution, but the exact location of the drainage could not be specified [16].
In this study, we demonstrate the usefulness of IRT as a diagnostic tool for detecting hoof abscess by correlating the temperature of healthy hooves and hooves with abscess. IRT is used for medical diagnostic purposes because it can measure the distribution of surface temperature and display the relationship between the surface temperature and lesions in the underlying anatomical structures [17]. As noted by Turner and Palmer, normal thermal patterns can be tabulated to accommodate the vascular system of horny tissue, which are highly symmetrical between the left and right sides of the body [8, 18]. Any asymmetry observed in thermal patterns would reflect an abnormality. Several studies have concluded that a temperature difference within 1 °C between the compared parts of the body is considered as normal [8, 19-21]. Most horses try to avoid stepping on the floor with minimal force when a sore leg occurs. That way, even without pain or other illness, the load on one side increases, whereas all other sides are resting, resulting in a high temperature. Therefore, temperature comparison with the other three hooves except for the hoof with an abscess in the same horse was excluded. Nevertheless, in this study, the range in temperature difference in the hoof was wide, even in the absence of inflammation or clinical signs. This cannot exclude the possibility of asymptomatic inflammation.
As shown in this study, the mean temperature of ROIs in hooves with abscess was higher than that of healthy hooves, a finding that was especially prominent for sole regions. The temperature range of healthy hooves (15.9 °C – 33.6 °C, Fig. (2a) was wider than that of hooves with hoof abscess (26.5 °C – 37.0 °C, Fig. 2b). This difference might reflect various influencing factors, such as season, exercise, and environment. Based on the average temperature of each ROI, the optimal cut-off value for discriminating hoof abscess was calculated through ROC analysis. The ROC curve is used to determine the usefulness of direct or indirect measurements of screening tests. The sensitivity and specificity of the sole region, where hoof abscesses mainly develop, were statistically significant at 96.7 and 88.3, respectively (p < 0.0001).
IRT aids in disease diagnosis by detecting the heat released by inflammation or increased blood circulation. The temperature changes are observed as an image using infrared heat sensing technology. Although it is an important early diagnosis technique that can provide a preventive diagnosis (screening) for each individual or group prior to a detailed diagnosis, it has not been as widely used in animals as in humans. Therefore, this study aims to investigate the utility of IRT as a new temperature measurement analysis system for determining the heat distribution of the hoof, which may be useful in the case of hoof abscesses. In conclusion, we believe that IRT would prove to be useful not only for pre-diagnostic purposes but also for determining the healing process in case of hoof diseases. In future research studies, the results of this study may be extrapolated not only to other hoof diseases (such as acute laminitis) but also to hoof diseases in other animals.
CONCLUSION
This study was conducted to establish IRT as a new temperature measurement analysis system and to use its indicators for detecting hoof abscess in horses. Through this study, we promote the early screening of horse hoof diseases, which will greatly contribute to the prevention of losing of high-valued horses .
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Animal Ethics Committee (KNU2019-0091) of Kyungpook National University in Korea.
HUMAN AND ANIMAL RIGHTS
No humans were used for the studies that are the basis of this research. All thermal imaging and sampling procedures on animals were performed according to the guidelines of the Animal Ethics Committee of Kyungpook National University in Korea for the care and use of experimental animals.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
The authors are grateful for the financial support provided by SGER through the National Research Foundation on Korea funded by the Ministry of Education, Science and Technology, grant number NRF-2017R1D1A02018652, and partially supported by the Ministry of Education, Science and Technology (NRF-2020R1IA3067905).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are grateful to the owners of the horse used in the study and also for the support of the three veterinarians and a farrier at the Jangsu Studfarm Adjunctive equine hospital of Korea Racing Authority.


