All published articles of this journal are available on ScienceDirect.
Mixed Cropping of Dwarf Napiergrass (Pennisetum purpureum cv. Mott) with Indigofera (Indigofera zollingeriana) using an Alley Cropping System
Abstract
Background:
Mixed cropping of forage grasses and legumes can potentially improve the performance of herbivores. However, the feasibility of grasses mixed-cropped with legumes should be examined at different plant densities.
Aim:
This study aimed to evaluate the growth characters and forage chemical composition in dwarf napiergrass (Pennisetum purpureum cv. Mott) with Indigofera (Indigofera zollingeriana) grown using alley cropping.
Methods:
Three densities of dwarf napiergrass with Indigofera, i.e., high density (2 and 1 plants m-2, respectively); medium density (1.33 and 1 plants m-2, respectively); and low density (1 and 1 plants m-2, respectively) were applied under rainfed conditions in Makassar.
Results:
Plant density significantly affected growth characteristics, such as plant height and tiller density in dwarf napiergrass (p<0.05), and non-significantly affected plant height and branch density (p>0.05) in Indigofera. Plant density did not affect the yielding ability at the first defoliation and annual total of yields (p>0.05), except at the second defoliation when the dry matter production of dwarf napiergrass peaked in the low plant density treatment (p<0.05). In terms of chemical composition, plant density significantly affected fiber concentrations of acid detergent fiber and neutral detergent fiber, which were lowest at low plant densities (p<0.05). The acid detergent lignin and cellulose concentrations tended to be lower at low plant densities, but the differences were not significant (p>0.05).
Conclusion:
We consider that alley cropping systems for dwarf napiergrass and Indigofera are suitable if low plant densities are employed in the region.
1. INTRODUCTION
Forage consisting of grasses and legumes is essential for the growth, nutrition, and reproduction of ruminant livestock. In addition, forage plays a crucial role in maintaining the health and function of the rumens in ruminant animals. The fiber components of forage, such as cellulose and hemicellulose, are used as energy sources for rumen microbes [1], and forage legumes provide nitrogen compounds for rumen bacteria [1]. Inadequate forage, both in terms of quantity and quality, is a barrier to optimal livestock rearing, and efforts to consistently provide good quality forage are important. One solution for improving the quality of feeding resources, especially for grass-fed cattle is to incorporate cropping of grasses mixed with legumes [1]. In monoculture systems, grasses produce a fresh yield of 400–2,000 kg m-2 year-1 depending on soil fertility. However, grass protein concentrations are 5–15% in dry matter (DM), which is markedly lower than that the 15–25% in legumes [2]; consequently, in terms of production and reproduction, grasses alone do not fully meet the needs of livestock. Moreover, in order to satisfy livestock nutritional requirements, producers need to supplement the diets of their livestock with ingredients, such as concentrates, which can significantly increase production costs because the raw materials used for producing concentrates are relatively expensive. In addition, to achieve high forage biomass production, relatively high fertilizer inputs using both chemical fertilizers [3, 4] and organic fertilizers [5] are necessary, which further increases production costs [6].
Mixed cropping refers to the simultaneous cultivation of grasses and legumes for use as forage on the same land. Mixing grasses and legumes has the potential to increase the quality and quantity of forage [1]. In southwestern Nigeria, mixed grass and legume crops have also been shown to control weeds more effectively and have a higher Crude Protein (CP) concentration than single grass crops [7]. The main objectives of these systems are to maximize the use of resources, such as space, insolation, and nutrients, which leads to increases in forage productivity and improves forage quality. Legumes can fix atmospheric nitrogen (N) in nodules on their roots [8]. Further, a range of N-transfer mechanisms to the host plant have been reported, including exfoliation of nodules, decaying of the root system, leaching of leaves, and decomposition of fallen leaves [9]. Nodule formation and adequate light are also essential factors for N fixation and transfer [10]. The direct contribution of legumes to livestock productivity is through the provision of feed sources that are rich in N compounds. When planted together with grasses, legumes can increase grass productivity by increasing N uptake from soils. Alley cropping systems can also be used as a management strategy to increase biodiversity and ecosystem function [11], which are also useful for maintaining soil properties, soil erosion control, and weed suppression [12], as well as increasing DM production through N fixation [8]. By using this system, the production and quality of forage can be improved, which potentially increases the livestock production potential and the bodyweight of livestock.
One of the promising combinations of grasses and legumes used in mixed-cropping systems is dwarf napiergrass and Indigofera. Dwarf napiergrass (Pennisetum purpureum cv. Mott) is a tropical forage grass that has high biomass, 10–15% crude protein, and in vitro DM digestibility >70% [3, 13, 14]; can be planted using manual or mechanized systems [15]; and is perennial with reasonable weed control [16]. Indigofera (Indigofera zollingeriana) belongs to the genus Indigofera, which contains 700 species found in Africa, Asia, Australia, and North America. Indigofera species are highly digestible and rich in neutral detergent fiber (NDF); even though calcium, phosphorus, magnesium, zinc, and manganese concentrations, CP content, and organic matter (OM) digestibility tend to be low, Indigofera can meet the minimum requirements for ruminants [17]. In addition, “this” species grows very fast at a variety of plant densities [18]. The main constraints in mixed-cropping systems using these two napiergrass and Indigofera plants are plant density and competition [19-21]. In cases where the plant density is too high, competition for soil nutrients and sunlight will increase and this will have a negative impact on the production and concentration of nutrients in the forage. However, planting at low plant densities will cause inefficient land use as well as low biomass production. Therefore, this study was conducted to determine the effect of plant density on the growth attributes, DM yield, and chemical compositions in dwarf napiergrass and Indigofera cultivated using an alley cropping system.
2. MATERIALS AND METHODS
2.1. Experimental Site, Soil Sampling, and Chemical Analysis
This study was conducted from December 2017 to July 2018 in Botto Lampe Hamlets of Lompo Tengah Village in Tanete Riaja Subdistrict, Barru Regency, South Sulawesi, Indonesia.
Soil samples (100 ml), which were collected at 12 sites at a depth of 10 cm below the ground surface in the experimental plot using the line transect method, were air-dried for a week. The following soil physicochemical parameters were measured in duplicate soil samples: pH (H2O) = 6.53 (Hanna 2211, Hanna Instruments Ltd., Bedfordshire, England); electric conductivity = 0.39 dS m-1 (CyberScan Con 410, Eutech Instruments PteLtd, Singapore); carbon (C) = 2.21% by the Walkley and Black method [22]; N = 0.19% by the Kjeldahl method [22]; and C/N = 11 (Soil Chemistry Laboratory, Faculty of Agriculture, Hasanuddin University, 2018).
2.2. Treatments and Research Design
The study employed a completely randomized design with three treatments performed in triplicate. Each plot (5 m × 7 m) in the alley cropping system contained the following densities of dwarf napiergrass and Indigofera, respectively: 2 and 1 plants·m-2 in the high-density treatment (P1); 1.33 and 1 plants·m-2 in the medium-density treatment (P2), and 1 and 1 plants·m-2 in the low-density treatment (P3).
The distance between each treatment and the edge of the field was uniformly fixed at 1 m, and that between each plot was fixed at 0.5 m. Before planting on January 3, 2018, the experimental plot was plowed using a hand tractor, and basic chemical fertilizer was applied at a dose of 200 kg N, P2O5, and K2O on the entire experimental plot (18 m × 24 m) to obtain a chemically homogeneous plot condition. The organic fertilizer contained C >12%; C/N 15–25; pH 4–8; moisture 10–20%; microbes >103 (PT Berdikari, Persero, Jakarta, Indonesia). Next, dwarf napiergrass tillers and Indigofera shoots were planted at the three different treatment densities (P1 – P3). No water was supplied at the time of planting because it was the rainy season. Weed control of the experimental plot was performed by manual weeding twice, once on January 17 and once on February 1, 2018.
2.3. Measurements and Calculations
2.3.1. Determination of Growth Attributes and Yield
Growth characteristics of dwarf napiergrass, such as plant height and tiller density, were measured for 18, 18, and 12 plants in P1, P2, and P3 treatment plots, respectively. Branches of Indigofera were measured for 16 plants·plot-1. Data collection was performed on February 3 (Month–1), March 3 (Month–2), March 31 (Month–3), when the first defoliation was performed, and July 3, 2018 (Month–4), when the second defoliation was performed.
The leaf width of three clumps of dwarf napiergrass and six clumps of Indigofera were measured using a leaf area meter (KWF Leaf Area Meter, KWF Sci-tech Development Co., Ltd., Indonesia) and chlorophyll values were also measured (SPAD 502, Konica Minolta, Inc., Tokyo, Japan).
The total DM production of dwarf napiergrass was measured using three randomly selected plants per replication on each plot. Napiergrass samples were collected using a sickle with a cutting height of 10 cm from the soil surface [23] and three Indigofera were collected within a cutting height of 100 cm above the ground [24]. No plant fractionation into leaves and stems was applied for dwarf napiergrass or Indigofera. Next, fresh sub-samples of 250 g of each species were oven-dried at 70°C for 4 days to measure the DM percentage as follows [25]:
DM production (Mg ha-1) = (Total FW × (DWss / FWss) × 10−2)
where Total FW is the total amount of fresh grass (gm−2), DWss is the dry weight of the grass sub-sample, and FWss is the weight of the fresh grass sub-samples in grams.
2.3.2. Chemical Analysis of Cell Wall Components
To assess the fiber composition, neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) were determined for dwarf napiergrass samples at the first defoliation using a modification of the method described in another study [26]. The compositions of cell wall contents, such as hemicellulose and cellulose, were calculated based on the following equations:
Hemicelluloses = NDF – ADF
and
Celluloses = ADF – ADL [27]
2.4. Statistical Analysis
Analysis of variance (ANOVA) was performed to analyze the growth characters, chemical composition, fresh yield, and DM yield for the different plant densities of dwarf napiergrass and Indigofera cultivated using the alley cropping system. Furthermore, the data were processed using SPSS software for Windows (ver. 16.0, Chicago, IL). Differences in mean values were tested at the 5% level using the least significant difference (LSD).
3. RESULTS
3.1. Site Description and Climatic Conditions
Barru Regency is a tropical region on the west coast of South Sulawesi Province. Based on the Agro-climatological Zone classification, Barru Regency can generally be classified as having a type C climate, with monthly rainfall >200 mm·month-1 in the wet months (October-March), and <100 mm·month-1 in the dry months (April-September). The average temperature ranges from 20ºC to 35ºC (Balai Besar Sungai Pompengan Jeneberang, Makassar, 2018).
The average rainfall in the experimental periods in 2018 reached 537.2 mm/month, with the highest rainfall in December (1,566 mm) and the lowest in August (71 mm). Rainfall at the time of planting in January 2018 was 1,180 mm, decreasing to 705 mm at the first defoliation in March 2018, and a minimum of 115 mm at the second defoliation in July 2018 (Balai Besar Sungai Pompengan Jeneberang, Makassar, 2018).
3.2. Growth Attributes of Forage
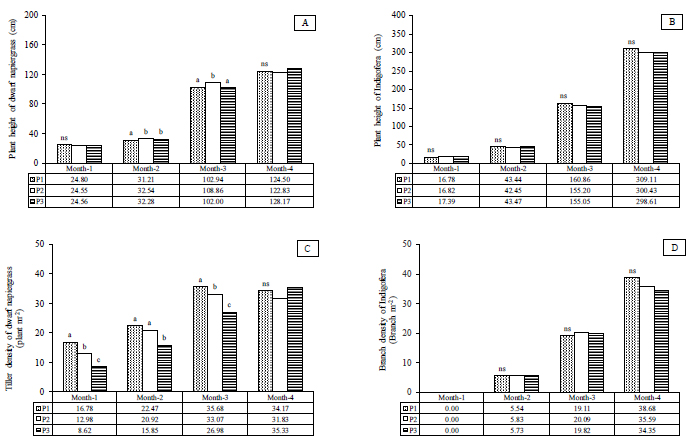
In terms of the effect of plant density on the growth characters of plant height and tiller densities, a significant difference (p<0.05) and non-significant difference (p>0.05) was observed in dwarf napiergrass and Indigofera, respectively (Fig. 1A-1D). In both dwarf napiergrass and Indigofera, plant height tended to increase over the course of the experiment. The plant height of dwarf napiergrass at the first defoliation (Month–3) was significantly higher (p<0.05) for the medium-density treatment (P2) than for the other treatments, although that for the low-density treatment (P3) tended to be higher (p>0.05) at the second defoliation (Month–4) in Fig. (1A). The tiller density in dwarf napiergrass also increased over the course of the experiment, except for the period from the first to the second defoliation when that for the high- (P1) and medium- (P2) density treatments decreased at the second defoliation in Month–4. In dwarf napiergrass, the high-density (P1) treatment had a significantly higher (p<0.05) tiller density than the other treatments, except for at the second defoliation in Month–4 when the low-density treatment (P3) tended to have a higher (p>0.05) tiller density than the medium-density treatment (P2) (Fig. 1C). The branch density of Indigofera increased from Month–2 to Month–4, except in Month–1 when no branches were produced. Branch density tended to be higher (p>0.05) in the medium-density treatment (P2) than the other densities in both Month–2 and Month–3, while that in the high-density treatment (P1) tended to be higher at the second defoliation in Month–4 (Fig. 1D).
3.3. Dry Matter Yield of Forage
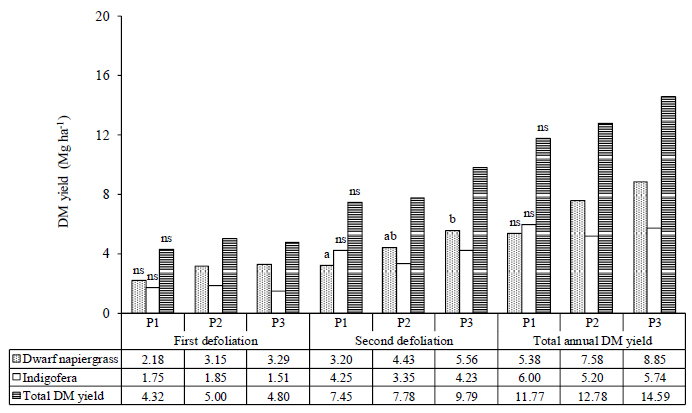
The DM yield of dwarf napiergrass increased from the first defoliation to the second in all treatments. At the first defoliation (Month–3), the treatment did not affect DM yield in dwarf napiergrass (p>0.05). However, at the second defoliation, the highest DM yield in dwarf napiergrass was obtained for the low-density (P3) treatment, followed by the medium-density (P2) treatment, and the lowest in the high-density (P1) treatment.
The DM yield of Indigofera did not differ among treatments in either the first or the second defoliation. Thus, the total DM yield (i.e., combining dwarf napiergrass and Indigofera) tended to be higher in the medium-density (P2) treatment compared to the other densities at the time of the first defoliation (p>0.05), and tended to be higher in the low-density (P3) treatment compared to the other densities at the time of the second defoliation (p>0.05). Annual total DM yield tended to be higher (p>0.05) in the low-density (P3) treatment compared to the other densities, followed by the medium- (P2) and the high-density (P1) treatments, determined by the DM yield in dwarf napiergrass (Fig. 2).
Values with different superscripts were significantly different (p<0.05) among plant densities. ns: not significant (p>0.05).
Values with different superscripts are significantly different (p<0.05) among plant densities. ns: not significant (p>0.05).
| Species | Plant Density† | Chlorophyll Value | Leaf Width (mm leaf-1) | ||||
|---|---|---|---|---|---|---|---|
| Month–1†† | Month–2†† | Month–3†† | Month–1 | Month–2 | Month–3 | ||
| Dwarf napiergrass | P1 (High) | 46.34±3.27ns‡ | 34.17±1.03 | 36.52±2.10 | 53.53±2.30 | 59.66±1.61 | 44.50±2.01 |
| P2 (Medium) | 47.19±2.67 | 37.38±1.07 | 34.68±0.97 | 58.79±4.07 | 62.56±0.70 | 48.58±1.62 | |
| P3 (Low) | 48.81±2.24 | 38.27±3.90 | 37.91±1.08 | 56.78±5.98 | 59.64±1.69 | 53.02±1.51 | |
| Indigofera | P1 (High) | 37.73±0.77ns | 41.12±1.14 | 39.88±1.35 | ---⁋ | --- | --- |
| P2 (Medium) | 39.35±0.89 | 42.40±1.20 | 41.56±0.79 | --- | --- | --- | |
| P3 (Low) | 37.83±1.34 | 42.54±0.58 | 41.02±1.05 | --- | --- | --- | |
⁑ ADF: acid detergent fiber, NDF: neutral detergent fiber, ADL: acid detergent lignin.
‡Values with different superscripts indicate significant differences among plant densities (p <0.05). ns: not significant (p>0.05).
Mean ± standard deviation (n = 3).
3.4. Chlorophyll Value, Leaf Width, and Chemical Composition of Forage
Plant density did not affect the chlorophyll content or the leaf width in either dwarf napiergrass or Indigofera in any month (p>0.05). The chlorophyll value tended to be higher for the low-density (P3) treatment than the other densities (p>0.05), while the leaf width characteristics were inconsistent with respect to months among treatments. However, in Indigofera, the chlorophyll value tended to be lower for the high-density (P1) treatment than the other densities from Month–1 to Month–3 (p>0.05) (Table 1).
Plant density affected ADF and NDF concentrations in dwarf napiergrass (p<0.05) and was lowest for the low-density (P3) treatment than for the other densities. In dwarf napiergrass, the hemicellulose and cellulose contents in ADL were not significantly different among treatments (p>0.05), while in Indigofera, ADL and cellulose contents tended to be lower for the low-density (P3) than the other densities (Table 2).
4. DISCUSSION
In the alley cropping system, we need to consider how plant density affects growth attributes. If the crops are planted at high densities, then competition for sunlight between the different crops is expected. If the crops are planted at low densities, then the crop stand may not obtain sufficient solar insolation and oxygen, which has a negative effect on the growth rate. Photosynthesis refers to the process whereby carbohydrates are formed from CO2 and H2O in green leaves exposed to solar energy. Carbohydrate production increases under N-rich conditions, and nitrogen is used for synthesizing proteins [18]. Since carbohydrates and proteins are components of plant DM, the increased formation of protein and carbohydrates will increase the DM yield of crops. Mwangi [20] demonstrated that chlorophyll formation in napiergrass fodder system is highly dependent on the supply of sunlight, oxygen, carbohydrates, water, and nutrients.
In the vegetative stage in Month–1, dwarf napiergrass increases the size and number of organs, such as leaves, stems, and roots (Fig. 1A). At this stage, plant height increased similarly in all treatments (Fig. 1A-1D), and the shoot meristem of dwarf napiergrass differentiates to form leaves that capture sunlight and photosynthesize. The nutrients that are produced in this way are then used to form roots [28]. The difference in tiller density among treatments at this stage was mainly affected by planting density; tiller density for the high-density (P1) treatment (i.e., 2 plants·m-2) was almost twice that for the low-density treatment (P3) (1 plant·m-2) (Fig. 1C). From Month–1 to Month–2, increases in plant height and tiller density were comparable for all treatments and the highest plant height and tiller density were obtained for the medium-density (P2) and high-density (P3) treatments, respectively (Figs. 1A and 1C).
In the stem-elongation stage in Month–3, dwarf napiergrass growth increased because the root system was well developed and the absorption of water and nutrients was sufficient to support the aboveground growth. However, the order in plant height and tiller density among treatments was also maintained during the period from Month–2 to Month–3. After defoliation in Month–3, the growth characteristics of dwarf napiergrass returned to the vegetative stage. However, the growth rate of dwarf napiergrass increased markedly in the stage from Month-3 to Month-4 than at the stages in Months–1 and –2, with plant height and tiller density in Month–4 in dwarf napiergrass being comparable to that observed in Month–3; however, no significant difference was observed in the growth characters of dwarf napiergrass among treatments (p>0.05) (Figs. 1A and 1C).
In the vegetative stage, the plant height of dwarf napiergrass was superior to that of Indigofera; however, the growth of Indigofera results in the formation of canopies, since the legume is included in the category of scrub plants [17]. Therefore, under low plant densities (P3), the alley dropping system minimizes competition between dwarf napiergrass and Indigofera, because Indigofera does not shade-out and inhibit the growth of dwarf napiergrass, which has vertical leaves to increase the interception of solar radiation [28]. Hatta [29] demonstrated that plant density affects plant growth and that the absorption of solar energy by the leaf canopy greatly affects plant growth; at high densities, less sunlight reaches the leaf canopies and competition between the alley-cropped plants for this sunlight increases. In the present study, Indigofera forms canopies with the minimum cutting height of 100 cm [24], and thus can inhibit the growth of dwarf napiergrass at high plant densities (P1).
Furthermore, Gardner et al. [30] reported that the agronomic aims to study the underlying plant density regulation to minimize intrapopulation competition so that the canopy leaves and plant roots can take advantage of the above-ground and below-ground environments in an optimal manner. In shaded conditions, plant canopies receive less solar radiation, which decreases photosynthesis, respiration, transpiration, protein synthesis, hormone production, translocation of assimilates, and aging, as well as reducing yield and quality of dwarf napiergrass [28, 31]. In addition, nitrogen fixation activity in the nodules of legumes also decreases under shaded conditions [8]. Compared to the high-density (P1) treatment, dwarf napiergrass at lower plant densities (P2 and P3) typically receives more insolation for photosynthesis, which is necessary for producing both glucose and proteins.
Dwarf napiergrass can be defoliated at the stem-elongation stage as this is when levels of tiller production, protein quality, forage digestibility, palatability, DM productivity, and regrowth ability are all optimal [2].
In contrast to plant height, tiller density tends to decrease at low plant densities, while interestingly, plants with low tiller densities tend to have a larger stem diameter, be more robust, and produce more tiller buds after defoliation (Fig. 1C). Lower plant densities also decreases the competition for nutrients, which means that the amount of solar radiation available for photosynthesis increases, increasing DM production in the canopy, plant height and tiller production in C4 grass [32]. At solar radiation intensities of 60–100%, tiller weight can increase by as much as 1.3 times that of the main shoot, which means that tiller density decreases but tiller DM production increases at low plant densities in the normal and dwarf napiergrasses [33]. Sunlight thus plays an important role in increasing the number of tillers, and higher tiller densities are observed at higher sunlight intensities in napiergrass [14].
In the present study, low plant densities tended to increase the DM yield of dwarf napiergrass at the first and second defoliation, and total annual DM yield (Fig. 2). In response to an open canopy and high sunlight intensities, the high levels of DM production in dwarf napiergrass are considered to occur due to the increase in nutrient absorption resulting from the high root density associated with the inhibition of nutrient absorption in Indigofera. Indigofera is a legume that is capable of fixing atmospheric N via symbiotic bacteria in root nodules, which increases the soil N content. Compared to single cropping systems, a significant increase in annual DM yields has been reported in mixed cropping systems using grass and legume species [34]. Leucaena mixed-cropped with napiergrass can increase soil fertility and potentially extend the productive period of napiergrass. For example, the presence of Leucaena in a napiergrass pasture has been shown to significantly increase the crude protein content and potential feeding value of napiergrass [35].
Regarding the disadvantages of high plant densities, Irfan [36] reported that high plant densities reduce DM yields due to increased competition for nutrients, water, solar radiation interception, and available space among the mixed-cropped seeds that are planted. Competition between plants in the same growing soil is a quite common phenomenon because of limited resources, such as water, nutrients, and sunlight [37]. Moreover, imbalances in the composition of grasses and legumes in mixed-cropped pastures results in the suppression of some plants by the dominant plants [38]. In the present study, the total annual DM yields of forage ranged from 23.15 to 29.18 Mg ha-1·year-1, which is higher than that reported in some studies [5, 14], and [16], but lower than that reported in others [39-42]. This difference may have occurred due to differences in pasture management, such as in fertilization, cutting intervals, pure stands and mixed cropping systems, climate, and temperature.
In the present study, defoliation was carried out at the stem-elongation stage, which was considered to be optimal because, at this stage, the forage qualities of dwarf napiergrass (i.e., in terms of ADF, NDF, ADL, cellulose, and hemicellulose) all met the recommended minimum standards of forage for ruminants [43]. NDF is considered to be the best fiber component for digestibility [43]. If the forage has high digestibility, then it will be digested quickly and the animal will be able to consume more feed [2]. Forage should be harvested at the optimal stage for production and quality [44], and a large quantity of good quality forage is critical for obtaining forage of optimal quality [45]. With increasing plant age, the NDF and lignin contents increase and CP and water-soluble carbohydrates decrease; therefore, the digestibility of older plants is reduced [46-48]. In terms of feed quality, ADF, NDF, and hemicellulose all play an important role.
Apart from plant density, using Indigofera as a green manure is very beneficial for dwarf napiergrass as the high rate of manure application increases the CP content and reduces the fiber content in the napiergrass [5]. Soil N is the most critical nutrient for plant growth and development, and positively affects DM yield, CP concentration, and digestibility [49]. Increases in NDF, ADF, and lignin contents at the stem-elongation stage are associated with increases in the stem to leaf ratio and fibrous tissues responsible for maintaining plant structure as the plant grows. Importantly, cell wall components are higher in stems compared to leaves [47], and dwarf napiergrass has a higher percentage of leaf blades than stems [5, 13, 14].
In the present study, the average fiber concentrations in the low, medium, and high plant density treatments were 54.46%, 59.77%, and 55.41% for NDF, and 39.62%, 42.89%, and 41.42% for ADF, respectively. These values are lower than those reported in a previous study [50] and higher than those reported in another study [40]. The hemicellulose and cellulose contents in NDF were lower than those reported previously [14, 42].
CONCLUSION
It was observed that the present range in the medium and high plant densities had a non-significant effect on the growth characteristics and DM yield of dwarf napiergrass, while low plant densities tended to have higher DM yields. Plant density did not affect the chlorophyll content or leaf width of either dwarf napiergrass or Indigofera in any of the measurement periods. In terms of chemical composition, plants in the low-density plot had lower fiber concentrations of ADF and NDF. Although the ADL, hemicellulose, and cellulose fractions were not affected by plant density, the values of these three quality fractions tended to be better at low plant densities. Therefore, we recommend that low plant densities of 1 plant·m-2 of dwarf napiergrass and 1 plant·m-2 of Indigofera be used for cultivation in alley-cropping systems.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for the studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available from the author upon request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


